Abstract
This randomized, double-blind, placebo- and active-controlled clinical trial evaluated the efficacy and safety of EnMax tablets in managing postoperative inflammation and supporting wound healing following orthopedic surgery. A total of 105 patients were randomized to receive EnMax, a comparator enzyme formulation (trypsin-bromelain-rutoside), or placebo alongside standard care for 7 days. Baseline characteristics were statistically comparable across all groups. EnMax demonstrated significant clinical benefits, including substantial reductions in pain (93.10%), inflammation (89.77%), and key inflammatory biomarkers—C-reactive protein (CRP, 57% reduction) and erythrocyte sedimentation rate (ESR, 61.32% reduction) by the final visit. EnMax also outperformed both placebo and the comparator in reducing erythema, local irritation, discharge, and tenderness. Notably, EnMax patients required less analgesic use and reported no adverse events, with full study compliance. In contrast, the placebo group exhibited minimal improvement across all measured parameters. These results suggest that EnMax is a promising, safe, and effective therapeutic option for enhancing postoperative recovery. Additional larger, long-term studies are recommended to further establish its clinical benefits.
Similar content being viewed by others
Introduction
Musculoskeletal disorders are the leading cause of disability worldwide, accounting for half of all chronic ailments among elderly individuals in the developed countries1. Orthopedic surgery patients often experience moderate to severe postoperative discomfort and complications. Thus, they represent a high level of intensive care admissions. These patients present various clinical challenges that require a thorough understanding of preoperative comorbidity, precise intra-operative strategies, and timely recognition and management of postoperative complications. Preventive measures and early intervention are considered among the most effective approaches to improve outcomes in this population2.
One of the most severe issues after orthopedic surgery is the risk of postoperative infections, especially surgical site infections (SSIs), which can significantly affect patient recovery and healthcare expenses. Worldwide, the prevalence of SSIs varies significantly, ranging from 1.4 to 41.9%. Orthopedic surgeries are especially at risk because deep-seated infections in bones and joints are challenging to eliminate. It is worth mentioning that the estimated recurrence rate for these infections ranges from 10 to 20%3.
Effective management of acute postoperative pain is equally vital. Inadequate control may lead to prolonged recovery, reduced quality of life, increased opioid use, and higher healthcare costs4. Patients with co-morbidities like hypertension (23.7%) and diabetes mellitus (18.4%) are more prone to infections due to weakened immunity5.
Pharmacological interventions include prophylactic antibiotics and multimodal pain management with NSAIDs, opioids, local anesthetics, and corticosteroids6. However, NSAIDs cause significant risks such as gastrointestinal issues, renal impairment, cardiovascular events, perioperative bleeding, and delayed bone healing, primarily due to COX-2 inhibition7.
Given these concerns, proteolytic enzymes offer a promising alternative. Known for their anti-inflammatory effects and digestive tolerance, they have shown efficacy in reducing postoperative edema and inflammation. First introduced intravenously in the 1950s, oral systemic enzyme therapy now presents a non-invasive approach to managing inflammatory symptoms post-surgery8,9.
Surgical wound healing, especially in patients with co-morbidities, remains challenging. Despite advancements, an evidence-based wound management standard is still lacking. The COVID-19 pandemic further limited timely care. Proteolytic enzymes offer a novel therapeutic avenue10. This study evaluates the short-term efficacy and tolerability of an oral enzyme supplement (EnMax) compared to placebo and a comparator product in orthopedic postoperative care, with the aim of promoting faster recovery through systemic anti-edema effects.
The present randomized, double-blind, placebo- and active-controlled clinical trial evaluated the short-term efficacy, safety, and tolerability of EnMax, a proprietary oral proteolytic enzyme tablets, in patients undergoing elective orthopedic surgery. EnMax has previously demonstrated clinical benefits in reducing postoperative inflammation in general surgical settings11. This study aimed to investigate its potential in orthopedic postoperative care by assessing patient-reported outcomes (pain and swelling), surgical wound-related symptoms, inflammatory biomarkers (CRP, ESR), and global assessments, compared to placebo and a enzyme-based comparator. The results provide evidence supporting the anti-inflammatory and wound healing efficacy of EnMax, likely mediated through modulation of systemic inflammatory pathways.
Materials and methods
Study design
This was a randomized, double-blind, parallel-group, controlled, comparative clinical study to evaluate the impact of EnMax tablet in managing post-operative inflammation in orthopedic surgeries.
The study was conducted at three sites: Ayushri Multispeciality Hospital, Pune; Lokmanya Medical Research Centre and Hospital, Pune; and Krishna Vishwa Vidyapeeth (Deemed to be University), Karad, Satara. Ethical approvals were obtained from the Institutional Ethics Committees of Sangvi Multispecialty Hospital, Lokmanya Medical Research Centre, Pune, and KIMS Deemed to be University, Karad, respectively. The study commenced only after written EC approvals were obtained. It was conducted in accordance with the approved protocol, Good Clinical Practice (GCP) guidelines, and AYUSH/ICH-GCP standards. The rights, safety, and well-being of the participants were prioritized throughout the trial. The investigational products were manufactured in compliance with Good Manufacturing Practices (GMP) as applicable in India. The study was prospectively registered on the Clinical Trials Registry - India (CTRI/2024/08/072363) on 12/08/2024, and participant enrollment began thereafter. Data was collected from August 20 to October 21, 2024.
Investigation product details
EnMax is an oral enzyme supplement containing 20,00,000 FCC PU of Protease and 100 mg of Rutin per tablet along with bromelain, amla, microcrystalline cellulose, polyvinylpyrrolidone, crosscarmellose sodium, magnesium stearate, talc, silicon dioxide, hydroxypropylmethyl cellulose, ethyl cellulose, polyethylene glycol, and mixed fruit flavor. The comparator tablet (FSSAI approved) contains 48 mg Trypsin BP, 90 mg Bromelain, and 100 mg Rutoside Trihydrate BP (quercetin-3-rutinoside) as active ingredients per tablet. The placebo tablet was composed of inert ingredients and was identical in appearance to the EnMax tablet. Tablets were administered twice a day with empty stomach followed by 240 ml of water 30 min before meal or 60 min after meal for seven days along with anti-inflammatory & analgesic and Antibiotic for seven-ten days.
Standard-of-care-anti-inflammatory, analgesic, and antibiotic treatments drug dosage, frequency, and duration was determined by the investigator’s clinical judgment and experience, taking into consideration factors such as the surgical site, organ system, incision, and the specific site of the study.
Inclusion criteria
Patients aged 25 to 50 years, electively posted for orthopedic surgery, without suspected or confirmed infections and not receiving treatment for the same, were enrolled. Patients willing and able to provide written informed consent prior to participation in the study were included.
Exclusion criteria
Participants were excluded if they met any of the following conditions: undergoing emergency surgery; receiving immunosuppressive or cytotoxic therapy (current or history); known allergy, sensitivity, or contraindication to any investigational product; history of hepatic or renal disorders, bleeding disorders (including menorrhagia, hematuria, or hematemesis), or a history of gastric ulcer or bleeding diathesis; prior investigational drug use within the past four weeks; positive pregnancy test or lactation; uncontrolled diabetes or other significant metabolic disorders; severe illness or moribund state; inability to comply with the treatment regimen; history of recreational drug use within the past 12 months; or any condition deemed unsuitable for study participation by the investigator.
Sample size
The sample size was determined using Day-8 VAS scores reported by earlier study conducted by us and later published as Rathi et al., Cureus (2024) (Test: 0.308 ± 0.679; Comparator: 0.846 ± 1.463; Placebo: 1.462 ± 1.174)11. Assuming a 2:2:1 allocation ratio (Test: Comparator: Placebo; 42:42:21; total n = 105), the study was powered for both superiority and non-inferiority analyses.
For superiority (Test vs. Placebo; two-sided α = 0.05), the mean difference (Δ) of 1.154 with a standard error (SE) of 0.2768 yielded z = 4.17, corresponding to ~ 95% power. For non-inferiority (Test vs. Comparator; two-sided α = 0.05; margin M = 0.50), the observed difference (d) of − 0.538 with SE = 0.2489 also provided ~ 95% power.
Methodology
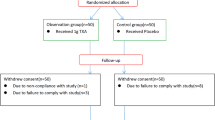
This randomized, double-blind (both participants & investigators), comparative clinical study evaluated the efficacy and safety of EnMax in managing postoperative inflammation in patients undergoing orthopedic surgeries. A total of 105 patients were randomized into three groups: Group A (EnMax), Group B (comparator product), and Group C (placebo). All participants received standard of care (SOC) and completed a 7-day treatment period (Fig. 1).
Participants were randomized using a computer-generated block randomization list developed by a qualified biostatistician prepared independently. The randomization was performed using a computer-generated sequence in blocks of five to maintain a 2:2:1 allocation ratio for Enmax tablet, comparator tablet, and placebo, respectively. Separate randomization lists were generated for each study site. This list was generated prior to study initiation to ensure unbiased allocation. Allocation concealment was maintained by securely storing the randomization schedule, which was accessible only to the site investigator. Participants were screened for eligibility and subsequently enrolled into the study. The informed consent was obtained from all participants before enrolment.
Randomization was implemented through pre-packaged study products labeled with unique identification codes corresponding to the randomization sequence and enrollment IDs. These packages were provided to the study site in sequentially numbered containers. Blinding was upheld throughout the study; both investigators and participants were unaware of treatment allocation.
The randomization codes were securely maintained, and access was restricted. Unblinding was permissible only after the database lock or in the event of a serious adverse event necessitating knowledge of treatment assignment. However, no such unblinding was required during the course of the study.
The scheduled study visits included a screening visit (Day − 14 to Day 1), followed by Visit 1 on Day 1 (day of surgery and initiation of treatment). Subsequent visits were conducted after 2 ± 1 days of treatment (Visit 2 on Day 3), after 5 ± 1 days of treatment (Visit 3 on Day 6), and at the end of the 7-day treatment period (Visit 4 on Day 8 ± 1).
The primary outcome was the reduction in pain intensity and inflammation, assessed using the Visual Analog Scale (VAS) and Verbal Rating Scale (VRS), respectively. Secondary outcomes included changes in inflammatory biomarkers CRP, ESR, wound healing parameters (erythema, local irritation, discharge, and tenderness), the requirement for rescue analgesics, and the overall safety profile.
Concomitant diseases and medications were recorded at screening. Pain was assessed using the visual analog scale, where scores ranged from 0 (no pain) to 10 (worst imaginable pain), and inflammation was assessed using the verbal rating scale, which ranged from 0 (no swelling) to 3 (severe swelling beyond the surgical site). Both parameters were evaluated on Days 1, 3, 6, and 8. Inflammatory biomarkers, including C-reactive protein and erythrocyte sedimentation rate, were measured on Days 1 and 8. The need for rescue analgesics was recorded on Days 1, 3, 6, and 8.
Surgical wound-related symptoms such as erythema, local irritation, discharge, induration, and tenderness were assessed using a 4-point scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe) on Days 1, 3, 6, and 8. Both patients and physicians completed a global assessment of response to therapy on Day 8 using a 5-point scale: 1 (excellent), 2 (good), 3 (average), 4 (no response), and 5 (poor). Complete blood count (CBC) was conducted at baseline and at the end of the study.
Safety, tolerability, treatment compliance, and adverse events were monitored throughout the study period. The CONSORT flow diagram illustrating patient progression through the study is presented in Fig. 1.
Statistical analysis
The data’s normality was assessed using the Kolmogorov-Smirnov test. Continuous variable i.e. age was summarized overall using summary statistics i.e. the number of observations, mean and standard deviation with 95% CI (among normal distribution) analyzed by student t-test, and gender was analyzed using chi-square test. Descriptive statistics, including mean ± standard deviation (SD), were used to present weight and age. The VRS score was analyzed using the Mann-Whitney U Test for between-group comparisons and the Wilcoxon Signed-Ranks Test for within-group comparisons. The VAS pain score was analyzed using the Mann-Whitney U Test for between-group comparisons and the Wilcoxon Signed-Ranks Test and dependent Student’s t-test for within-group comparisons. CRP and ESR data were analyzed using the Mann-Whitney U Test, independent Student’s t-tests for between-group comparisons, and dependent t-test, Wilcoxon Signed-Ranks Test for within-group comparisons. Changes in independence on analgesics, global assessment of patient and investigator response to therapy scores, and symptom scores (erythema, local irritation, discharge, induration, and tenderness) were represented as numbers and percentages of patients. Safety analysis included CBC and vital signs (mean ± SD), with compliance expressed as a percentage. GraphPad Prism version 10.5.0. was used for all analyses.
Results
Demographic characteristics
Demographic data, including age, gender, diet type, and type of orthopedic surgery. The study included 105 patients: 75 males and 30 females across all groups. The age difference between the groups was almost comparable. The table also details the subclassification of surgical procedures performed across the groups, Table 1.
Assessment of investigator reported inflammation score using verbal rating scale over time
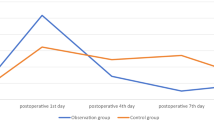
Inflammation was assessed using a VRS. The EnMax group showed the greatest improvement, with inflammation scores reducing by 53.40% at Visit 2, 72.72% at Visit 3, and 89.77% at Visit 4—all statistically significant. The comparator group also showed significant reductions: 59.55% at Visit 2, 66.29% at Visit 3, and 80.89% at Visit 4. The Placebo group had the least reduction, with 41.66%, 50.00%, and 64.58% reductions at Visits 2, 3, and 4, respectively—also significant within the group.
Between-group comparisons revealed no statistically significant difference between EnMax and the comparator and placebo group at any visit. However, EnMax showed a greater numerical reduction in inflammation scores at all visits compared to the comparator group. Overall, the findings indicate that EnMax was associated with the highest reduction in inflammation, followed by the comparator and Placebo groups, supporting its potential clinical benefit in reducing inflammation (Table 2).
Assessment of the visual analogue pain scale between groups
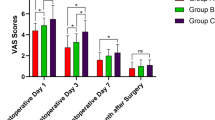
Pain reduction across the three groups, EnMax, comparator, and Placebo was assessed using the VAS, ranging from 0 (no pain) to 10 (worst pain imaginable). The EnMax group showed a significant and consistent reduction in pain scores: 69.65% at Visit 2, 83.44% at Visit 3, and 93.10% at Visit 4. The comparator group also showed significant reductions: 60.26%, 79.09%, and 84.32% at Visits 2, 3, and 4, respectively. The Placebo group had the least but still significant pain reduction—30.65%, 43.06%, and 65.69% across the same visits. Overall, EnMax demonstrated the highest percentage reduction in pain, followed by the comparator and Placebo groups. Between-group comparisons revealed a significant difference between EnMax and Placebo, but not between EnMax and the comparator, Table 2 & Fig. 2.
Assessment of changes in inflammatory biomarkers between groups
The study assessed changes in inflammatory biomarkers, CRP and ESR, across the EnMax, comparator, and Placebo groups over four visits. The EnMax group showed the most significant reduction in CRP levels, with a 57% decrease from Visit 1 to Visit 4, compared to 36% in the comparator group and 28.41% in the Placebo group. Similarly, the EnMax group had a 61.32% reduction in ESR levels, while the comparator group showed a 25.19% reduction and the Placebo group a 20.85% reduction. These results highlight that the EnMax group experienced the greatest reduction in both CRP and ESR levels, with the Placebo group showing the least improvement, Table 3; Fig. 3. The observed reductions in CRP and ESR are clinically meaningful, reflecting decreased systemic inflammation and aligning with improved clinical outcomes such as reduced pain, edema, and analgesic use in the EnMax group.
Assessment of the use of analgesics as rescue medication between groups
The use of analgesic rescue medication was assessed across the groups over four visits. In the EnMax group, 100% of patients required analgesics at Visit 1, which decreased to 64.29% by Visit 4. In the comparator group, 100% used analgesics at Visit 1, decreasing to 78.57% by Visit 4. The Placebo group maintained a steady 100% use of analgesics throughout. These results demonstrate that the EnMax group showed the most significant reduction in analgesic use by Visit 4, while the comparator group also showed a reduction, and the Placebo group showed no change.
Assessment of symptom grading on a 4-point scale
The number of participants in each treatment group (EnMax, marketed comparator, and placebo) were categorized by symptom severity on a 4-point scale for erythema, local irritation, discharge (pus/blood), induration, and tenderness, assessed at baseline (Visit 1) and subsequent follow-up visits (Visits 2–4).
Across all symptoms, patients in the EnMax group exhibited a more rapid and consistent shift toward lower severity scores compared with both comparator and placebo groups. By Visit 3, a significantly higher proportion of EnMax-treated patients achieved complete resolution (score 0) for erythema, discharge, and tenderness compared with the comparator (*p < 0.05) and placebo (#p < 0.05). These improvements were maintained or further enhanced by Visit 4, with nearly all EnMax participants showing no residual symptoms.
For local irritation and induration, improvements were also observed in the EnMax group, with higher proportions achieving score 0 compared with placebo from Visit 3 onwards, although differences versus the comparator did not consistently reach statistical significance. Overall, the EnMax group demonstrated the fastest and most sustained symptom resolution throughout the study (Table S1).
Global assessment of response to therapy score after treatment between groups
Patient response to therapy
After treatment, the EnMax group showed the strongest response, with 61.90% of patients having an excellent response, 35.71% good, and 2.38% average. In the comparator group, 26.19% had an excellent response, 52.38% good, and 21.42% average. The Placebo group had mainly average, no, or poor responses, indicating much lower efficacy.
Investigators response to therapy
The investigator assessments revealed that the EnMax group showed the most favorable response, with 78.57% of patients (33 out of 42) demonstrating an excellent response to treatment. Additionally, 21.43% (9 patients) in the EnMax group had a good response, indicating a strong overall therapeutic effect. In contrast, the comparator group had 54.76% with an excellent response, and the Placebo group showed the least favorable outcomes, with no excellent responses.
Assessment of complete blood count parameters
All complete blood count parameters remained well within normal physiological ranges throughout the study. There were no clinically significant changes observed in any of the three groups (EnMax, comparator, and Placebo). By Visit 4, these levels had returned to within normal ranges.
Assessment of vital signs and compliance
Throughout the study, there were no clinically significant changes observed in vital signs, including blood pressure, heart rate, body temperature, and respiratory rate. All measured parameters remained within normal ranges.
All three groups demonstrated 100% compliance in consuming the investigational product throughout the study.
Discussion
EnMax, assessed in a randomized, double-blind clinical trial for managing post-operative inflammation in orthopaedic surgery, showed superior efficacy with the highest reductions in inflammation (89.77%), pain (93.10%), CRP (57%), and ESR (61.32%) by Visit 4. It significantly improved symptoms such as erythema, local irritation, discharge, induration, and tenderness. EnMax also led to reduced analgesic requirements and demonstrated excellent safety, with no adverse events and 100% compliance, confirming its strong tolerability and clinical benefits.
The post-operative period is marked by significant pain and inflammation, stemming from the body’s natural immune response. This involves increased microvascular permeability, vasodilation, and heightened extravascular osmotic activity, leading to localized and generalized edema. Such responses are commonly observed after orthopaedic surgeries due to both the surgical intervention and underlying pathology12,13. During this phase, acute-phase proteins such as alpha1 antitrypsin and alpha2 macroglobulin rise sharply. These protease inhibitors can delay wound healing by inhibiting plasmin, thereby impeding fibrinolysis and prolonging inflammatory edema. Oral protease enzyme combinations work by competitively inhibiting these molecules, promoting plasmin activation, and shortening the inflammatory cascade to enable faster tissue repair14.
Swelling is a key factor that delays wound healing by limiting oxygen and nutrient diffusion, impeding metabolic waste removal, and compressing blood vessels, reducing tissue perfusion. While NSAIDs and steroids are conventionally used to manage pain and inflammation, NSAIDs can impair healing due to their anti-proliferative effects and are relatively ineffective at managing edema12,13. This has led to increased interest in enzyme-based therapies that target inflammation more directly and safely.
In this context, earlier studies have reported favorable outcomes with proteolytic enzyme combinations. A single-center observational study showed that trypsin, bromelain, and rutoside significantly reduced post-operative pain and swelling, with 75% of patients experiencing complete pain relief and 50% showing complete resolution of swelling in just eight days. These improvements were also linked to shorter hospital stays and reduced medication burden12. Similarly, a study involving 60 patients undergoing internal fixation for long bone fractures demonstrated that enzyme therapy resulted in significantly faster swelling reduction and lower analgesic consumption, with minimal side effects14. The present clinical study supports these findings, as patients in the EnMax and comparator groups experienced significantly greater reductions in pain and inflammation, and required fewer analgesics compared to the placebo group, suggesting faster recovery within a seven-day treatment period.
In a recent randomized trial, EnMax, a novel blend of microbial and plant-derived proteases combined with rutin, demonstrated excellent clinical efficacy in reducing post-operative inflammation and pain. It outperformed both the placebo and a comparator in terms of symptom relief and reduction of inflammatory biomarkers. The EnMax group showed significant improvements in CRP and ESR, with favorable evaluations by both investigators and patients. Importantly, no adverse events were reported, confirming the safety and tolerability of the formulation11. The present study observed similar benefits with EnMax, further supporting its therapeutic potential.
CRP and ESR are widely recognized as sensitive markers of inflammation. These biomarkers typically increase rapidly after surgery due to tissue trauma and then decline as healing progresses. Persistent elevation may suggest complications such as infection. Monitoring CRP and ESR alongside WBC count provides valuable insight into post-operative recovery and helps rule out infection15,16. For instance, in hip arthroplasty, CRP levels peak by day 2 or 3 post-surgery and normalize over time. In a prior study, CRP decreased by 32% in patients treated with oral enzyme therapy over seven days compared to the placebo17. In the current study, EnMax demonstrated a 57% reduction in CRP and 61.32% in ESR, which were significantly higher than reductions seen with the comparator (CRP: 36%, ESR: 25.19%) and placebo groups.
Overall, the study demonstrates that patients undergoing orthopedic surgeries and treated with the proteolytic enzyme-rutin combination (EnMax) experienced statistically comparable or slightly superior improvements in post-operative pain and inflammation when compared to those receiving the trypsin-bromelain-rutoside combination (comparator). These improvements were reflected in faster resolution of swelling, greater reduction in inflammatory biomarkers (CRP and ESR), and decreased need for analgesic medication, suggesting enhanced wound healing and recovery. EnMax also showed favorable outcomes in clinical symptoms such as erythema, induration, and tenderness. Patient and investigator assessments further supported its clinical benefits. These results are consistent with previous studies reporting the effectiveness of enzyme-based therapies in managing post-surgical inflammation.
The study was rigorously designed with randomization and a placebo-controlled arm, ensuring methodological robustness and minimizing bias. The comprehensive assessment using clinical scores, biochemical markers, and symptom tracking strengthens the reliability of the findings. High treatment compliance, absence of adverse events, and stable hematological and vital parameters highlight the safety and tolerability of EnMax. However, the relatively small sample size, particularly in the placebo group, limits the broader applicability of the conclusions. Despite this, the promising outcomes support the therapeutic potential of EnMax in managing post-operative inflammation. Further large-scale studies with extended follow-up periods are warranted to confirm these findings and establish its long-term benefits.
Conclusion
This randomized, double-blind, controlled trial suggests that EnMax may support the management of post-operative inflammation and pain, showing greater percentage improvements in pain scores, inflammatory biomarkers (CRP and ESR), and reduced analgesic use compared to both placebo and the comparator. EnMax was also associated with earlier symptomatic relief and favorable global assessments by both patients and investigators.
Further, larger, and independently conducted multicenter trials incorporating predefined subgroup analyses are planned to confirm these findings and better define the clinical applicability of EnMax.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due intellectual property constraints but are available from the corresponding author on reasonable request.
References
Mclain, R. F. & Weinstein, J. N. Orthopaedic surgery. In Handbook of Pain Management: A Clinical Companion To Textbook of Pain (435–451 ). (Elsevier Inc, 2003)
Taylor, J. M. M. D., Gropper, M. A. & MD Phd. Critical care challenges in orthopedic surgery patients.Critical Care Medicine 34 (9) P S191-S199, 2006.
Cheng, J., Zhang, L., Zhang, J., Asadi, K. & Farzan, R. Prevalence of surgical site infection and risk factors in patients after foot and ankle surgery: A systematic review and Meta-Analysis. Int. Wound J. 21 (1), E14350 (2024).
Holmes, A. et al. Predictors of pain severity 3 months after serious injury. Pain Med. 11 (7), 990–1000 (2010).
Mangram, A. J. et al. Guideline for prevention of surgical site infection, 1999. Infect. Control Hosp. Epidemiol. 20 (4), 247–280 (1999).
Buchanan, J. M., Baldasera, J., Poole, P. H., Halshaw, J. & Dallard, J. K. Postoperative pain relief; A new approach: narcotics compared with Non-Steroidal Anti-Inflammatory drugs. Ann. R. Coll. Surg. Engl. 70 (5), 332 (1988).
Leth, M. F. et al. Risk of serious adverse events associated with non-steroidal anti‐inflammatory drugs in orthopaedic surgery. A protocol for a systematic review. Acta Anaesthesiol. Scand. 66 (10), 1257–1265 (2022).
Kacha, H. V., Kubavat, A. R., Mundhava, S. G. & Malek, S. M. A prospective comparative study to evaluate efficacy, safety and cost effectiveness of diclofenac and their combination with various proteolytic enzymes in orthopaedic department of tertiary care teaching hospital. Eur. J. Biomed. 7 (1), 460–464 (2020).
Tillaeva, U. M., Gaibnazarova, D. T., Jalilov, F. S. & Abdullaev, B. S. Significance, effectiveness and prospects of development of enzyme preparations in modern pharmaceutical practice. Drugs 2 (3), 4 (2020).
Spaeth, G. L. The effect of bromelains on the inflammatory response caused by cataract extraction: A Double-Blind study. Eye Ear Nose Throat Mon. 47, 634–639 (1968).
Rathi, P. C. et al. The impact of the combination of proteolytic enzyme and Rutin on the Post-Operative management of inflammation: A randomized, controlled, Double-Blind, comparative clinical trial. Cureus ;16(10). (2024).
Sisodia, Y., Dharangutti, R., Khemnar, B. M., John, J. & Vishwakarma, D. T. Post-Operative management of inflammation after orthopaedic surgeries using trypsin, Bromelain and rutoside combination: A Single-Centre prospective observational study. Int. J. Res. Orthop. 9 (1), 1 (2023).
Tiwari, S., Khemnar, B. M. & John, J. Management of post-operative wound in dental surgeries using proteolytic enzyme-flavonoid combination of trypsin, Bromelain and rutoside: a single-centre experience. Int. J. Res. Med. Sci. 10, 1919–1924 (2022).
Swarup Chakraborty & Roy, P. Peri-operative role of proteases (bromelain + rutosides) in surgical patients- a prospective clinical trial. Asian J. Sci. Technol. 10 (08), 9948–9950 (2019).
Bakhshi, G. D. et al. Systemic enzyme therapy with Trypsin-Bromelain-Rutoside combination to counter post-operative wound inflammation-a randomized active-controlled multicentre trial. Eur. J. Pharm. Med. Res. 6 (8), 493–500 (2019).
Kunakornsawat, S. et al. Postoperative kinetics of C-Reactive protein and erythrocyte sediment rate in One-, Two-, and multilevel posterior spinal decompressions and instrumentations. Global Spine J. 7 (5), 448–451 (2017). 2017
Youn, G., Choi, M. K. & Kim, S. B. Comparison of inflammatory markers changes in patients who used postoperative prophylactic antibiotics within 24 hours after spine surgery and 5 days after spine surgery. J. Korean Neurosurg. Soc. 65 (6), 834–840 (2022).
Acknowledgements
The authors would like to acknowledge the research and back-office teams involved in the conduct of this study. We would like to acknowledge the support of Mprex Healthcare Pvt. Ltd., Pune, for serving as the independent contract research organization (CRO) responsible for clinical site management, data collection, monitoring, and statistical analysis. The sponsor had no role in data collection, analysis. The material and testing expenses of the study were borne by Advanced Vital Enzymes P. Ltd. (Advenza) Thane, Maharashtra 400604.
Funding
The material and testing expenses of the study were borne by Advanced Vital Enzymes P. Ltd. (Advenza), Thane, Maharashtra 400604.
Author information
Authors and Affiliations
Contributions
AS, HP, SA, GPG: Acquisition, analysis, or interpretation of data.AS, HP, SA, PCR, CLR, SPR, GPG: Drafting and critical review of the manuscript.All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Piyush C. Rathi declare(s) a grant from Advanced Vital Enzymes Private Limited (ADVENZA). Chandrakant L. Rathi declare(s) a grant from Advanced Vital Enzymes Private Limited (ADVENZA). Shilpa P. Risbud declare(s) employment from Advanced Vital Enzymes Private Limited (ADVENZA). The clinical trial execution, including data collection, site monitoring, and statistical analysis, was conducted independently by a contract research organization. Authors with declared conflicts of interest were involved in study design, and manuscript preparation. Other author declares no conflict of interest.
Informed consent
The informed consent was obtained from all participants before enrolment.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Suryawanshi, A., Patkar, H., Aute, S. et al. Systemic enzyme therapy for postoperative inflammation and wound healing after orthopedic surgery: a randomized, placebo and active controlled clinical study. Sci Rep 15, 34336 (2025). https://doi.org/10.1038/s41598-025-16747-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16747-2