Abstract
Postural orthostatic tachycardia syndrome (POTS) is characterized by an abnormal increase in heart rate upon standing, leading to symptoms such as dizziness, fatigue, and rapid heart rate. Time-restricted eating (TRE), which limits caloric intake to an 8–10 h daily window, has been shown to decrease inflammation and improve immune, autonomic, and mitochondrial function, as well as cardiometabolic parameters. This single arm pilot study evaluated the effects of TRE on quality of life (QOL), heart rate, and mitochondrial function in 20 participants with POTS (≥ 30 bpm increase in upright heart rate) and a baseline dietary window of ≥ 12 h. Following a 2-week baseline monitoring period, participants underwent a 12-week TRE intervention. Pre- and post-intervention assessments included QOL questionnaires, a 10-minute stand test, and plasma mitochondrial analysis. TRE significantly reduced heart rate increase upon standing (mean decrease: 11 bpm, p < 0.001) and improved QOL metrics, as assessed by the Malmö POTS Symptom Score Survey (MAPS) and the General Health Questionnaire Short Form-36 (SF-36). Notable improvements include POTS symptom severity (p < 0.0001), physical functioning (p = 0.02), and energy/fatigue (p < 0.01). Additionally TRE increased mitochondrial-derived ATP production. These findings suggest TRE as a promising lifestyle intervention to improve QOL, heart rate, and mitochondrial function in POTS patients.
Similar content being viewed by others
Introduction
Postural orthostatic tachycardia syndrome (POTS) is a heterogeneous, complex clinical syndrome that impacts multiple organ systems1. This syndrome, characterized by an abnormal increase in heart rate upon standing without a corresponding drop in blood pressure, has gained increasing recognition, particularly due to its association with long COVID/post-acute sequelae of SARS-CoV-2 infection (PASC)2,3. It is estimated that up to 70% of patients with PASC have POTS, with clinicians worldwide seeing an increasing number of POTS patients4. Despite this increasing prevalence of POTS due to long COVID/PASC, there is very little mechanistic insight into POTS and limited pharmacological options. There are also very few clinical trials examining the efficacy of lifestyle interventions for POTS5,6.
Current diagnostic criteria define POTS as a heart rate increase of at least 30 beats per minute within 10 min of standing (or to at least 120 beats per minute), often accompanied by symptoms such as orthostatic intolerance, fatigue, “brain fog,” gastrointestinal dysmotility, and autonomic dysfunction7,8. Similar to other chronic diseases, including chronic obstructive pulmonary disease and congestive heart failure, POTS imposes significant limitations on quality of life (QOL) and daily functioning9. The mechanisms underlying POTS are poorly understood and are thought to be due to a combination of immune, autonomic dysfunction, hypovolemia, and hyperadrenergic state10.
Circadian rhythm dysfunction (CRD) is starting to be recognized as a common feature in conditions like POTS and myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS)11. CRD disrupts the natural 24-hour biological cycles that regulate sleep-wake cycles, hormone secretion, and metabolic activity. This disruption can lead to mitochondrial dysfunction, impaired immune responses, and dysregulation of the autonomic nervous system, exacerbating symptoms in affected individuals12. CRD has also been linked to altered neurotransmitter signaling and increased levels of pro-inflammatory cytokines, including IL-613,14. Mitochondrial dysfunction further contributes to this pro-inflammatory state by increasing oxidative stress, which is implicated in various pathological conditions, including autoimmune disorders, and may exacerbate the inflammatory environment in patients with POTS15. The association of elevated IL-6 levels with sympathetic nervous system activation underscores the connection between inflammation and autonomic instability, suggesting that addressing CRD may be a key therapeutic strategy to help reduce neuroinflammation and autonomic dysregulation, thereby improving symptom management in individuals with POTS. While it remains unclear whether CRD precedes or follows the development of POTS, the overlap in pathophysiological mechanisms suggests it may contribute to symptom burden in patients with POTS.
Interventions targeting circadian rhythm disturbances, such as time-restricted eating (TRE), may hold promise in managing symptoms associated with POTS. TRE involves restricting daily food intake to an 8–10 h window, aligning meal times with the body’s circadian clock. Both animal and human studies have shown that TRE can improve circadian rhythm alignment, mitochondrial, immune, and autonomic function, reduce inflammation, and improve overall cardiometabolic health16,17,18,19.
Research in mice and Drosophila found that TRE impacts physiology via coordinated changes in global gene expression throughout the brain and body. This leads to improvements in autonomic function, inflammation, cardiometabolic health, mitochondrial function, motor coordination, endurance, gut microbiome composition and function, and neuroendocrine regulation20. In mice, the beneficial impact of TRE on mitochondria is vast, including changes in gene expression, metabolites, mitochondrial structure, and function21,22. These benefits are conserved in Drosophila, with TRE reducing expression of the mitochondrial electron transport chain (ETC) components, which likely results in the production of reactive oxygen species (ROS) and preserves mitochondrial structural integrity23. These biochemical and ultrastructural improvements in mitochondrial function translate to improved physiology, including improved muscle function, measured by endurance in mice and flight ability and cardiac contractility in Drosophila21,22,23.
While these preclinical findings provide compelling mechanistic insights, emerging human studies suggest that TRE may also confer anti-inflammatory and autonomic benefits24. TRE has been shown to down-regulate pro-inflammatory mediators such as TNF-α and leptin and may help attenuate exaggerated nocturnal sympathetic activity, a common feature in POTS24,25. However, most current studies investigate TRE in the context of metabolic disorders, diabetes management, or weight loss strategies rather than targeted autoimmune reactions and dysautonomia26. Therefore, further research is needed to explore the use of TRE as a therapeutic strategy for POTS.
We hypothesized that TRE, by optimizing circadian rhythms and improving autonomic/immune function, could address and improve underlying mechanisms contributing to POTS symptoms and ultimately improve QOL for affected individuals. This proof-of-concept pilot study aims to evaluate the potential of TRE as a lifestyle intervention for POTS.
Methods
This clinical study was approved by the University of California, San Diego Institutional Review Board (IRB #802200) and registered on ClinicalTrials.gov (NCT05409651) on 16-05-2022. All methods were performed in accordance with the relevant guidelines and regulations set forth by the University of California, San Diego IRB. Informed written consent was obtained from all participants prior to their inclusion in the study.
Study design
Following recruitment by the study team, and a 2-week baseline period, eligible patients with an eating window of ≥ 12-hours were entered into a 12-week TRE intervention, during which they adhered to an 8-10-hour eating window (Fig. 1A).
Study timeline and consort diagram. (A) The 14-week study consisted of a 2-week baseline period followed by 12-weeks of TRE intervention, with 2 clinic visits (CV). mCC app = myCircadianClock; stand-test = 10-minute POTS orthostatic diagnostic test; questionnaire = SF-36 Health Survey and BDI-II Depression Survey. (B) 63 patients were screened for the study, 23 entered intervention, and 20 completed the study and were included in the analysis.
Patient selection
Participants were screened and recruited from the cardiovascular and internal medicine clinics at the University of California, San Diego, and from www.clinicaltrials.gov. Patients ages 18–70 years with a self-reported dietary intake of ≥ 12-hour window, regular daytime schedule of activity (no shift workers) before study enrollment, and a formal diagnosis of POTS with chronic symptoms that have lasted for longer than six months, were enrolled. Pregnant and/or breastfeeding women and patients with other disorders, medications, or functional states that are known to predispose to orthostatic tachycardia, were excluded (Supplemental Material 1). All patients were taking medications for POTS, including beta blockers, midodrine, ivabradine, and fludrocortisone. On average, they were diagnosed with POTS 3.5 years before enrolling in the study, with 3 participants having a post-COVID POTS diagnosis.
MyCircadianClock (mCC) app for monitoring eating pattern
The smartphone app, myCircadianClock (mCC), serves as an electronic food, activity, and sleep diary27,28 and has been used in several studies to monitor adherence to TRE29,30,31,32,33,34. Participants who met preliminary eligibility entered a 2-week baseline phase of screening where they were instructed to utilize the mCC app to record all ingestion events. The study team tracked patient eating patterns to confirm ≥ 12 h eating windows and determine individual 8–10 h TRE intervention windows. The length of this TRE interval was at least 4 h shorter than their average eating window during their pre-screening, with a minimum eating window of 8 h (from a 12-hour eating window baseline) and a maximum of 10 h (from a baseline of 14-hours or greater). The interval was entered in the app, so participants could visualize their daily eating pattern and consume all meals within the designated interval. Participants continued to utilize the mCC app throughout the 12 weeks of intervention and received study-related nudges and reminders from the study team to encourage adherence to the intervention (Supplemental Table 1).
If a participant fails to log any food data for more than 1 day, the dashboard flags the participant and sends an alert to the research coordinator. The coordinators will login to the dashboard at least twice weekly to monitor food intake data, and follow up with flagged participants as necessary. If any participant faces difficulty logging data, or has questions about any of the features of the app, they can contact the study coordinator through the feedback feature of the app. Their questions are encrypted and delivered to a HIPAA compliant email server specifically set up for this study.
Intervention
Following a 2-week baseline period, participants whose initial eating windows met study criteria were invited to the clinic for their first visit. Patients were instructed to hold all POTS medications including those that impact heart rate (beta blockers, calcium channel blockers, ivabradine) for 48 h before both Visit 1 (baseline) and Visit 2 (after the 12-week intervention). During Visit 1, a 10-minute active standing test was conducted to confirm the diagnosis of POTS and establish baseline orthostatic values without medications35. The stand test was conducted by first having patients rest in the supine position for 5 min prior to standing. Blood pressure and heart rate were recorded at both the 3-minute and 5-minute supine time points; however, only the 5-minute measurements—taken immediately prior to standing—were used for calculating orthostatic changes. Participants were then instructed to stand for 10 min. During the 10 min, blood pressure and heart rate were taken at the 3-, 5-, 7-, and 10-minute time points. All supine and standing blood pressure and heart rate measures were recorded as single measurements, not averaged. All orthostatic measurements were obtained using an automated blood pressure cuff and a pulsometer.Participants failing to meet POTS criteria (30-point increase in heart rate) during this test were excluded from further study procedures. After completion of the 10-minute active standing test, a fasted blood draw was performed, and participants completed general health and sleep questionnaires. Screening for depression utilized the Beck Depression Inventory-II (BDI-II), with scores ≥ 19, without stable medication or therapy, resulting in exclusion. QOL measurements were assessed using the Malmö POTS Symptom Score Survey (MAPS) and the General Health Questionnaire Short Form-36 (SF-36). All assessments took place at the University of California, San Diego.
Upon completion of Visit 1, data was reviewed, and eligible participants worked with a research coordinator to select an 8–10 h eating window tailored to fit their lifestyle, based on pre-screening eating patterns. Subsequently, participants entered a 12-week intervention period during which dietary intake was documented using the mCC app. Participants were instructed to hydrate well during the fasting period and not to make any other changes to their diet. Participants were told not to consume commercial electrolyte supplements during fasting periods, and instead to consume non-caloric beverages (carbonated water, water, decaffeinated tea etc.). Periodic phone contacts reinforced nutritional practices, supplemented by daily push notifications on their phones.
After the 12-week intervention, participants returned to the clinic for Visit 2, replicating procedures from Visit 1, including the standing test, fasted blood draw, and health questionnaires.
Outcomes
The primary outcome was a change in self-reported QOL, evaluated using two instruments: the 36-Item Short Form Health Survey questionnaire (SF-36) and the Malmö POTS Symptom Score (MAPS) survey. The SF-36 is widely used to assess health-related QOL, providing insights into patients’ physical and mental well-being36.
The MAPS survey was specifically developed to assess the symptom burden experienced by POTS patients. It utilizes a semiquantitative system to evaluate the severity of 12 commonly reported symptoms: five cardiac symptoms (palpitations, dizziness, presyncope, dyspnea, and chest pain) and seven non-cardiac symptoms (gastrointestinal symptoms, insomnia, concentration difficulties, headache, myalgia, nausea, and fatigue)37. Unlike the SF-36, which offers a broader evaluation of health-related QOL, the MAPS survey focuses specifically on symptoms relevant to POTS, providing detailed and condition-specific insights into patient experiences. Each of the 12 symptoms included in the survey is rated on a scale from 0 to 10, with 0 indicating no symptoms and 10 indicating the most severe symptoms. The total MAPS score ranges from 0 to 120, with higher scores reflecting greater symptom burden. The optimal cut-point value to differentiate POTS and healthy individuals is ≥ 4237.
A secondary outcome of this study was the change in orthostatic heart rate (HR) when patients transitioned from a supine to an upright position.
To analyze the exploratory outcome of mitochondrial function, glycolytic and mitochondrial metabolism profiling was performed using established techniques38. To analyze the metabolic effects of plasma samples, C2C12 cells, mouse myoblasts (ATCC, CRL-1772™) were used. C2C12 cells were maintained in culture with DMEM (ATCC, 30-2002™) supplemented with 10% fetal bovine serum (Gibco, A31604-01), 37 °C, 5% CO2, and controlled humidity. To perform the metabolic profiling assays, cells were resuspended with trypsin 0.25% (Gibco, 25200-056), centrifuged at 1,000RPM for 5 min, and washed with PBS1X (Gibco, 10010-023). The cells were seeded in Agilent Seahorse 96-well plates (Agilent, 103774-100) at a density of 10,000 cells per well and maintained in incubation for 24 h before the assay. All cells used for the metabolic profiling assays were at or before passage 5, meaning they were subcultured five times or fewer since initial isolation to minimize phenotypic drift and preserve metabolic integrity.
A glycolytic and mitochondrial metabolism profile was performed to analyze the plasma samples’ potential metabolic effects. C2C12 cells were treated with plasma samples (plasma 1%) prepared in Agilent Seahorse Assay Media (XF DMEM 103575-100, HEPES 5mM) supplemented with Agilent glucose (103577-100, final concentration 10mM), glutamine (103579-100, final concentration 2mM, and pyruvate (103578-100, final concentration 1mM). The cells were washed twice in supplemented Seahorse Assay Media, treated with plasma solutions (100mL final volume per well), and incubated for 1 h in a CO2-free incubator as part of the standard Agilent Seahorse degassing procedure. After 1 h, the media was removed and replaced with fresh Agilent Seahorse Assay Media (180mL final volume per well) and a Mitochondrial Stress assay (Agilent Cell Mito Stress Test Kit, 103015-100) and a Real-time ATP Rate Assay (Agilent Real-Time ATP Rate Assay Kit, 103592-100) were performed in an Agilent Seahorse XF Pro. Both assays were run in parallel in two Seahorse XF Pro systems for data reliability. The final well concentrations of the Mitochondrial Stress assay used were oligomycin at 1.5mM, FCCP at 2.0mM, and rotenone/antimycin A at 0.5mM. For the Real-time ATP Rate Assay, the final well concentrations of the compounds used were oligomycin at 1.5mM and rotenone/antimycin A at 0.5mM. In both assays, during the Rotenone/Antimycin A injection Hoechst (ThermoScientific, 33342) was added. All data was normalized by cell count per well and presented as Oxygen Consumption Rate (OCR) (pmol O2/min/1,000 cells). ATP data is presented as ATP production rate (pmol/min/1,000 cells).
Statistical methods
Descriptive statistics and exploratory graphing techniques, including frequencies, means, standard deviations (SDs), box and whisker plots, stem and leaf diagrams, and scatter plots, were employed to assess the data for normality, skewness, and outliers. Additionally, z-scores for skewness and kurtosis were calculated for all outcomes. Final analyses for heart rate and QOL were conducted using both the original data (non-log-transformed) and nonparametric methods, yielding similar results. All reported analyses utilized the original non-log-transformed data format.
The comparability of baseline and post-intervention values was tested using analyses of variance (ANOVAs) for continuous variables and chi-square analyses for dichotomous variables. Treatment effect estimates, along with corresponding p-values, were derived from within-patient comparisons. All statistical tests were two-tailed. All available data was included in the analyses, with no patients excluded due to missing data. The SF-36 was scored according to RAND instructions39. Differences were considered statistically significant if a p-value of 0.05 or less was obtained using R version 4.1.1.
Results
Patient demographics
Baseline characteristics are shown in Table 1. In total, 63 patients were screened, 40 failed screening/were excluded (e.g. did not meet POTS criteria, did not meet food logging adherence, confounding illness). Of the 23 that entered the 12-week intervention, 3 withdrew (unable to adhere to TRE eating window and personal issues unrelated to the study), and 20 completed the study (average age 36.3 ± 11.8 years, n = 18 women) and were included in the final data analysis (Fig. 1B).
The majority of participants who completed the study were White (90%), Non-Hispanic (85%), and predominantly female (90%). All participants met formal diagnostic criteria for POTS and maintained stable doses of pharmacological therapy throughout the study period.
Primary outcome: quality of life
The SF-36 survey provided a comprehensive assessment of various aspects related to QOL. Analysis of baseline and post-intervention data revealed significant improvements in several domains. Specifically, participants reported enhanced physical function (Delta = 9.00 (1.98 to 16.02); p = 0.02) and improved levels of energy/fatigue (Delta = 11.25 (5.09 to 17.41); p < 0.01) following the 12-week intervention (Table 2A).
Analysis of the MAPS survey revealed significant improvements across multiple common POTS symptoms following the 12-week intervention (Table 2B). Participants reported notable reductions in symptoms such as dizziness, palpitations, difficulty breathing, nausea, gastrointestinal issues (GI), exhaustion, and insomnia. The average overall severity score decreased by approximately 19 points (p < 0.001). Notably, the average severity score post-intervention was 40.65, indicating a significant improvement from baseline (59.75). The optimal cut-point value of MAPS to differentiate POTS from healthy controls is a score ≥ 42, underscoring the clinical significance of these improvements37.
Secondary outcome: orthostatic heart rate
Upon analyzing the secondary outcome of orthostatic heart rate, we observed notable changes in participants’ cardiovascular responses throughout the study period (Table 3). At baseline, participants exhibited an average supine heart rate of 70.45 (± 13.23) beats per minute (bpm), which increased significantly to an average of 114.55 (± 14.44) bpm upon assuming an upright posture. Following the 12-week TRE intervention, a marked improvement in orthostatic heart rate was observed (p < 0.0001). Specifically, the increase in orthostatic heart rate between baseline and post-intervention decreased by approximately 11 bpm (44.1 bpm vs. 33.25 bpm). Post-intervention, participants’ supine heart rate averaged 71.15 (± 12.97) bpm, with an average standing heart rate of 104.4 (± 16.41) bpm.
Exploratory outcome: mitochondrial function
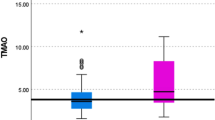
Agilent Seahorse Metabolic Flux assessments revealed no significant changes in mitochondrial stress test outcomes between Pre- and Post-TRE plasma-treated cells (Fig. 2A and B). However, under physiological conditions, there was a significant increase in mitochondrial-derived ATP synthesis in cells treated with post-TRE plasma compared to Pre-TRE plasma (Fig. 2C). These findings suggest that factors elevated in post-TRE plasma may enhance cellular metabolism and energy production without affecting the mitochondrial stress response.
Pre and post TRE plasma-mediated alteration of mitochondrial respiration in skeletal muscle cells. We utilized the Agilent Seahorse Metabolic Flux system to assess mitochondrial stress (A and B) and metabolic profile (C) by monitoring oxygen consumption rates (OCR) via adoptive transfer of plasma to cultured C2C12 cells. C2C12 cells were incubated with plasma at 1% final concentration for one hour before initiating the mitochondrial stress energy generation protocol. (A) Averaged values representing tracing of the Seahorse protocol. We assessed baseline respiration up until the addition of oligomycin (ATPase inhibitor) followed by FCCP (uncoupler) and finally rotenone/antimycin A (Rot/Ant, complex I and III inhibitors, respectively). (B) Mitochondrial functional parameters were analyzed for all samples. We saw no change in mitochondrial stress parameters. (C) Metabolic profiling revealed a significant increase (p < 0.05) in mitochondrial-derived ATP in post-TRE plasma, suggesting that factors in the post-TRE plasma can potentially increase mitochondrial function. Groups were n = 20.
Discussion
POTS presents substantial challenges in management due to its complex symptomatology and limited treatment options. This study explored the impact of a 12-week time-restricted eating intervention on POTS patients, and observed significant improvements across various outcomes on top of standard of care medical therapy for POTS. QOL assessments using the SF-36 and Malmö POTS Symptom Score surveys indicated significant enhancements in physical function (p = 0.02), energy/fatigue (p < 0.01), and reductions in symptom severity encompassing domains such as dizziness, palpitations, and gastrointestinal issues (p < 0.001). Participants demonstrated a notable decrease in orthostatic heart rate, averaging an 11 beats per minute reduction (p < 0.0001) after the TRE intervention (assessments of heart rate were made with their medications for POTS held for 2 days). Importantly, 20 out of 23 participants completed the intervention, underscoring high adherence to the TRE protocol within our study cohort, which predominantly comprised middle-aged women of White and Non-Hispanic ethnicity. There were no adverse events associated with TRE in this population.
Time-restricted eating emerges as a pragmatic intervention for POTS patients for several reasons. Unlike more restrictive dietary approaches, TRE accommodates personal food choices and does not necessitate calorie reduction or increased physical activity, which can be challenging for POTS patients40. It requires no higher level of health literacy, is culturally acceptable, intuitive to follow, and can complement pharmacotherapy. Our study specifically implemented an 8-10-hour TRE protocol, which proved safe, feasible, and associated with high adherence, without adverse effects33. The improvements observed in symptom severity, QOL, and orthostatic heart rate following the TRE intervention may be driven by its ability to target several core mechanisms implicated in POTS.TRE has been shown in both animal and clinical trial to optimize circadian rhythms, promotes a safe state of ketosis, enhances mitochondrial function, reduces inflammation, and improves autonomic function33,41. By consolidating caloric intake into a consistent 8–10 h window, TRE helped reinforce circadian rhythm alignment—an important factor in autonomic stability and cardiovascular regulation. CRD has been associated with heightened sympathetic activity and impaired baroreflex sensitivity42, both of which are commonly disrupted in POTS43. However, the significant reduction in orthostatic heart rate seen in our cohort, suggests TRE may improve autonomic dysfunction by reducing nocturnal sympathetic overactivity and enhancing parasympathetic tone.
In line with these broader implications, our exploratory analysis of mitochondrial function revealed that cells treated with post-TRE plasma demonstrated a significant increase in mitochondrial-derived ATP synthesis under physiological conditions compared to Pre-TRE plasma-treated cells, suggesting that TRE may improve mitochondrial function in patients with POTS. Given that fatigue and exercise intolerance are hallmark symptoms of POTS1, and that mitochondria play a central role in cellular energy production and autonomic regulation44, mitochondrial dysfunction has been proposed as a key contributor to symptom burden. Prior work in long COVID/PASC-associated POTS has identified mitochondrial damage in both the peripheral and central nervous systems, suggesting that impaired ATP production and increased mitochondrial stress may underlie aspects of POTS pathophysiology45. Therefore, assessing mitochondrial-derived ATP synthesis and stress response served as an objective mechanistic indicator of how TRE may modulate cellular energetics and inflammation in this population. The changes seen on the Real-time ATP Rate assay correlate to improvements in QOL metrics especially in energy and fatigue.
Addressing orthostatic heart rate is pivotal in managing POTS due to its diagnostic significance and therapeutic implications. Monitoring and managing orthostatic heart rate abnormalities are essential for tailoring effective treatment strategies that alleviate symptoms and enhance overall well-being in individuals with POTS46,47. The observed 11 bpm reduction in orthostatic heart rate post-TRE (44.1 bpm vs. 33.25 bpm, p < 0.0001) suggests enhanced autonomic function and improved cardiovascular stability, potentially alleviating symptoms such as lightheadedness, dizziness, and fatigue associated with POTS. This 11 bpm reduction is comparable to what is seen with pharmacologic interventions for POTS such as beta blockers48.
QOL outcomes further underscored the intervention’s efficacy, revealing significant improvements in common POTS symptoms (Table 2). Participants reported marked enhancements in physical function, increased energy levels, and reduced symptom severity across multiple domains, including dizziness, palpitations, and gastrointestinal issues (p < 0.001). These findings are consistent with previous research linking improvements in orthostatic heart rate to enhanced symptom management and overall QOL in POTS patients49,50. Notably, symptoms such as gastrointestinal issues are prevalent among POTS patients with connective tissue diseases and autonomic neuropathy51often co-occurring with conditions like Ehlers-Danlos syndrome and functional GI disorders52. Addressing these multifaceted symptoms by targeting mitochondrial function and autonomic dysfunction and improving the hemodynamic aspects of POTS, TRE not only mitigates physiological dysregulation but also translates into substantial improvements in daily functioning and overall well-being for individuals living with POTS. Importantly, the majority of POTS patients in our study were severe enough to be treated chronically with pharmacotherapy and had been diagnosed with POTS for an average of 3.5 years, and TRE was able to further improve symptoms beyond what medications could provide.
Limitations
This is a single-center clinical trial with a small sample size. However, the sample size is comparable to other published clinical studies in POTS. This is also an open-label study as it is difficult to have blinding with a dietary intervention such as TRE. As such, we acknowledge the potential for psychological bias in QOL reporting, particularly given the appeal of a structured intervention in a population with limited treatment options. However, the observed objective improvements in mitochondrial ATP production provide physiological support for the reported benefits. While our study showed short-term benefits over the 12-week period, the durability of these improvements beyond the intervention phase requires further investigation. Larger studies with longer duration of TRE are warranted to confirm these findings.
Conclusion
Interventions aimed at optimizing orthostatic heart rate are pivotal in tailoring effective treatment approaches for POTS patients. Our findings highlight TRE as a promising addition to existing pharmacological strategies, offering a non-pharmacological avenue to modulate cardiovascular responses and improve overall well-being in individuals with POTS.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
Zhao, S. & Tran, V. H. Postural Orthostatic Tachycardia Syndrome (StatPearls Publishing, 2023).
Safavi-Naeini, P. & Razavi, M. Postural orthostatic tachycardia syndrome. Tex. Heart Inst. J. 47 (1), 57–59 (2020).
Mallick, D. et al. COVID-19 induced postural orthostatic tachycardia syndrome (POTS): A review. Cureus 15 (3), e36955 (2023).
Seeley, M. C. et al. High incidence of autonomic dysfunction and postural orthostatic tachycardia syndrome in patients with long COVID: implications for management and health care planning. Am J. Med Published Online June. 29 https://doi.org/10.1016/j.amjmed.2023.06.010 (2023).
Taub, P. R. et al. Randomized trial of ivabradine in patients with hyperadrenergic postural orthostatic tachycardia syndrome. J. Am. Coll. Cardiol. 77 (7), 861–871 (2021).
Bourne, K. M. et al. Compression garment reduces orthostatic tachycardia and symptoms in patients with postural orthostatic tachycardia syndrome. J. Am. Coll. Cardiol. 77 (3), 285–296 (2021).
Arnold, A. C., Ng, J. & Raj, S. R. Postural tachycardia syndrome - Diagnosis, physiology, and prognosis. Auton. Neurosci. 215, 3–11 (2018).
Fedorowski, A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J. Intern. Med. 285 (4), 352–366 (2019).
Benrud-LarsonLM et al. Quality of life in patients with postural tachycardia syndrome. Mayo Clin. Proc. 77 (6), 531–537 (2002).
Vernino, S. et al. Postural orthostatic tachycardia syndrome (POTS): state of the science and clinical care from a 2019 National institutes of health expert consensus Meeting - Part 1. Auton. Neurosci. 235, 102828 (2021).
Cambras, T., Castro-Marrero, J., Zaragoza, M. C., Díez-Noguera, A. & Alegre, J. Circadian rhythm abnormalities and autonomic dysfunction in patients with chronic fatigue syndrome/myalgic encephalomyelitis. PLoS One. 13 (6), e0198106 (2018).
Sulli, G., Manoogian, E. N. C., Taub, P. R. & Panda, S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol. Sci. 39 (9), 812–827 (2018).
Xu, X., Wang, J., & Chen, G. Circadian cycle and neuroinflammation. Open Life Sci. 18 (1), 20220712 (2023).
Okamoto, L. E. et al. Sympathetic activation is associated with increased IL-6, but not CRP in the absence of obesity: Lessons from postural tachycardia syndrome and obesity. Am. J. Physiol. Heart Circ. Physiol. 309 (12), H2098–H2107 (2015).
Renz-Polster, H., Tremblay, M. E., Bienzle, D. & Fischer, J. E. The pathobiology of myalgic encephalomyelitis/chronic fatigue syndrome: The case for neuroglial failure. Front. Cell. Neurosci. 16, 888232 (2022).
Chaix, A., Manoogian, E. N. C., Melkani, G. C. & Panda, S. Time-Restricted eating to prevent and manage chronic metabolic diseases. Annu. Rev. Nutr. 39, 291–315 (2019).
Sutton, E. F. et al. Early Time-Restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell. Metab. 27 (6), 1212–1221e3 (2018).
Chow, L. S. et al. Time-Restricted eating effects on body composition and metabolic measures in humans who are overweight: A feasibility study. Obesity 28 (5), 860–869 (2020).
Kentish, S. J. et al. Time-Restricted feeding prevents ablation of diurnal rhythms in gastric vagal afferent mechanosensitivity observed in High-Fat Diet-Induced obese mice. J. Neurosci. 38 (22), 5088–5095 (2018).
Deota, S. et al. Diurnal transcriptome landscape of a multi-tissue response to time-restricted feeding in mammals. Cell Metab. 35 (1), 150–165.e4 (2023).
Hatori, M. et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15 (6), 848–860 (2012).
Chaix, A., Zarrinpar, A., Miu, P. & Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20 (6), 991–1005 (2014).
Gill, S., Le H. D., Melkani, G. C. & Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 347 (6227), 1265–1269 (2015).
Turner, L. et al. The effects of time-restricted eating versus habitual diet on inflammatory cytokines and adipokines in the general adult population: A systematic review with meta-analysis. Am. J. Clin. Nutr. 119 (1), 206–220 (2024).
Miglis, M. G. & Barwick, F. Sleep disorders in patients with postural tachycardia syndrome: A review of the literature and guide for clinicians. Auton. Neurosci. 215, 62–69 (2018).
Lin, X., Guan, Y., Wu, G., Huang, J. & Wang S. Time-restricted eating for patients with diabetes and prediabetes: A systematic review. Front. Nutr. 9, 1025919 (2022).
Gill, S. & Panda, S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell. Metab. 22 (5), 789–798 (2015).
Manoogian, E. N. C., Wei-Shatzel, J. & Panda, S. Assessing Temporal eating pattern in free living humans through the mycircadianclock app. Int. J. Obes. (Lond). 46 (4), 696–706 (2022).
Manoogian, E. N. C. et al. Time-restricted eating in adults with metabolic syndrome: A randomized controlled trial. Ann Intern Med. Published online October 1, (2024). https://doi.org/10.7326/M24-0859
Manoogian, E. N. C. et al. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: the healthy heroes randomized control trial. Cell. Metab. 34 (10), 1442–1456e7 (2022).
Prasad, M. et al. A smartphone intervention to promote time restricted eating reduces body weight and blood pressure in adults with overweight and obesity: A pilot study. Nutrients 13 (7), 2148 (2021).
Phillips, N. E. et al. The effects of time-restricted eating versus standard dietary advice on weight, metabolic health and the consumption of processed food: A pragmatic randomised controlled trial in community-based adults. Nutrients 13 (3), 1042 (2021).
Wilkinson, M. J. et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell. Metab. 31 (1), 92–104e5 (2020).
Hutchison, A. T. et al. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obes. (Silver Spring). 27 (5), 724–732 (2019).
Brignole, M. et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur. Heart J. 39 (21), 1883–1948 (2018).
Lins, L. & Carvalho, F. M. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open. Med. 4, 2050312116671725 (2016).
Spahic, J. M. et al. Malmö POTS symptom score: assessing symptom burden in postural orthostatic tachycardia syndrome. J. Intern. Med. 293 (1), 91–99 (2023).
Garrett-Bakelman, F. E. et al. The NASA twins study: A multidimensional analysis of a year-long human spaceflight. Science 364 (6436). https://doi.org/10.1126/science.aau8650 (2019).
36-Item Short Form Survey (SF-36. ) Scoring Instructions. (2024). https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html. Accessed August 22.
Fu, Q. & Levine, B. D. Exercise in the postural orthostatic tachycardia syndrome. Auton. Neurosci. 188, 86–89 (2015).
de Cabo, R. & Mattson, M. P. Effects of intermittent fasting on health, aging, and disease. N Engl. J. Med. 381 (26), 2541–2551 (2019).
Faraci, F. M. & Scheer, F. A. J. L. Hypertension: Causes and consequences of circadian rhythms in blood pressure. Circ. Res. 134 (6), 810–832 (2024).
Yeh, S. J. et al. The relationship between cardiovagal baroreflex and cerebral autoregulation in postural orthostatic tachycardia disorder using advanced cross-correlation function. Sci. Rep. 14(1), 25158 (2024).
Casanova, A., Wevers, A., Navarro-Ledesma, S. & Pruimboom L. Mitochondria: It is all about energy. Front. Physiol. 14, 1114231 (2023).
DePace, N. L. & Colombo, J. Long-COVID syndrome and the cardiovascular system: A review of neurocardiologic effects on multiple systems. Curr. Cardiol. Rep. 24 (11), 1711–1726 (2022).
Gibbons, C. H., Silva, G. & Freeman, R. Cardiovascular exercise as a treatment of postural orthostatic tachycardia syndrome: A pragmatic treatment trial. Heart Rhythm. 18 (8), 1361–1368 (2021).
Nesheiwat, Z. et al. Supraventricular tachycardia and postural orthostatic tachycardia syndrome overlap: A retrospective study. J. Innov. Card Rhythm Manag. 12 (2), 4385–4389 (2021).
Raj, S. R. et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation 120 (9), 725–734 (2009).
Anderson, J. W. et al. Cognitive function, health-related quality of life, and symptoms of depression and anxiety sensitivity are impaired in patients with the postural orthostatic tachycardia syndrome (POTS). Front. Physiol. 5, 230 (2014).
Frye, W. S. & Greenberg, B. Exploring quality of life in postural orthostatic tachycardia syndrome: A conceptual analysis. Auton. Neurosci. 252, 103157 (2024).
Mehr, S. E., Barbul, A. & Shibao, C. A. Gastrointestinal symptoms in postural tachycardia syndrome: a systematic review. Clin. Auton. Res. 28 (4), 411–421 (2018).
Kilgore, A. L., Hawa, J. T., Liu, E., Belkind-Gerson, J. & Santucci, K. K. (eds). EDS-related Feeding Difficulties: Preventing the Placement of a Surgical Feeding Tube. JPGN Rep. ;3(1):e147. (2022).
Acknowledgements
This work was supported by a research grant from Dysautonomia International to PT. Research in SP lab is supported by Clara Wu and Joe Tsai Foundation and Wu Tsai Human Performance Alliance. HHP is supported by a Veterans Administration Research Career Scientist Award (BX5005229). ENM is supported by the Larry L Hillblom Foundation and the National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number R01DK139356. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This study was funded by Dysautonomia International.
Author information
Authors and Affiliations
Contributions
All authors contributed to and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dzotsi, M., Strohm, A., Varshney, S. et al. Time-restricted eating improves quality of life, heart rate, and mitochondrial function in patients with postural orthostatic tachycardia syndrome. An open-label pilot study. Sci Rep 15, 34345 (2025). https://doi.org/10.1038/s41598-025-16836-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16836-2