Abstract
Lower respiratory tract infections (LRTIs) are a significant cause of morbidity and mortality in pediatric populations worldwide. This study examines cerebral oxygenation (StO2) in children with LRTIs using Time-Domain Near-Infrared Spectroscopy (TD-NIRS) device (PIONIRS S.r.l., Milan, Italy), a non-invasive tool that allows for measurement of cerebral StO2in real-time. An observational case-control study was conducted, including 60 participants: 30 patients with LRTIs and 30 age and sex matched controls. We evaluated the correlation between cerebral StO2 and various clinical and physiological parameters and we used a multivariate logistic regression analysis explore predictors of LRTI. Cerebral StO2 was one of the key and independent predictors of LRTIs (OR = 0.45, p = 0.002) suggesting it could be an additional parameter to record for better assessing the condition of LRTI patients. These findings highlight the role of TD-NIRS in providing deeper insights into brain oxygenation in pediatric patients, independent of peripheral measurements such as peripheral oxygen saturation (SpO2).
Similar content being viewed by others
Introduction
The incidence of community-acquired childhood low respiratory tract infections (LRTIs) has decreased over recent decades, thanks to improvements in immunization, nutrition, and socio-economic conditions. Despite this decline, LRTIs remain the leading cause of early childhood mortality1,2. While most children recover uneventfully, a subset may develop chronic complications due to factors such as immunosuppression, poor secretion clearance, airway abnormalities, genetic predispositions, microbiological agents, and environmental influences.
Lung tissue is particularly susceptible to long-term injury, as damage is often difficult to reverse. Children with recurrent LRTIs are more prone to reinfections and more severe respiratory episodes, sometimes involving polymicrobial infections3. Repeated or chronic inflammation may impair the airway mucosa and alveolar structures, potentially leading to long-term morbidity3, such as persistent coughs, reduced lung function, and increased risk of conditions like asthma, bronchiolitis obliterans, or pulmonary fibrosis. Early identification and management of children at risk for respiratory sequelae are crucial for preserving long-term lung health. However, due to the lack of high-level evidence and quantitative comprehensive data, determining whether and when a specific patient condition should be further investigated, remains challenging1.
LRTIs can cause hypoxia, a condition where oxygen delivery to tissues is insufficient to maintain adequate homeostasis. In the context of LRTIs, hypoxia may result from hypoventilation and/or ventilation-perfusion mismatch4. The severity of hypoxia can range from mild to severe and can manifest in acute, chronic, or mixed forms. While some tissues tolerate transient hypoxia, others—such as the brain—are highly vulnerable. Even brief episodes of cerebral hypoxia can impact neurodevelopment and cognitive outcomes in children. Monitoring cerebral oxygenation may thus be critical in detecting early signs of neurological compromise.
In pediatric and neonatal intensive care, cerebral Near-InfraRed Spectroscopy (NIRS) is part of standard care to monitor specific neurological conditions, such as cerebral hypoxia5. Using NIRS, it is possible to noninvasively evaluate oxyhemoglobin concentrations (O2Hb) and deoxyhemoglobin (HHb) at the tissue and brain level. NIRS exploits the different absorption characteristics of O2Hb and HHb and the transparency of tissues to near-infrared light (600–1000 nm). In particular, NIRS has been used to study cerebral oxygenation (StO2 = O2Hb/(O2Hb + HHb)) in experimental and clinical settings, both in adults and pediatric patients, as a biomarker for brain physiological status. While the adult applications of NIRS are well documented, including during the COVID-19 pandemic where it was used for continuous respiratory monitoring6. studies involving pediatric respiratory diseases are limited. A British prospective study described NIRS as a diagnostic tool for evaluating respiratory disorders in neonates7. and it has shown promise in distinguishing pneumonia from transient tachypnea of the newborn. Furthermore, NIRS was successfully employed in children with sleep-disordered breathing to evaluate changes in cerebral oxygenation8,9.
The majority of clinical NIRS devices employ the continuous wave (CW) NIRS approach based on light source with a constant intensity and detector sensitive to light attenuation. Noticeably, CW NIRS cannot provide absolute value for O2Hb and HHb and the estimate of StO2 are based on the use of multiple source detector channels and the a priori assumption on the spectral dependence of the reduced scattering coefficient. Moreover, CW NIRS can be strongly affected by motion artefacts and by the presence of overlying tissue (e.g., adipose tissue in muscle monitoring, scalp and skull in cerebral cortex monitoring)10. Time-domain (TD) NIRS addresses these limitations. It uses short laser pulses and time-resolved detection to measure photon travel time through tissue, allowing separation of absorption and scattering effects. This approach enables the absolute quantification of O2Hb and HHb concentrations with improved depth sensitivity and reduced susceptibility to superficial artifacts. Among the different types of NIRS, the TD-NIRS potentially provides greater accuracy and reproducibility of measurements11. Although initially limited by size and cost, recent technological advances in photonics have made portable TD-NIRS devices more accessible.
The aim of this study was the assessment of cerebral StO2 using a portable TD-NIRS tissue oximeter, in children with a concurrent LRTIs. Early identification of altered brain tissue StO2 can significantly improve outcomes in vulnerable patients, such as infants and young children, by enabling timely and personalized treatments.
Materials and methods
We conducted an observational case-control study with age and sex-matched groups. Both cases and controls were enrolled among pediatric patients (aged 0–18 years) admitted to the Pediatric Department of Buzzi Children’s Hospital in Milan, Italy, from December 2023 to May 2024. Specifically, the cases were admitted because of concurrent LRTIs, i.e. pneumonia or bronchiolitis. Controls were selected among patients admitted to the hospital for reasons unrelated to respiratory conditions, such as minor trauma, mild dehydration (e.g., secondary to vomiting or diarrhea), or non-specific abdominal pain without an identified organic cause, who met all of the following inclusion criteria: stable vital signs, no fever, no history of cardiac or pulmonary disease, absence of chronic conditions, no ongoing pharmacological treatments, intact skin at the measurement site, and normal hematocrit levels confirmed by blood tests.
The Ethics Committee Milano Area 1 approved the protocol (No. 0004021/2023). The study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2008. Written informed consent was obtained from a parent and/or legal guardian for all participants after they received information about the study. The term ‘sex’ used in this study refers to sex assigned at birth.
Clinical and anthropometric evaluation
To establish a potential correlation between clinical or demographical features and TD NIRS tissue oximetry parameters, for each patient we recorded weight (kg), height (cm), body mass index (BMI; kg/m2), pubertal assessment according to Tanner12,13 (prepubertal stage = Tanner 1; middle puberty = Tanner 2–3; late puberty = Tanner 4–5) and skin pigmentation evaluated with the Fitzpatrick scale (type 1–6). BMI was transformed in BMI z-score for all children referring to World of Health Organization references14. Using a measuring tape, we assessed the middle upper arm circumference (MUAC, cm), head circumference (cm), nasion-inion distance (cm) and periauricular distance (cm).
Peripheral oxygen saturation (SpO2, %) was recorded during TD NIRS acquisitions and hematocrit level (%) and hemoglobin concentration (g/dL) were retrieved by performing a blood test on each patient. Within the pathological groups, patients undergoing oxygen therapy have been labelled accordingly.
Device and optical probe description
The NIRSBOX device (PIONIRS S.r.l., Milan, Italy.) used in this work is a commercial research-grade TD-NIRS tissue oximeter. The device is equipped with two picosecond lasers emitting at 685 nm and 830 nm and a flexible, skin compatible, smart optical probe (G5 “Goccia”, PIONIRS S.r.l.) with 2.5 cm source -detector distance. The probe features a built-in capacitive contact sensor to ensure correct application on the tissue and give feedback to the clinical operator. The measurement is non-invasive and the device provides continuous real-time monitoring of tissue optical properties (absorption and reduced scattering coefficients at 685 nm and 830 nm), tissue hemodynamic parameters (as concentration of oxygenated hemoglobin (O2Hb, [µM]), deoxygenated hemoglobin (HHb, [µM]), total hemoglobin (tHb [µM] = HbO2 + HHb), percentage of tissue oxygen saturation (StO2 [%] = 100 HbO2/tHb)), and differential pathlength factor (DPF) at 685 nm and 830 nm (DPF_L1 and DPF_L2 refer to the differential pathlength factors measured in prefrontal and arm locations, and are derived from optical parameter and laser pulses shapes11,15. More details can be found in references11,15 where the same set up was previously exploited in a large cohort of pediatric subjects to obtain expected tissue oximetry and optical values in pediatric population, on both, prefrontal cortex and MUA locations.
Data obtained by the NIRSBOX tissue oximeter were not considered in therapeutic decisions, they were acquired solely for research purposes.
Measurement protocol
The optical probe was manually held in place by the clinical operator during measurements. Two locations were considered: the left middle upper arm below the deltoid region, and the left frontotemporal cortex (Fp1 of the 10/20 EEG system mapping). These sites were selected based on their clinical relevance and practicality: the MUA is a standard location in anthropometric evaluation, and the prefrontal cortex (Fp1) is widely used in NIRS studies due to its hair-free accessibility. For each location, the protocol included five consecutive probe repositioning and for each repositioning the measurement lasted 5 s at 1 Hz acquisition frequency.
Statistical analysis
Demographic and physiological data were compared between LRTIs patients and healthy controls. If not differently specified, results were reported as mean values and standard deviation (mean ± std), and linear correlation between pairs of variables was assessed by Pearson’s correlation.
All the measurements were compared between cases and controls by a Student T-test. A multivariate logistic regression model was used to investigate the independent ability of each parameter to discriminate cases and controls. To screen the variables to be included in the multivariate model, we implemented a Recursive Feature Elimination (RFE) procedure to rank the importance of predictors based on their contribution to model accuracy. RFE was coupled with k-fold cross-validation (with k set to 5 group resampling) to ensure that the feature selection process was robust and not prone to overfitting (cross-validation score > 80%). We assessed multicollinearity among the selected features using Variance Inflation Factors (VIF). A threshold of 5 was applied to identify features with significant multicollinearity concerns. All selected predictors all had VIF < 5. This feature selection process facilitated the identification of the most relevant predictors while maintaining model interpretability and performance, ultimately enhancing the reliability of our logistic regression analysis.
A multivariate logistic regression model was then built, incorporating the selected predictors, to estimate the odds ratios (OR) and their associated p-values (p). The model’s performance was evaluated using a confusion matrix, accuracy, specificity, and the area under the receiver operating characteristic curve (AUC). Statistical analysis was performed on python, exploiting the Scikit-learn module16.
Results
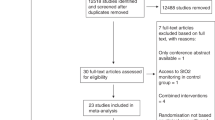
We enrolled a cohort of 60 pediatric subjects (30 cases and 30 controls). Thirty patients (cases) were admitted because of a concurrent LRTIs, i.e. pneumonia or bronchiolitis (M: F = 1:1; mean age 3.7 years ± 3.5). Thirty age and sex matched controls were selected among those admitted to the hospital for different reasons (M: F = 1:1; mean age 3.8 years ± 3.4). At the time of enrolment, 29 subjects in each group (LRTI and control) were in the prepubertal stage (Tanner = 1), while only one subject per group, two in total, were in a more advanced pubertal stage (Tanner = 4). Baseline characteristics of the enrolled cohort are presented in Table 1 for clinical and anthropometric data and Table 2, for tissue oximetry data.
No significant differences were observed in demographic and physical characteristics between the two study groups, ensuring comparability and minimized confounding factors Table 1.
Patient with LRTIs showed significantly higher respiratory rate (approximately 6 cycles more per minute, p = 0.001) and lower peripheral saturation, SpO2, 97.1% vs. 98.6% (p = 0.0003). Cerebral StO2 was significantly lower in the LRTI group (61.9% vs. 65.3%, p = 0.001), while no differences were found in MUA tissue oximetry parameters.
A statistical model based multiple linear regression have been developed, selecting the most relevant regressors form the full set of acquired variables. After recursive feature elimination, multicollinearity assessment and cross validation to prevent overfitting, the following predictors were selected: SpO2, frontotemporal (cerebral) StO2, heart rate, head circumference, height, and Respiratory Rate.
Comparison between univariate analysis and multivariate analysis of the selected features, have been performed and results are reported in Table 3.
The multivariate analysis explains 59% of the variability in the outcome (pseudo R² = 0.59) and achieved an accuracy of 87%. Respiratory rate (OR = 1.298, p = 0.002) and cerebral StO2 (OR = 0.667, p = 0.011) emerged as the strongest predictors, while SpO2 retained moderate significance (OR = 0.550, p = 0.036). Head circumference showed no independent significance but suggested interplay with other predictors, with an increase OR and decreased p values compared to the univariate analysis.
The performance of the multivariate logistic regression model demonstrates high reliability in identifying pathological patients with LRTIs Table 4 (right column). The specificity of 0.87 reflects its strong ability to identify healthy individuals, reducing the likelihood of false positives, while the precision of 0.86 ensures that 86% of those classified as pathological truly have LRTIs. Most notably, the ROC curve AUC of 0.92 underscores the model’s discriminatory power, Fig. 1, blue curve.
To further highlight the importance of including the variables captured by TD-NIRS, a comparison the multivariate logistic regression predictive models with and without significant tissue oximetry, TD-NIRS variable (StO2) is reported in Table 4; Fig. 1.
The inclusion of cerebral StO2 significantly improved model performance, increasing accuracy to 87% and AUC to 0.92 compared to 80% accuracy and AUC of 0.87 in the model excluding tissue oximetry variables. Sensitivity and specificity were also enhanced with StO2 included, reaching 83% and 87%, respectively.
To further analyze the relations between predictors, we looked for correlations between key oximetry variables and standard clinical indicators. The correlation between StO2 and SpO2 was assessed in the two separate and in the pooled populations. No significant correlation was found neither in the pathological group (Pearson’s r < 0.08) nor in the healthy group (r < 0.01).
Discussion
Cerebral StO2 in patients with LRTI was assessed by TD-NIRS. We reported that patients with LRTI have lower cerebral StO2 values than healthy controls, even in the absence of clinical signs of respiratory distress such as nasal flaring, chest retractions, tachypnea, or hypoxia on pulse oximetry. Monitoring cerebral tissue oxygenation in patient with LRTIs may help identify early subclinical hypoxia, guiding decisions regarding closer monitoring or early intervention.
While peripheral SpO2 has long been a standard for assessing oxygenation, our findings demonstrate that it may not provide a full picture of cerebral oxygenation in patient with LRTIs. In previous studies, SpO2 has been shown to be an effective measure of systemic oxygenation, yet it lacks sensitivity in detecting localized hypoxia in critical organs like the brain17. In this study, the observed differences in respiratory rate and SpO2 between groups align with clinical expectations, confirming their relevance as markers of respiratory pathology. However, the addition of cerebral StO2 to the predictive model notably improved diagnostic accuracy, sensitivity, and specificity, underscoring its value as an independent and robust predictor. Additionally, the lack of significant correlation between StO2 and SpO2 suggests that cerebral StO2 provides unique physiological information distinct from peripheral measurements, supporting its integration into diagnostic protocols.
Our results emphasize the potential of TD-NIRS oximetry as a valuable non-invasive tool for monitoring cerebral StO2 in pediatric patients with LRTI. The significant difference in cerebral StO2 between infected patients and healthy controls underscores the impact of respiratory infections on brain oxygenation. All enrolled patients were neurologically asymptomatic at the time of evaluation, with no clinical signs suggestive of central nervous system infection, thereby supporting the attribution of cerebral oxygenation changes to respiratory illness rather than encephalitic processes. Additionally, since cerebral hypoxia is a key risk factor for neurodevelopmental delays in pediatric patients18 cerebral StO2 may provide crucial insight for more effective monitoring an care during child development.
Peripheral tissue saturation measurements in the MUA location did not yield a significant regressor in the multivariate model. Even though peripheral StO2 is expected tot be affected in patients with LRTIs, the highly heterogeneous, tissue morphology in the MUA region and the characteristics of the selected population could lead to high variability of the acquired data, resulting in lack of significant differences between the two population.
Head circumference, despite remaining a non significant regressor, increased its contribution in the multivariate linear regression model compared to the univariate one, suggesting that it may play role when merged together with other variables. When head circumference was removed from the regression model, the significance of other regressors remained intact, yet the overall discrimination power of the model decreased. As pointed out in a simulation study by Amendola et al.,19 geometrical characteristics of the head, such as skull thickness or tissue composition, and head circumference can impact the accuracy of optical oximetry techniques, TD-NIRS, FD-NIRS and it is even more prominent in current CW-NIRS medical devices due to their less efficient penetration depth. This suggests the need to explore how anatomical variability affects TD-NIRS signals and to develop advanced analytical models capable of accounting for these variations.
While our study provides key insights into cerebral StO2 monitoring using TD-NIRS in pediatric patients with LRTIs, several limitations must be noted.
The small sample size of 60 participants limits the statistical power and generalizability of the findings. A larger cohort would provide more robust data and improve the reliability of conclusions. Furthermore, being a single-center study conducted at Buzzi Children’s Hospital in Milan, the patient population may not be representative of broader populations. Multi-center studies would increase the external validity. We also faced limited control over confounding variables. Factors such as genetic predispositions, environmental influences like air quality, and co-infections were not fully accounted for, which could have impacted cerebral StO2 outcomes. Additionally, short-term monitoring only captured cerebral StO2 at specific time points, missing potential fluctuations throughout the LRTI progression. Continuous monitoring could offer a more comprehensive picture of cerebral StO2 dynamics during illness. The study’s wide age range (0–18 years) may have masked developmental differences in oxygenation responses. Future research should segment age groups more narrowly to better assess age-specific oxygenation patterns, to help the interpretation of our data, our previous study which addressed the trends of peripheral and cerebral tissue oximetry in developing childhood can be exploited.
Finally, we did not track long-term clinical outcomes such as neurodevelopmental impacts. While cerebral hypoxia is known to affect brain development, we did not follow patients longitudinally to determine if oxygenation deficits correlated with cognitive impairments. Future studies should address this by incorporating long-term follow-up.
In summary, while the study demonstrates the promise of TD-NIRS, addressing these limitations through larger, multi-center, and longitudinal studies with continuous monitoring will be crucial for its wider clinical application.
Conclusions
This study underscores the significant differences in cerebral StO2 between pediatric patients with LRTIs and healthy controls. Both univariate and multivariate logistic regression analyses demonstrate that cerebral StO2 is strongly influenced by LRTIs and respiratory rate. Utilizing a multivariate logistic regression model incorporating traditional clinical indicators such as SpO2, respiratory rate, heart rate, and anthropometric variables (e.g., height and head circumference), the model achieved a discriminatory power with an AUC of 0.87 and an accuracy of 80%. Notably, the inclusion of frontotemporal cerebral StO2 further enhanced the model’s performance, increasing the AUC to 0.92 and the accuracy to 87%.
Interestingly, hematocrit levels and peripheral tissue saturation did not emerge as significant predictors for distinguishing LRTIs from controls. Furthermore, cerebral StO2 and peripheral SpO2 were not correlated in either the pathological or control groups, indicating that these metrics provide complementary information. Specifically, cerebral StO2 offers a more localized assessment of regional cerebral tissue health, which cannot be captured by conventional pulse oximetry. Although the pathophysiological mechanisms need to be studied in detail, in patients with LRTIs it cannot be excluded that fever and tachycardia, by increasing metabolic demand and oxygen consumption, could potentially impair tissue oxygenation, especially in the brain, due to autoregulatory mechanisms.
These findings highlight the potential value of integrating cerebral StO2 metrics into pediatric care for respiratory infections, advancing beyond traditional SpO2 monitoring to enhance diagnostic and prognostic accuracy.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Dangor, Z. et al. Lower respiratory tract infection in children: when are further investigations warranted?? Front. Pediatr. 9, 708100. https://doi.org/10.3389/fped.2021.708100 (2021).
Troeger, C. et al. Estimates of the global, regional, and National morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Infect. Dis. 18, 1191–1210. https://doi.org/10.1016/S1473-3099(18)30310-4 (2018).
Guo, J., Niu, W., Zhang, Q. & Cui, X. Lower respiratory tract infections in early childhood. Lancet 402, 2194–2195. https://doi.org/10.1016/S0140-6736(23)01622-7 (2023).
Bhutta, B., Alghoula, F., Berim, I. & Hypoxia Available online: https://www.ncbi.nlm.nih.gov/books/NBK482316/ (accessed on 29 July 2024).
Hansen, M. L. et al. Cerebral Near-Infrared spectroscopy monitoring (NIRS) in children and adults: A systematic review with Meta-Analysis. Pediatr. Res. https://doi.org/10.1038/s41390-022-01995-z (2022).
Mah, A. J. et al. Optical monitoring of breathing patterns and tissue oxygenation: A potential application in COVID-19 screening and monitoring. Sensors 22, 7274. https://doi.org/10.3390/s22197274 (2022).
Ozdemir, F. E., Alan, S. & Aliefendioglu, D. The diagnostic value of pulmonary Near-infrared spectroscopy in the early distinction of neonatal pneumonia from transient tachypnea of the newborn. Pediatr. Pulmonol. 58, 3271–3278. https://doi.org/10.1002/ppul.26656 (2023).
Tabone, L. et al. Cerebral oxygenation during respiratory events in children with Sleep-Disordered breathing and associated disorders. J. Pediatr. 214, 134–140e7. https://doi.org/10.1016/j.jpeds.2019.07.040 (2019).
Zini, T., Miselli, F. & Berardi, A. Noninvasive Monitoring Strategies for Bronchopulmonary Dysplasia or Post-Prematurity Respiratory Disease: Current Challenges and Future Prospects. Children 10, 1753. https://doi.org/10.3390/children10111753 (2023).
Amendola, C. et al. Robustness of tissue oxygenation estimates by continuous wave Space-Resolved near infrared spectroscopy. J. Biomed. Opt. 28 https://doi.org/10.1117/1.JBO.28.7.075002 (2023).
Calcaterra, V. et al. Reference Values for Cerebral and Peripheral Tissue Oximetry in Children: A Clinical TD - NIRS Study. Acta Paediatr. apa.17459. https://doi.org/10.1111/apa.17459 (2024).
Marshall, W. A. & Tanner, J. M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 45, 13–23. https://doi.org/10.1136/adc.45.239.13 (1970).
Marshall, W. A. & Tanner, J. M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 44, 291–303. https://doi.org/10.1136/adc.44.235.291 (1969).
WHO Multicentre Growth Reference Study Group WHO Child Growth Standards Based on Length/Height. Weight and age. Acta Paediatr. Oslo Nor. 1992 Suppl. 450, 76–85. https://doi.org/10.1111/j.1651-2227.2006.tb02378.x (2006).
Calcaterra, V. et al. Cerebral baseline optical and hemodynamic properties in pediatric population: A large cohort Time-Domain near-Infrared spectroscopy study. Neurophotonics 11 https://doi.org/10.1117/1.NPh.11.4.045009 (2024).
Pedregosa, F. et al. Scikit-Learn: machine learning in python. Mach Learn. PYTHON.
Cheung, A., Tu, L., Macnab, A., Kwon, B. K. & Shadgan, B. Detection of hypoxia by Near-Infrared spectroscopy and pulse oximetry: A comparative study. J. Biomed. Opt. 27, 077001. https://doi.org/10.1117/1.JBO.27.7.077001 (2022).
Cognitive Impairment Caused by Hypoxia. From Clinical Evidences to Molecular Mechanisms | Metabolic Brain Disease Available online: October (2024). https://doi.org/10.1007/s11011-021-00796-3 (accessed on 2).
Accuracy of Homogeneous Models for Photon Diffusion in Estimating Neonatal Cerebral Hemodynamics by TD-NIRS Available online. https://opg.optica.org/boe/fulltext.cfm?uri=boe-12-4-1905&id=449003 (accessed on 2 October 2024).
Acknowledgements
This work was partly funded by the European Union’s Horizon Europe programme under grant agreement No. 101099093 – PROMETEUS project.
Author information
Authors and Affiliations
Contributions
M.L.: Conceptualization; methodology; writing – original draft; writing – review and editing; formal analysis; statistical analysis; supervisionV.C.: Conceptualization; writing – original draft; writing – review and editing; supervision; methodologyV.R.: Investigation; writing – original draft; writing – review and editingS.Z.: Investigation; writing – original draft; writing – review and editingM.B.: Conceptualization; methodology; writing – original draft; writing – review and editingM.P.S: Conceptualization; writing – original draft; writing – review and editing; methodology; formal analysis; statistical analysis; supervisionD.C.: Conceptualization; writing – original draft; methodology; writing – review and editing; supervision A.T.: Conceptualization; writing – original draft; methodology; writing – review and editing; formal analysis; supervisionG.Z.: Conceptualization; writing – original draft; writing – review and editing; methodology; supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lacerenza, M., Calcaterra, V., Rossi, V. et al. The role of cerebral oxygenation in pediatric lower respiratory tract infections based on insights from time domain near infrared spectroscopy tissue oximetry. Sci Rep 15, 31171 (2025). https://doi.org/10.1038/s41598-025-16639-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16639-5