Abstract
Periprocedural complications, including perforator occlusion, in-stent restenosis, and even death, are frequently encountered in endovascular therapy for basilar artery (BA) lesions. This study sought to evaluate the efficacy of the Neuroform EZ stent in managing atherosclerotic BA stenosis. A retrospective analysis was conducted on clinical data from 71 patients with symptomatic atherosclerotic BA stenosis who underwent Neuroform EZ stent implantation. The primary outcomes assessed were periprocedural complications (ischemic stroke, death, restenosis, or hemorrhagic stroke) within 30 days post-surgery. Additionally, multivariable logistic regression analysis was used to identify risk factors for in-stent restenosis (ISR). Results showed that the mean stenosis degree significantly improved from 85.2% ± 8.7% before treatment to 25.8% ± 12.5% after Neuroform EZ stent placement. Furthermore, the mean NIHSS score decreased by 3 points following the procedure. The overall ISR rate among all patients was 6.5%. Multivariable logistic regression analysis revealed that irregular post-procedural antiplatelet therapy (HR, 3.231; 95% CI, 1.09–4.61; p = 0.031), lesion length (HR, 2.966; 95% CI, 1.21–4.23; p = 0.026), and lesion location (HR, 3.117; 95% CI, 1.12–4.15; p = 0.042) were independent risk factors for long-term ISR. In conclusion, this study provides evidence supporting the effective use of the Neuroform EZ stent in the treatment of refractory BA stenosis.
Similar content being viewed by others
Introduction
While the US Food and Drug Administration (FDA) has noted a risk of stroke or death associated with the Wingspan stent system for intracranial atherosclerotic disease, it remains a treatment option for patients experiencing recurrent stroke despite optimal medical management1,2. In comparison, the Neuroform EZ stent (Stryker Neurovascular, Fremont, USA) offers greater flexibility and accessibility than the Wingspan device. It exerts lower radial outward force and has been shown to effectively reduce the incidence of in-stent restenosis (ISR)3. Conversely, delivering and deploying the Wingspan stent (Boston Scientific Corporation, Fremont, USA) in tortuous vascular pathways can be technically challenging, leading to lower overall technical success rates and heightened risks of procedural complications. Moreover, unlike the Wingspan system, the Neuroform EZ stent is deliverable via a 0.027-inch microcatheter4,5. Given these advantages, the Neuroform EZ stent holds promise for achieving high technical success and reducing periprocedural complication rates in the treatment of basilar artery (BA) stenosis. However, its clinical outcomes and efficacy in this context remain unclear. In this study, we retrospectively analyzed periprocedural complications and postoperative outcomes in patients who underwent Neuroform EZ stenting for BA stenosis, aiming to investigate the stent’s efficacy in this indication. To our knowledge, this is the first study to evaluate the efficacy of the Neuroform EZ stent system specifically for BA stenosis. Our findings suggest that the Neuroform EZ stent represents a potential therapeutic option for medically refractory BA stenosis.
Methods
Patients
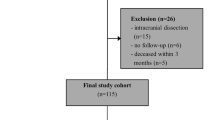
We analyzed data from patients with medically refractory basilar artery (BA) atherosclerotic stenosis (> 70%) who underwent Neuroform EZ stenting at Xiangya Hospital. Demographic and clinical variables recorded included age, sex, presenting symptoms, stenosis location, length, and severity, Mori classification of stenosis6, stent model, residual stenosis post-percutaneous transluminal angioplasty and stenting (PTAS), perioperative complications, response to antiplatelet agents (aspirin and clopidogrel), modified Rankin Scale (mRS) scores at discharge, and 30-day postoperative mortality. Stenosis severity was quantified using the Warfarin-Aspirin for Symptomatic Intracranial Disease (WASID) criteria. Inclusion criteria were: (1) severe BA atherosclerotic stenosis (> 70% confirmed by digital subtraction angiography [DSA]); (2) recurrent transient ischemic attacks (TIAs) or ischemic stroke attributable to BA atherosclerosis; (3) at least one documented atherosclerotic risk factor (hypertension, hyperlipidemia, diabetes mellitus, smoking, or hyperhomocysteinemia); (4) computed tomography perfusion (CTP) imaging (via RAPID automated software) demonstrating > 30% reduction in the BA vascular territory; and (5) age 18–85 years. Exclusion criteria were: (1) high-resolution magnetic resonance imaging (HR-MRI) indicating non-atherosclerotic intracranial pathology; (2) concurrent intracranial abnormalities (aneurysm, tumor, inflammation, or arteriovenous malformation); (3) DSA (Azurion 7 M20, Amsterdam, Netherlands) confirming complete BA occlusion; (4) functional dependence due to severe neurological deficits; and (5) contraindications to aspirin, heparin, or clopidogrel. The study flowchart is presented in Fig. 1. This retrospective study was approved by the Institutional Ethics Review Board of Xiangya Hospital, Central South University (No. 2018030447), with all procedures conducted in accordance with the Declaration of Helsinki and relevant ethical standards.
Perioperative management
Angiography and interventional procedures were performed by board-certified neuroradiologists with subspecialty expertise. All patients underwent thromboelastography (TEG) preoperatively to assess antiplatelet responsiveness. A loading regimen of clopidogrel (75 mg daily) and aspirin (100 mg daily) was administered for at least 5 days prior to PTAS. Patients with adequate dual antiplatelet response (ADP inhibition > 30% for clopidogrel; arachidonic acid [AA] inhibition > 70% for aspirin) continued this regimen. For clopidogrel resistance (ADP inhibition < 30%, typically identified within 2 days via TEG), ticagrelor was substituted for clopidogrel while aspirin was maintained. Aspirin was replaced with cilostazol in cases of aspirin resistance (AA inhibition < 70%). Atherosclerotic risk factors (hypertension, homocysteine, low-density lipoprotein cholesterol, and glycemia) were managed in accordance with guidelines for secondary prevention of ischemic stroke2.
Neurointerventional procedure
Intravenous heparin (50 U/kg) was administered as a bolus prior to the endovascular intervention. Femoral artery access was established using introducer sheaths for PTAS. Following femoral cannulation and induction of general anesthesia, a 6 Fr guiding catheter was advanced to the vicinity of the stenotic segment. An Excelsior XT-27 microcatheter (Stryker Neurovascular, Fremont, CA, USA) and a 200 cm Synchro-14 guidewire (Stryker Neurovascular) were navigated from the proximal to distal aspect of the target lesion. Angioplasty was performed using a Gateway PTA balloon (Boston Scientific Corporation, Fremont, USA), sized to approximately 80% of the reference vessel diameter (proximal or distal to the lesion) to facilitate arterial straightening during inflation. The microcatheter was then advanced across the target lesion to enable stenting. A Neuroform EZ stent (Stryker Neurovascular) was delivered through the microcatheter, positioned at the stenotic site, and deployed once aligned with the distal margin of the lesion.
Radiologic and clinical follow-up
All patients underwent National Institutes of Health Stroke Scale (NIHSS) and mRS assessments pre- and post-PTAS. DSA images were reviewed to document stenosis characteristics, anatomical features, residual stenosis, side-branch patency, and periprocedural complications. The primary endpoints were periprocedural complications (ischemic stroke, death, restenosis, or hemorrhagic stroke) within 30 days of surgery. Post-stenting restenosis-associated ischemic stroke was defined as a new focal neurological deficit lasting ≥ 24 h or an NIHSS score ≥ 3 during follow-up. In-stent restenosis (ISR) was defined as ≥ 50% luminal narrowing within the stent or within 3 mm of its edges. Clinical follow-up was conducted at 1, 3, 6, and 12 months postoperatively. Angiographic follow-up (DSA, MRA, or CTA) was performed for patients with recurrent symptoms or at 12 months for asymptomatic individuals. Follow-up metrics included ISR rate, recurrent ischemic events, and all-cause mortality. Hemorrhagic stroke was defined as a new neurological deficit or NIHSS score ≥ 3 attributable to subarachnoid, intraventricular, or parenchymal hemorrhage during follow-up. Technical success was defined as < 50% residual stenosis with preserved antegrade flow and complete lesion coverage.
Statistical analysis
Continuous variables were analyzed using the independent Mann-Whitney U test or Student’s t-test, as appropriate. Categorical variables were evaluated using the χ² test or Fisher’s exact test. Logistic regression analysis was performed to identify independent predictors of ISR. A two-tailed p-value < 0.05 was considered statistically significant. All analyses were conducted using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
Informed consent
All enrolled patients provided written informed consent, including authorization for the use of their deidentified data for research purposes.
Results
Patient characteristics
A total of 71 patients were included in this retrospective study, comprising 51 males and 20 females with a mean age of 62.34 ± 7.53 years (range, 48–77 years). All patients underwent Neuroform EZ stenting within the basilar artery (BA) segment (Fig. 2). The median interval between the onset of recurrent symptoms and endovascular intervention was 83.28 days (range, 15–360 days). Pre-procedural stenosis-related neurological deficits were categorized as transient ischemic symptoms (78.8%, n = 56) or acute ischemic stroke (21.2%, n = 15). Detailed patient demographics and clinical features are summarized in Table 1.
BA stenosis and angiographic characteristics
Target lesion severity was distributed as follows: 75% stenosis in 18 patients, 80% in 33 patients, and 90% in the remaining 20 patients. Stenosis locations within the BA were: proximal segment (43 patients, 60.6%), middle segment (16 patients, 22.5%), and distal segment (12 patients, 16.9%). Based on Mori classification, lesions were categorized as type A (53.5%, n = 38), type B (21.1%, n = 15), and type C (25.3%, n = 18).
Periprocedural outcomes
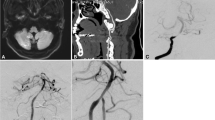
The overall technical success rate was 100%, with no patients requiring implantation of multiple stents. No vessel dissection, stent migration, or acute stent occlusion was observed. The mean pre-procedural stenosis was 85.2% ± 8.7% (median, 84.1%; interquartile range, 75–90%) (Fig. 3), while the immediate post-stenting residual stenosis was 25.8% ± 12.5% (Fig. 4). One patient (1.8%) developed ischemic symptoms with contralateral hemiparesis persisting for > 24 h post-PTAS. Post-stenting angiography revealed de novo stenosis of ~ 75% within the stented segment (Table 2). The National Institutes of Health Stroke Scale (NIHSS) score improved by 3 points following the procedure. Magnetic resonance imaging (MRI) demonstrated a new diffusion-weighted imaging (DWI) lesion in the left pons (Fig. 5), consistent with a perioperative perforator occlusion event. No cases of vasospasm, intracranial hemorrhage, or mortality were observed.
Representative images of cases with basilar artery stenosis before stenting. (a) Magnetic resonance angiography showing high-grade stenosis (90%) in the lower segment of the basilar artery. (b) High-resolution magnetic resonance imaging demonstrating the cross section of stenosis in the basilar artery. (c–e) Posterior digital subtraction angiography confirming the stenosis in the lower segment of the basilar artery. The red arrow indicates the location of stenosis.
Revascularization of the basilar artery after Neuroform EZ stent placement. (a, b) Microwire anchoring in the distal branch of the basilar artery and the dilated stenosis lesion. (c, d) Improvement of stenosis lesion immediately after Neuroform EZ stent placement. (e) Absent post-PTAS perforator occlusion in this patient. (f) Six-month follow-up MRA image revealing the patency of the basilar artery. The red arrow represents the residual stenosis after surgery.
Perforator occlusion occurred in one patient (3.0%) during the periprocedural period. (a–d) T2 and fluid attenuated inversion recovery sequence axial MRI displaying a small increase in signal intensity in the left side of the pons, indicated by the red arrow. Diffusion-weighted imaging sequences confirming the new infarction event. (e, f) MRA and high-resolution MRI indicating the patency of the basilar artery without in-stent restenosis.
Follow-up outcomes
Clinical follow-up was completed at 1, 3, 6, and 12 months postoperatively in 54 patients. Angiographic follow-up (DSA, MRA, or CTA) was performed in 36 patients (DSA) and 18 patients (MRA/CTA) either for recurrent symptoms or as routine assessment at 12 months for asymptomatic individuals. Among these, 36 underwent DSA, while 18 underwent MRA (n = 13) or three-dimensional CTA (n = 5). One patient experienced an ischemic stroke involving the right lateral medulla on day 65; MRI and cerebral angiography confirmed a new infarction and ~ 70% stenosis in the mid-BA segment, respectively. Additionally, two patients developed vertigo and ataxia without evidence of new infarction on MRI; catheter angiography identified two de novo stenoses of ~ 50% in the mid-BA segment (Table 2). The overall in-stent restenosis (ISR) rate was 6.5%, with no asymptomatic cases of significant ISR. Notably, all three patients with ISR had discontinued post-procedural antiplatelet therapy due to concurrent medical conditions. Lesion characteristics of these three patients are summarized in Table 3. Collectively, the post-procedural complication rate following Neuroform EZ stenting for BA atherosclerotic stenosis was 5.6%.
Risk factors associated with ISR
Comparative analysis of lesions with and without subsequent ISR/occlusion revealed that ISR lesions were longer (> 2 cm) than non-ISR lesions. All ISR lesions were classified as Mori type C, located in the mid-BA segment, and exhibited irregular contours. Multivariable logistic regression analysis (median follow-up, 20.4 months) identified lesion length (hazard ratio [HR], 2.966; 95% confidence interval [CI], 1.21–4.23; p = 0.026), irregular post-procedural antiplatelet therapy (HR, 3.231; 95% CI, 1.09–4.61; p = 0.031), and lesion location (HR, 3.117; 95% CI, 1.12–4.15; p = 0.042) as independent risk factors for long-term ISR (Table 4).
Discussion
Previous studies have evaluated the short-term safety of the Apollo stent for mitigating basilar artery (BA) stenosis7. Additionally, multiple investigations have explored the safety and mid-term outcomes of direct stenting versus pre-stenting angioplasty in BA interventions8,9,10,11,12, with findings suggesting that pre-stenting angioplasty may be the preferred approach for BA stenting. However, perforator ischemic stroke remains associated with both angioplasty and stenting. Given the relatively high risk of perforator stroke, careful evaluation of BA lesion subtypes and selection of safer interventional strategies are critical when determining optimal stent use.
In the present study, the follow-up complication rate was 5.6%, with a notably low incidence of perforator ischemic events (1.8%) compared to the SAMMPRIS trial. Furthermore, Neuroform EZ stent deployment achieved a 100% technical success rate with effective BA revascularization. Multivariable logistic regression analysis identified irregular post-procedural antiplatelet therapy, lesion length, and lesion location as independent risk factors for long-term in-stent restenosis (ISR). Among the 71 patients, 4 (5.6%) developed ISR and/or occlusion post-intervention, supporting the efficacy of the Neuroform EZ stent in treating BA stenosis. It is important to note, however, that 1 patient experienced ischemic symptoms with contralateral hemiparesis immediately post-PTAS, and 3 additional patients developed ischemic stroke during follow-up. These cases may introduce bias into the analysis of follow-up complication rates associated with Neuroform EZ stenting, highlighting the need for validation through larger patient cohorts and prospective clinical trials.
Post-stenting restenosis is a major complication affecting outcomes in intracranial atherosclerotic stenosis (ICAS) stenting trials. A systematic review reported that post-stenting restenosis occurs in up to 17.4% of patients with ICAS13,14,15, while the SAMMPRIS trial documented restenosis rates of 7.5–31.2% following Wingspan stenting. Notably, the post-stenting restenosis rate in our study was 5.6%, lower than rates reported in previous literature. Unlike the Wingspan stent, the Neuroform EZ stent exerts relatively lower radial force16,17, which may contribute to the reduced restenosis incidence observed here by minimizing vascular trauma.
Overall, our single-center study demonstrates encouraging perioperative and postoperative outcomes in patients with severe BA stenosis (> 70%) treated with PTAS using the Neuroform EZ stent. These findings suggest that Neuroform EZ stenting may represent a viable therapeutic option for medically refractory BA stenosis.
This study has several limitations. First, selection bias may arise from its retrospective design, inclusion of only Chinese patients, and lack of randomization or a control group. Second, the single-center setting and small sample size limit statistical power; while trends toward high technical success and low complication rates were observed, these could not be rigorously validated statistically. Finally, the imbalance in patient sex distribution should be considered when interpreting results.
Conclusion
Our findings provide evidence supporting the effective use of the Neuroform EZ stent in the management of refractory basilar artery (BA) stenosis, characterized by low periprocedural complication rates and high technical success. Consequently, the Neuroform EZ stent emerges as a promising therapeutic option for medically refractory BA stenosis.
Data availability
Data are available upon request. Source data may be obtained from the corresponding author on reasonable request.
References
Barnard, Z. R. & Alexander, M. J. Device profile of the wingspan stent system for the treatment of intracranial atherosclerotic disease: overview of its safety and efficacy. Expert Rev. Med. Devices. 17, 167–171. https://doi.org/10.1080/17434440.2020.1732813 (2020).
Chimowitz, M. I. et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl. J. Med. 365, 993–1003. https://doi.org/10.1056/NEJMoa1105335 (2011).
Li, H. et al. The safety and efficacy of the neuroform EZ stent for the treatment of symptomatic atherosclerotic stenosis in the middle cerebral artery. Clin. Imaging. 82, 210–215. https://doi.org/10.1016/j.clinimag.2021.11.024 (2022).
Xu, H. et al. Neuroform EZ stenting for symptomatic intracranial artery stenosis: 30 days outcomes in a High-Volume stroke center. Front. Neurol. 10, 428. https://doi.org/10.3389/fneur.2019.00428 (2019).
Daou, B. J. et al. Stent-assisted coiling of cerebral aneurysms: head to head comparison between the neuroform atlas and EZ stents. Interv Neuroradiol. 27, 353–361. https://doi.org/10.1177/1591019921989476 (2021).
Ohkawa, S., Tabuchi, M. & Mori, E. [Classification of lacunar stroke–subtypes and lesions]. Nihon Rinsho. 51 (Suppl), 654–664 (1993).
Tang, L. et al. Treatment of Basilar artery stenosis with an Apollo balloon-expandable stent: a single-centre experience with 61 consecutive cases. Acta Neurol. Belg. 121, 1423–1427. https://doi.org/10.1007/s13760-020-01311-8 (2021).
Jia, B. et al. Factors associated with perforator stroke after selective Basilar artery angioplasty or stenting. J. Neurointerv Surg. 9, 738–742. https://doi.org/10.1136/neurintsurg-2016-012329 (2017).
Liu, L. et al. Stenting for symptomatic intracranial vertebrobasilar artery stenosis: 30-day results in a high-volume stroke center. Clin. Neurol. Neurosurg. 143, 132–138. https://doi.org/10.1016/j.clineuro.2016.02.029 (2016).
Liu, P. et al. Comparison of safety and mid-term effects between direct stenting and angioplasty before stenting in the Basilar artery. Clin. Neurol. Neurosurg. 193, 105773. https://doi.org/10.1016/j.clineuro.2020.105773 (2020).
Maier, I. L. et al. Transluminal angioplasty and stenting versus Conservative treatment in patients with symptomatic Basilar artery stenosis: perspective for future clinical trials. Clin. Neuroradiol. 28, 33–38. https://doi.org/10.1007/s00062-016-0528-x (2018).
Palmisciano, P. et al. Percutaneous transluminal angioplasty and/or stenting for the treatment of Basilar artery stenosis: a systematic review and meta-analysis. Neuroradiology 65, 985–1000. https://doi.org/10.1007/s00234-023-03124-x (2023).
Groschel, K., Schnaudigel, S., Pilgram, S. M., Wasser, K. & Kastrup, A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke 40, e340–347. https://doi.org/10.1161/STROKEAHA.108.532713 (2009).
Deng, Y., Huang, J., Chen, C., Wen, Y. & Qiu, D. Fe(3)O(4) coated stent prevent artery neointimal hyperplasia by inhibiting vascular smooth muscle cell proliferation. Mater. Today Bio. 27, 101133. https://doi.org/10.1016/j.mtbio.2024.101133 (2024).
Yin, J. et al. Diabetic conditions promote drug coating degradation but prevent endothelial coverage after stenting. Acta Biomater. 177, 189–202. https://doi.org/10.1016/j.actbio.2024.01.034 (2024).
You, W. et al. Bifurcated aneurysm location predicts In-Stent stenosis after Neuroform-EZ Stent-Assisted coiling for intracranial aneurysm. Front. Neurol. 13, 873014. https://doi.org/10.3389/fneur.2022.873014 (2022).
Zhou, K. et al. A comparison of safety and effectiveness between wingspan and neuroform stents in patients with middle cerebral artery stenosis. Front. Neurol. 12, 527541. https://doi.org/10.3389/fneur.2021.527541 (2021).
Buonomo, O. et al. Safety and effect of Neuroform Atlas stent in the treatment of symptomatic intracranial stenosis: A single-center experience. Heliyon 7, e08040. https://doi.org/10.1016/j.heliyon.2021.e08040 (2021).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 82301513).
Author information
Authors and Affiliations
Contributions
D.Q. conceived and designed the study; J.Y., L.L, J.F., J.H., Y.W., and J.H. performed the study; D.Q., J.F., L.L, J.H. analyzed and interpreted the data; D.Q. and J.F. wrote the manuscript. All authors reviewed and approved for the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Duan, J., He, J., Wen, Y. et al. Neuroform EZ stenting for basilar artery atherosclerotic stenosis. Sci Rep 15, 30825 (2025). https://doi.org/10.1038/s41598-025-16517-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16517-0