Abstract
Liver biopsy is not a routine screening tool for detecting fibrosis progression in metabolic dysfunction-associated steatotic liver disease (MASLD). Noninvasive methods based on serum biomarkers are used instead. This study evaluated the relationship between serum zinc (Zn), copper (Cu), and ceruloplasmin levels and non-invasive criteria for hepatic fibrosis, including Fibrosis-4 (FIB-4), Nonalcoholic Fatty Liver Disease fibrosis score (NFS), and BARD, in patients with MASLD. In this prospective cross-sectional study, a total of 134 MASLD patients referred to the gastroenterology clinic of Imam Reza Hospital and Sheikh AlRaees Clinic, Tabriz, in 2022 were included. Serum levels of Zn, Cu, ceruloplasmin, albumin, complete blood count (CBC), fasting blood sugar (FBS), Alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were checked. Also, non-invasive measures of FIB-4, NFS, and BARD were measured. Finally, the relationship between the levels of Zn, Cu, and ceruloplasmin with each of the FIB-4, NFS, and BARD criteria was investigated. Zn serum levels were significantly lower in FIB-4 3 than in FIB-4 1 and 2 (P-value < 0.01); however, no significant relationship was found between serum Cu and ceruloplasmin variables with FIB-4 (P > 0.05). Zn serum levels were also significantly higher in NFS fibrosis score 1 (P-value < 0.01). There was no significant relationship between serum Cu and ceruloplasmin variables with NFS fibrosis score in the studied patients (P > 0.05). No significant relationship was observed between serum Zn, Cu, and ceruloplasmin with the BARD fibrosis score in the studied patients (P > 0.05). It seems that among the levels of Zn, Cu, and ceruloplasmin serum with non-invasive measures of liver fibrosis, including FIB-4, NFS, and BARD in MASLD patients, there is only a significant relationship between serum Zn level and FIB-4 and NFS criteria. And therefore, Zn may serve as a potential biomarker.
Similar content being viewed by others
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a clinical syndrome that is defined as hepatic macrovesicular steatosis (intracellular triglycerides of more than 5%)1. MASLD encompasses a spectrum from simple steatosis to- non-alchoholic steatohepatitis, fibrosis and cirrohsis2. As the prevalence of obesity, diabetes, and metabolic syndrome is growing among the population, MASLD has become the most common chronic liver disease all over the world3,4,5. The prevalence of MASLD is 38% globally6. South America has the most abundance, and Africa has the least abundance of this disease7. Its prevalence in Iran is 33.9%4. Diagnosis of MASLD is based on imaging methods like sonography, computed tomography (CT) scan, and magnetic resonance imaging (MRI), and rules out alcohol consumption; however, a biopsy is the gold standard of diagnosis8.
As most of the diseases that affect the liver are preventable, identifying fibrosis and its severity is essential to the management and follow-up of patients7,9. Zinc (Zn) is an important mineral that can eliminate oxygen radicals in the human body and necessary factor in lipid metabolism in liver cells10. Also, copper (Cu) is a necessary mineral for human and plant metabolism which can neutralize oxidative stress enzymes. In liver hepatocytes, Cu is incorporated into apo-ceruloplasmin using P-type ATPase enzymes11,12. Ceruloplasmin, a ferroxidase enzyme, is released into the bloodstream and is responsible for transporting over 95% of serum copper, oxidizing Fe2+ to Fe2+, regulating intracellular iron, and eliminating free radicals9,13,14,15,16,17,18. Recent studies found that serum levels of Zn and Cu are correlated with the hepatic necroinflammation19,20. This correlation is explained with these minerals important roles in oxidative stress, endoplasmic reticulum stress, apoptosis, and inflammation, which have a significant association with MASLD21,22. Despite known roles of zinc in liver health, its relationship with non-invasive fibrosis scores remains underexplored. Recently, liver fibrosis association with serum Zn in MASLD patients has been proved through biopsy; however, biopsy is an invasive and costly method, so it couldn’t be used to follow up and management of patients with chronic liver disease. To facilitate liver fibrosis assessment, some non-invasive indices like Fibrosis-4 (FIB-4), Nonalcoholic Fatty Liver Disease fibrosis score (NFS), and BARD have been introduced. Among these items, FIB-4 is the most proper index relative to the others23,24.
Because of the significance of non-invasive assessment of fibrosis in MASLD patients and the presence of nutritional metabolic role in the basis of MASLD, we are to examine the association between serum levels of Zn, Cu, and ceruloplasmin with non-invasive indices like FIB-4, NFS, and BARD.
Materials and methods
Study design and setting
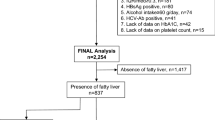
This study was a prospective descriptive-analytic cross-sectional study that examined serum and liver lab tests of MASLD patients who had been referred to Imam Reza Research and Educational Hospital in Tabriz, Iran, during the year 2022. Abdominal sonography was used to diagnose and assess hepatic steatosis. To calculate the sample size, the PASS (version 15) application was used. According to a study by Ito Takanori, et al.23 which was done to assess serum Zn level and liver fibrosis association, with 80% power and 5% of type one error, the sample size was calculated to be 97 patients; Also for increasing study accuracy and the possibility of 20% sample loss, we considered 122 as sample size. And finally, 134 patients were included in this study.
Data collection and study performance
A total of 134 MASLD patients who had been referred to Imam Reza Hospital during the year 2022 who had the inclusion criteria were selected and included in this study. The patients were selected through simple randomization. Patients younger than 18 or older than 65, those with a history of alcohol consumption, a history of Zn supplements, a history of viral or autoimmune hepatitis, and a history of consuming drugs known to alter Zn or Cu levels, such as penicillamine, diuretics, and corticosteroids were excluded from the study. After getting informed consent, their demographic data, like sex, age, height, weight, and patient’s medical history, like diabetes and hypertension, were obtained and registered in the previously accepted checklist. Also, a fasting blood sample was gained to examine serum levels of Zn, Cu, ceruloplasmin, albumin, complete blood count (CBC), fasting blood sugar (FBS), and hepatic enzymes like Alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Serum Zn, Cu, and ceruloplasmin levels were measured using atomic absorption spectrophotometry and colorimetric assays, and interpreted according to standard reference ranges. By using these data, non-invasive indices of liver fibrosis like FIB-4, NFS, and BARD were calculated. Age, platelet level, ALT, and AST are needed to calculate FIB-4. FIB-4 under 1.45 was considered a 0–1 fibrosis stage, a score between 1.45 and 3.25 was considered a 2–3 fibrosis stage, and a score of more than 3.25 was considered a 4–6 fibrosis stage25. The NFS index was calculated by using age, body mass index (BMI), platelet level, albumin, AST, ALT, and the existence of glucose tolerance disorder. In this index, a score under − 1.455 was equalized as fibrosis absence (F = 0), mild fibrosis (F = 1), or moderate fibrosis (F = 2). A score between − 1.455 and 0.675a was considered an unclear level of fibrosis. A score over 0.675 was considered severe fibrosis (F = 3) or cirrhosis (F = 4)4. BARD index was calculated by utilizing BMI, AST, ALT, and the existence of diabetes mellitus. A score between 0 and 1 was considered a low possibility of fibrosis; 2 to 4 is considered a severe stage of fibrosis26.
In this article, to simplify the comparison, we considered a FIB-4 below 1.45 as Category 1, between 1.45 and 3.25 as Category 2, and above 3.25 as Category 3. In NFS, we considered below − 1.455 as category 1, between − 1.455 and 0.675 as category 2, and above 0.675 as category 3, and in BARD, 0 was considered as category 0, 1 as category 1, 2 as category 2, 3 as category 3, and 4 as category 4.
After calculating these indices, their association with the level of serum Zn, Cu, and ceruloplasmin was examined.
Statistical analysis
All data were analyzed using IBM SPSS 26; Descriptive results were presented with mean (standard deviation (SD)) and frequency (percentage) for numerical and categorical variables, respectively. The normality of the variables was assessed and assured by the descriptive measures of distribution, the skewness (within ± 1.5), and the kurtosis (within ± 2). One-way ANOVA and independent t-test for normal variables were used to compare the significance. * Multivariable logistic regression analysis was conducted to identify potential cofounding factors influencing NFS and FIB-4. An odds ratio (OR) with a 95% confidence interval (CI) was presented. Factors with a P-value of less than 0.05 were considered statistically significant.
Ethical considerations
The current study was approved by the ethical committee of Tabriz University of Medical Sciences with code IR.TBZMED.REC.1401.292. All methods were carried out according to relevant guidelines and regulations. All experimental protocols were checked by the liver and gastrointestinal diseases research center and then approved by the scientific committee of the medicine faculty. All patients’ information was kept confidential, and their personal information was not mentioned or published anywhere. Furthermore, all needed tests were performed for patients, and no extra fees were charged to the patients. Informed consent was obtained from all patients.
Results
The demographic and laboratory data are presented in Table 1. The mean (SD) age was 50.05 ± 9.5 years. 78 patients (58.2%) were female. Serum markers of patients and their non-invasive liver fibrosis indices are demonstrated in Table 1.
In Table 2, the association between serum Zn, Cu, and ceruloplasmin levels with FIB-4, NFS and BARD scores was assessed. Lower serum Zn levels were found to have a significant correlation with higher FIB_4 and NFS scores (P-value < 0.05).
In Table 3, the results indicate that in the unadjusted (univariate) model, except for the variable Zn, none of neither variable Cu nor Ceruloplasmin showed a significant relationship with the variable FIB-4. Therefore, in both adjusted and unadjusted models, no significant difference in the probability of event occurrence was observed between FIB-4 and Cu and Ceruloplasmin. However, logistic regression analysis showed that after adjusting for age, BMI, and sex in the model, a significant relationship between the variable Zn and FIB-4 was still observed.
In Table 4, the results show that in the unadjusted model, by comparing categories 1 and 2 with the reference category 3, no significant difference in the odds of outcome occurrence for Cu and ceruloplasmin variables was observed. By adjusting for age, BMI, and sex in the multivariate model, no significant difference was observed in the results. Although the Zn variable was a significant predictor for different levels of NFS in the unadjusted model. Thus, when comparing categories 1 and 2 with the reference category 3, a significant difference in the odds of outcome occurrence for the Zn variable was observed. Similar results were found in multivariate findings based on adjusted odds ratios, showing a significant relationship between NFS and Zn after adjusting for confounding variables, age, BMI, and sex. In other words, the odds of NFS outcome occurrence in categories 1 and 2 compared to category 4 were significantly influenced by changes in the Zn variable.
Discussion
This study was designed to investigate serum Cu, Zn, and ceruloplasmin levels with three top non-invasive hepatic fibrosis indices: FIB-4, NFS, and BARD. This study found that among the three variables (Cu, Zn, and ceruloplasmin), serum Zn level had a significant reverse association with the FIB-4 and NFS scores. In the study by Iwata et al. lower serum Zn levels was found to be associated with hepatic fibrosis in hepatitis B patients. They suggested that disturbance in Zn metabolism may predict hepatic fibrosis27. Also a cross-sectional study done by Kim et al. found that higher FIB-4 index is associated with lower serum Zn level and Lower serum Zn levels could be an independent risk factor for hepatic fibrosis in MASLD patients3. Moreover, the Ito et al. study also found a significant correlation between low serum Zn and the hepatic fibrosis and higher FIB-4 and NSF scores in MASLD patients23. In the Moriyama et al. study, Zn levels were found to be significantly associated with FIB-4, NFS, and hyaluronic acid28. Finally, in recent study published by Semeya et al. Zn deficiency was reported to have a strong correlation with the severity of liver cirrhosis and hepatic encephalopathy29. The results of this study were compatible with all these findings, demonstrating a possible significant association between serum Zn level and hepatic non-invasive indices. Zn being an important part of a constitution of metabolic, anti-inflammatory, and antioxidant enzymes which have huge impacts on distorted metabolism in chronic liver disease as well as its role in reduction of the degree of liver injury and normalization of lipid peroxidation are the possible explanations for these findings30,31. Furthermore, in this study, there was no significant association between serum ceruloplasmin and FIB-4, NFS, and BARD scores. Studies report conflicting results regarding this matter. For example, In the Nobili et al. study, on children with MASLD proved by biopsy, The results claim that although serum ceruloplasmin is associated with the possibility of Nonalcoholic Steatohepatitis, inflammation, and steatosis, but it is not associated with fibrosis32. Also, in the review study by Chen et al. the relationship between serum/hepatic Cu or ceruloplasmin concentration and MASLD was evaluated. Their meta-analysis demonstrated that serum Cu and ceruloplasmin were not associated with MASLD33. However, Corradini et al. reported that ceruloplasmin variants were independently associated with liver fibrosis34. Moreover, in the Issa et al. study, Ceruloplasmin level was significantly lower in patients with higher levels of fibrosis in comparison with patients with no fibrosis35. The results of this study, showed no association between ceruloplasmin level and non-invasive hepatic indices. This contrast may arise from the target population, study environment, inclusion and exclusion criteria, and underlying diseases. Moreover, the limited sample size and the single-border nature of this research can also be another explanation for this contrast.
To our knowledge this study is one of the first studies investigating three serum elements correlation with three scoring systems in MASLD patients. The findings of this study could be used for an easier risk stratification of liver fibrosis in MASLD patients. However, some limitations may affect our findings. One of them is the lack of long-term follow-up in the study. Also, hepatic steatosis was assessed using ultrasonography, which might underrepresent the prevalence of MASLD in the studied population specially in the early stages of MASLD. Moreover, the design of this study does not allow for confirming or negating causality between the assessed parameters. Finally lack of control group is another major limitation of this study. In addition, there is a need for future prospective multicenter studies that could use MRI/elastography for early diagnosis of MASLD to provide a higher level of evidence in this regard.
Conclusion
This study found a significant reverse correlation between serum Zn levels and FIB-4 and NFS scores in MASLD patients. Therefore, serum Zn may serve as a risk stratification factor in MASLD patients. Serum Cu and ceruloplasmin showed no significant association with the FIB-4 and NFS scores. Lastly, none of these three markers showed a significant association with the BARD scoring system. Future prospective studies are recommended for further evaluation of these findings.
Data availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- MASlD:
-
Metabolic dysfunction-associated steatotic liver disease
- Zn:
-
Zinc
- Cu:
-
Copper
- BMI:
-
Body Mass Index
- MRI:
-
Magnetic resonance imaging
- NASH:
-
Non-alcoholic steatohepatitis
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase (AST)
- NFS:
-
The NAFLD fibrosis score
- FIB-4:
-
The fibrosis 4 index
References
Chan, W. K. et al. Metabolic dysfunction-associated steatotic liver disease (MASLD): A state-of-the-art review. J. Obes. Metab. Syndr. 32 (3), 197–213 (2023).
Hashim, M. M. A. et al. Pathological evolution and internal medicine management of nonalcoholic fatty liver disease (NAFLD) in the era of metabolic dysfunction-associated steatotic liver disease (MASLD). Cureus 17 (6), e86963 (2025).
Kim, M. C. et al. Serum zinc level and hepatic fibrosis in patients with nonalcoholic fatty liver disease. PLoS One. 15 (10), e0240195 (2020).
Angulo, P. et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45 (4), 846–854 (2007).
Angulo, P. et al. Liver fibrosis, but no other histologic features, is associated with Long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149 (2), 389–97e10 (2015).
Younossi, Z. M., Kalligeros, M. & Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 31 (Suppl), S32–s50 (2025).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 64 (1), 73–84 (2016).
Moghaddasifar, I. et al. Prevalence of non-alcoholic fatty liver disease and its related factors in Iran. Int. J. Organ. Transpl. Med. 7 (3), 149–160 (2016).
Loomba, R. & Sanyal, A. J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 10 (11), 686–690 (2013).
Olechnowicz, J., Tinkov, A., Skalny, A. & Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 68 (1), 19–31 (2018).
Sharp, P. The molecular basis of copper and iron interactions. Proc. Nutr. Soc. 63 (4), 563–569 (2004).
Maio, N. et al. Role of external loops of human ceruloplasmin in copper loading by ATP7B and Ccc2p. J. Biol. Chem. 285 (27), 20507–20513 (2010).
Aigner, E. et al. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am. J. Gastroenterol. 105 (9), 1978–1985 (2010).
Tallino, S. et al. Nutrigenomics analysis reveals that copper deficiency and dietary sucrose up-regulate inflammation, fibrosis and lipogenic pathways in a mature rat model of nonalcoholic fatty liver disease. J. Nutr. Biochem. 26 (10), 996–1006 (2015).
Prohaska, J. R., Geissler, J., Brokate, B. & Broderius, M. Copper, zinc-superoxide dismutase protein but not mRNA is lower in copper-deficient mice and mice lacking the copper chaperone for superoxide dismutase. Exp. Biol. Med. (Maywood). 228 (8), 959–966 (2003).
Chen, C. et al. Copper exposure association with prevalence of non-alcoholic fatty liver disease and insulin resistance among US adults (NHANES 2011–2014). Ecotoxicol. Environ. Saf. 218, 112295 (2021).
Antonucci, L., Porcu, C., Iannucci, G., Balsano, C. & Barbaro, B. Non-Alcoholic fatty liver disease and nutritional implications: special focus on copper. Nutrients. 9(10). (2017).
Ameh, T. & Sayes, C. M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 71, 103220 (2019).
Kosari, F., Jamali, R., Ramim, T. & Mosavi Jahan Abad, E. The correlation between serum zinc level and liver histology in non-alcoholic steatohepatitis. Iran. J. Pathol. 14 (1), 17–25 (2019).
Chen, S. D. et al. J-shaped relationship between serum zinc levels and the severity of hepatic necro-inflammation in patients with MAFLD. Nutr. Metab. Cardiovasc. Dis. 32 (5), 1259–1265 (2022).
Mousavi, S. N. et al. Zinc and selenium co-supplementation reduces some lipid peroxidation and angiogenesis markers in a rat model of NAFLD-Fed high fat diet. Biol. Trace Elem. Res. 181 (2), 288–295 (2018).
Cui, J., Xu, T., Lv, H. & Guo, M. Y. Zinc deficiency causes oxidative stress, endoplasmic reticulum stress, apoptosis and inflammation in hepatocytes in grass carp. Fish. Shellf. Immunol. 139, 108905 (2023).
Ito, T. et al. Correlation of serum zinc levels with pathological and laboratory findings in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 32 (6), 748–753 (2020).
Shah, A. G. et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7 (10), 1104–1112 (2009).
Loko, M. A. et al. Validation and comparison of simple noninvasive indexes for predicting liver fibrosis in HIV-HCV-coinfected patients: ANRS CO3 Aquitaine cohort. Am. J. Gastroenterol. 103 (8), 1973–1980 (2008).
Cichoż-Lach, H. et al. The BARD score and the NAFLD fibrosis score in the assessment of advanced liver fibrosis in nonalcoholic fatty liver disease. Med. Sci. Monit. 18 (12), Cr735–Cr740 (2012).
Iwata, K. et al. Serum zinc value in patients with hepatitis virus-related chronic liver disease: association with the histological degree of liver fibrosis and with the severity of varices in compensated cirrhosis. J. Clin. Biochem. Nutr. 55 (2), 147–152 (2014).
Moriyama, M. et al. Clinical significance of evaluation of serum zinc concentrations in C-viral chronic liver disease. Dig. Dis. Sci. 51 (11), 1967–1977 (2006).
Semeya, A. A., Elgamal, R. & Othman, A. A. A. Correlation of serum zinc levels with hepatic encephalopathy severity in patients with decompensated liver cirrhosis: A prospective observational study from Egypt. Biol. Trace Elem. Res. (2025).
Cabré, M., Camps, J., Paternáin, J. L., Ferré, N. & Joven, J. Time-course of changes in hepatic lipid peroxidation and glutathione metabolism in rats with carbon tetrachloride-induced cirrhosis. Clin. Exp. Pharmacol. Physiol. 27 (9), 694–699 (2000).
Himoto, T. & Masaki, T. Associations between zinc deficiency and metabolic abnormalities in patients with chronic liver disease. Nutrients. 10(1). (2018).
Nobili, V. et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 44 (2), 458–465 (2006).
Chen, Y., Wu, C., Li, G., Wang, W. & Tang, S. Comparison of copper concentration between non-alcoholic fatty liver disease patients and normal individuals: A meta-analysis. Front. Public. Health. 11, 1095916 (2023).
Corradini, E. et al. Ceruloplasmin gene variants are associated with hyperferritinemia and increased liver iron in patients with NAFLD. J. Hepatol. 75 (3), 506–513 (2021).
Issa, D., Lopez, R., Feldstein, A., Alsabbagh, M. & Alkhouri, N. Serum ceruloplasmin, ferritin, and their ratio are significantly associated with nonalcoholic steatohepatitis in patients with NAFLD: 427. Am. J. Gastroenterol. 109, S128 (2014).
Acknowledgements
The authorization to perform this research was granted by the Liver and Gastrointestinal Disease Research Center, Tabriz University of Medical Sciences, for which the authors are grateful. The authors would also like to thank all of the patients who participated in this study.
Funding
This work was supported by the Deputy for Research of Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
S.H. contributed to writing the text. L.A. and S.M. contributed to correcting text mistakes. L.A. and Z.N. contributed to the design of the work. S.H. contributed to preparing tables and figures. Z.N. and S.M. contributed to analyzing data. S.Z. contributed to collecting data. L.A. contributed to submitting a manuscript and will coordinate between the authors. E.B. contributed to collecting data, writing the text, preparing the tables and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study process was reviewed and approved by the ethics committee of Tabriz University of Medical Sciences, according to the Declaration of Helsinki (ethics code: IR.TBZMED.REC.1401.292). Before collecting data, informed consent was obtained from all patients. All methods were carried out according to relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Banisefid, E., Zonouzi, S.K., Hamzehzadeh, S. et al. Association of serum trace elements with non-invasive fibrosis scores in metabolic dysfunction-associated steatotic liver disease. Sci Rep 15, 32837 (2025). https://doi.org/10.1038/s41598-025-16787-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16787-8