Abstract
Prenatal exposure to climate factors, air pollution, and green space has been linked to respiratory diseases in infants. However, the role of the combined effects of exposure to these factors on respiratory ailments remains unclear. Here we investigate the association of combined exposure to traffic-related air pollution (TRAP), climate factors, and green space during the prenatal period with respiratory diseases in infants. We enrolled 454 participants from the ongoing prospective birth cohort known as the Mothers and Children’s Environmental Health study. Data on infant respiratory diseases were collected from parents or guardians. Average exposure values of TRAP, climate factors, and green spaces for the study population were calculated based on geocoded residential addresses. Multiple logistic regression and quantile-based g-calculation models were utilized to examine the association of exposure to environmental factors and green space with respiratory diseases. The combined exposure to climate factors and TRAP during the first trimester of pregnancy was associated with an increased risk of respiratory diseases in infants. High levels of particulate matter with a diameter less than 2.5 μm (PM2.5) and temperature increased the risk of respiratory diseases (adjusted odds ratio (AOR): 1.615, 95% confidence interval (CI): 1.001, 2.658). Additionally, the risk of respiratory diseases from exposure to air pollution and temperature was associated with lower tertiles of residential green spaces. The AOR was 1.064 (95% CI: 1.001, 1.133) per 1 µg/m3 increase in PM2.5, 1.057 (95% CI: 1.001, 1.116) per 1 ppb increase in NO2, and 1.108 (95% CI: 1.001, 1.176) per 1 ℃ increase in temperature. Incorporating green space into the analysis of joint exposure to climate factors and air pollution reduced the risk of respiratory disease. This study proposes that combined exposures to climate factors, TRAP, and green spaces during pregnancy are associated with infant respiratory diseases. Fewer residential green spaces could enhance the association of climate factors and air pollution with respiratory diseases.
Similar content being viewed by others
Background
Environmental pollution exposure is becoming a major cause of childhood morbidity and mortality, with a particularly negative impact on respiratory diseases1,2,3. Children are more susceptible to pollution because their physiology is different from that of adults, and their immature enzymatic systems make them less likely to detoxify xenobiotics4,5. Additionally, because the development of the respiratory system begins during pregnancy, environmental exposures during fetal development often have lasting effects in adult life6,7.
Traffic-related air pollution (TRAP) encompasses atmospheric contamination arising from both motorized vehicles and non-exhaust traffic sources8,9. Recent studies have shown that TRAP and climate change affect respiratory health, particularly worsening respiratory health in older adults and children8,9,10,11,12. A US pregnancy cohort study found that elevated concentrations of fine particulate matter during pregnancy were associated with increased risk of asthma in children11. Khreis et al. reported that TRAP during childhood adversely affects asthma development8. Similarly, a study conducted in Kuala Lumpur found that high levels of TRAP exposure was associated with an increased incidence of respiratory symptoms and impaired lung function in children9. Additionally, more than 30% of children will experience cumulative health impacts as the frequency of extreme climate events such as heat and cold waves, floods, and droughts rises, increasing risks to respiratory health12. However, another study found that the effect of climate on respiratory mortality was lower when air pollution such as particulate matter and ozone was included13. The combined effects on respiratory disease are inconsistent and require further research.
Green spaces distributed around residential areas have been shown to be effective for protecting respiratory health by reducing environmental pollutants14,15,16. A study in southern China found that increased green exposure around residential areas mitigated respiratory mortality through interactions and mediating pathways of air pollutants17. However, the effects and mechanisms on the disease are still unclear, with other studies showing no association with green space or providing conflicting results18,19,20.
To the best of our knowledge, few studies have explored the interrelationships and joint effects of TRAP, climate change, and green spaces, and none have specifically investigated their effects on respiratory diseases in children using birth cohort data. Therefore, this study aimed to investigate the combined effects of prenatal exposure to air pollution and meteorological factors, as well as the role of surrounding green space, on the risk of respiratory diseases such as asthma, pneumonia, bronchitis, and bronchiolitis in infants.
Methods
Study population
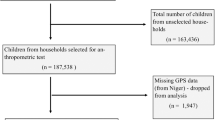
The study population was drawn from the Mothers and Children’s Environmental Health (MOCEH) study, the first large-scale prospective cohort study in four regions of Korea. The MOCEH study focused on the investigation of maternal and child diseases and health conditions, the evaluation of fetal growth and development, and the exploration of the associations between these factors and exposure to environmental elements21. A total of 1,751 pregnant women with a gestational age of less than 20 weeks were recruited during 2006–2010. Follow-up monitoring of the children’s development is ongoing. After excluding lost subjects from follow-up, 1,516 were identified as eligible participants. The study excluded 85 children with birth weight under 2.5 kg or gestational age < 37 weeks. An additional 977 were excluded for incomplete information, including residential address or relocation (n = 80), respiratory disease status (n = 768), educational level (n = 11), household income (n = 36), family history of allergy (n = 12), maternal body mass index (BMI; n = 59), and maternal smoking (n = 11). The final selection encompassed 454 participants, and further details are provided in the Supplementary Information (Figure S1). The characteristics of the excluded participants did not differ significantly from those of the study population, thereby supporting the reliability of the data utilized in this study (Table S1). All participants in the MOCEH study provided informed consent before enrollment. This study received ethical approval from the Institutional Review Boards of Dankook University Hospital, Ulsan University Hospital, and Ewha Womans University. All studies were carried out in accordance with relevant guidelines and regulations. This research was performed in accordance with the Declaration of Helsinki.
Climate factors and air pollution assessment
To determine each participant’s level of TRAP exposure in this study, we estimated pollutant concentrations at their residential locations by applying a land use regression (LUR) model based on standardized procedures and precise address geocoding. This assessment leveraged values from the Korean Ministry of Environment’s atmospheric measurement station, incorporating predictive variables such as topography, land usage, spatial trends, and traffic indicators. Model results for nitrogen dioxide (NO2) and particulate matter with a diameter less than 10 μm (PM10) yielded adjusted R-squared values of 0.79 and 0.69, respectively. These show a strong correlation between the predicted and observed values, as previously documented22,23. In Korea, particulate matter with a diameter less than 2.5 μm (PM2.5) began to be measured in 2015; thus, LUR analysis for this factor could not be performed due to the lack of requisite air pollution station data. Therefore, we estimated PM2.5 exposure through community multiscale air quality (CMAQ) modeling, a three-dimensional photochemical transport framework that processes atmospheric emission information. Previous research provided detailed information on the CMAQ air quality numerical modeling24. PM2.5 concentrations for each study subject were derived through kriging interpolation using a geographic information system tool. Performance evaluation of the CMAQ model showed an R-squared value of 0.64 and a root mean squared error of 4.17. PMcoarse was derived by subtracting the PM2.5 mass concentration from that of PM10. Given that PM10 encompasses PM2.5, PM2.5 and PMcoarse were included in the analysis to evaluate their respective associations with disease outcomes.
Climate factors, including minimum, maximum, and average temperatures, and relative humidity were analyzed by processing data provided by the Korea Meteorological Administration. Participant’s exposure levels to TRAP and climate factors were calculated based on date of birth and gestational age. After computing the daily average values, the exposure measures for each pregnant woman during the first, second, and third trimesters, as well as the entire pregnancy, were analyzed.
Residential greenness assessment
Residential green spaces were evaluated utilizing the Korean Ministry of Environment’s Land-Cover Map, which accurately represents land cover across Korea. Thematic maps were derived from the National Aeronautics and Space Administration’s Landsat image data and Korea’s Arirang satellite imagery. Following methods detailed in previous studies25, maps depicting grasslands, forest areas, and parks were extracted employing geographic information system (GIS) tools (ArcGIS Pro version 2.7.0.). These were used to quantify the green space within 100, 200, 300, and 500 m buffers surrounding the subjects’ residences. Individual green space exposure during pregnancy was evaluated using GIS-based spatial analysis of residential locations, which incorporated geocoded address data for each participant.
Health outcome
Information regarding respiratory diseases diagnosed by physicians at the age of six months was gathered through a questionnaire administered to the children’s parents or primary caregivers. The occurrence of respiratory diseases was identified through a positive response to the inquiry “Has your child ever been diagnosed by a medical doctor with respiratory diseases such as asthma, bronchitis, pneumonia, and bronchiolitis?” A diagnosis of respiratory disease was considered confirmed if any of these ailments was present. Although questions were formulated for each specific respiratory disease, the study had a limited number of cases involving respiratory diseases among six-month-old infants. Consequently, respiratory-related diseases were grouped and analyzed.
Covariates
Covariates included in the models were gestational age (in weeks), maternal age (in years), infant sex, maternal educational level (either less than university or university and above), BMI, parental history of allergies, season of birth, exposure to environmental tobacco smoke, and monthly household income category (below $2000, $2000–4000, and above $4000). Gestational age was determined as the interval between the date of the last menstrual period and the date of delivery, as documented in medical records. Other variables were assessed via interviews and questionnaires conducted by trained personnel with primary caregiver. Exposure to environmental tobacco smoke (ETS) was defined based on whether any household member smoked indoors during pregnancy. Participants who responded affirmatively were classified as exposed to ETS. Adjusted covariates were selected based on established or suspected confounders identified in previous literature and known risk factors for the outcomes8,9,10,11,12,13.
Statistical analysis
The frequencies and values of specific features were analyzed in relation to the children’s respiratory status using the chi-square and t-test. Multiple logistic regression models were employed to explore associations between respiratory diseases, TRAP, and climate factors or green area levels. To assess the risk of respiratory diseases stemming from combined exposure to TRAP and climate factors, the variables were stratified by TRAP and climate change influences and then recombined and evaluated for unit increases. Based on the levels of residential green space, a stratified analysis was conducted to pinpoint the connection between the combined exposure to climate factors, TRAP during different windows of pregnancy (first, second, and third trimesters), and resultant respiratory diseases in infants. A quantile-based g-computation analysis was performed to complement the combined exposure assessment and to address potential collinearity. The method integrated the adaptability of g-computation as an estimation approach with the direct inference provided by weighted quantile sum regression. Environmental composite exposure effects were estimated using g-computation through generalized linear models to estimate causal effects26.
Several sensitivity analyses were conducted to assess the robustness of the associations between climate factors, TRAP, greenness exposure, and respiratory disease. First, to verify the consistency of greenness benefits regardless of model flexibility and buffer size, greenness exposures within 100 m, 200 m, and 300 m buffers were compared to the primary exposure at 500 m using a flexible spatial model based on Global Positioning System (GPS). Second, to investigate the interactions between climate factors, TRAP, and green space, an interaction term was included for each paired exposure and analyzed. Third, given the observed significant differences in secondhand smoke (SHS) exposure between participants with and without respiratory diseases, we further investigated potential effect modification by including interaction terms between SHS exposure and both climatic factors and air pollution in the models. The analyses were conducted using STATA (version 17, Stata Corp, USA), R (version 4.2.1), and ArcGIS pro 2.7.0.
Results
Among the 454 participants, 75 were six-month-old infants with a history of diagnosis of respiratory diseases. These included 4 cases of asthma, 38 cases of pneumonia, 35 cases of bronchitis, and 37 cases of bronchiolitis, with 39 duplicate cases. The specific characteristics of the study subjects, both with and without respiratory diseases, are consolidated in Table 1. The average maternal age was approximately 29 years. During pregnancy, 63% of the mothers encountered secondhand smoke, a rate greater among those whose infants had respiratory diseases. The characteristics of the excluded participants did not differ significantly from those of the study population (Table S1). Data on maternal exposure to different environmental factors and gestational stages is available in Supplementary Tables (Table S2). The mean exposures (standard deviation, SD) during pregnancy were 29.9 (5.10) µg/m3 for PM2.5, 24.04 (8.79) µg/m3 for PMcoarse, and 24.7 (8.01) ppb for NO2.
The associations between respiratory diseases and exposures to climatic elements, air pollutants, and green spaces during pregnancy are detailed in Fig. 1. Single-pollutant models employing multivariable logistic regression indicate that the risk of respiratory diseases is associated with exposures to PM2.5, particulate matter with a diameter of 2.5 to 10 μm (PMcoarse), and NO2 during the first trimester of pregnancy. The adjusted odds ratio (AOR) (95% confidence interval (CI)) was 1.039 (1.004, 1.076) per 1 µg/m3 increment in PM2.5, 1.024 (1.003, 1.046) per 1 µg/m3 increment in PMcoarse, 1.032 (1.001, 1.064) per 1 ppb increase in NO2, and 1.021 (1.001, 1.056 per 1 ℃ increment in temperature. The associations between respiratory diseases and exposure to climate variables and green spaces in different trimesters are shown. Figure S2 delineates the correlation between climate factors, air pollutants, and greenness, with the correlation coefficient ranging from − 0.23 to 0.7.
Risk of respiratory diseases in infants with prenatal exposure to traffic-related air pollution, climate factors, and residential green space. Multiple logistic regression models were employed to explore associations between respiratory diseases, air pollution, and climate factors or green area levels. The models were adjusted for maternal age, education, income, BMI, gestational age, exposure to secondhand smoke, birth weight, sex, and season of birth. Abbreviations: trim., trimester.
Figure 2 illustrates the combined exposure effects of climate variables and TRAP on the risk of respiratory diseases, as determined by a stratified analysis. Both temperature and air pollution were categorized into tertiles, with subsequent analyses conducted for each tertile group. The risk of respiratory diseases increased when PM2.5 concentrations exceeded 32.9 µg/m3 and temperatures were greater than 17.1 ℃, resulting in an AOR of 1.615 (95% CI: 1.001, 2.658). The combined exposure to PMcoarse and climate factors was not associated with respiratory diseases (Figure S3).
Effects of combined exposure of climate factors and PM2.5 on respiratory disease risk in infants aged six months during the first trimester of pregnancy. (A) Stratified analysis by temperature, defining tertile 1 as < 9.7 °C, tertile 2 as 9.7–17.1 °C, and tertile 3 as > 17.1 °C. (B) Stratified analysis by humidity, categorizing tertile 1 as < 60.8%, tertile 2 as 60.8–69.0%, and tertile 3 as > 69.0%. The straight line with a circle, triangle, and square marks indicate PM2.5 concentrations < 25.7, 25.7–32.9, and > 32.9 µg/m3, respectively. The Y-axis represents the AOR (95% CI) for the combined exposure effect of climate variables and air pollution on respiratory disease risk. Multiple logistic regression models were used to explore the association between respiratory diseases and combined exposure to PM2.5 and climate factors. The models were adjusted for maternal age, education, income, BMI, gestational age, exposure to secondhand smoke, birth weight, sex, and season of birth.
The risk of respiratory diseases decreased when NO2 (< 19.4 ppb) and temperature (< 9.7 ℃) were at low levels. The AOR was 0.702 (95% CI: 0.501, 0.983). The combined exposure to NO2 and humidity was not associated with respiratory diseases (Fig. 3).
Effects of combined exposure of climate factors and NO2 on respiratory disease risk in infants aged six months during the first trimester of pregnancy. (A) Stratified analysis by temperature, with tertile 1 defined as < 9.7 °C, tertile 2 as 9.7–17.1 °C, and tertile 3 as > 17.1 °C. (B) Stratified analysis by humidity, where tertile 1 is < 60.8%, tertile 2 is 60.8–69.0%, and tertile 3 is > 69.0%. The straight line with a circle mark indicates NO2 < 19.4 ppb, the triangle mark indicates 19.4–23.5 ppb, and the square mark indicates > 23.5 ppb. The Y-axis depicts the AOR (95% CI) for the combined exposure effect of climate variables and air pollution on respiratory disease risk. Multiple logistic regression models were used to explore the association between respiratory diseases and combined exposure to NO2 and climate factors. The models were adjusted for maternal age, education, income, BMI, gestational age, exposure to secondhand smoke, birth weight, sex, and season of birth.
Table 2 shows the association between the risk of respiratory diseases and exposure to air pollution and temperature in regions with lower tertiles of residential green areas within a 500-meter buffer zone. The AOR (95% CI) was 1.064 (95% CI: 1.001, 1.133) per 1 µg/m3 increase in PM2.5, 1.057 (95% CI: 1.001, 1.116) per 1 ppb increase in NO2, and 1.108 (95% CI: 1.001, 1.176) per 1 ℃ increase in temperature.
Table 3 shows the joint effects of mixture exposures of climate factors, TRAP, and green spaces on respiratory disease, and quintile-based g-computation models produced approximate risk estimates. Each quintile increase in exposure to air pollutants and climate factors was associated with an AOR of 1.601 (95% CI: 1.021, 2.532) for respiratory disease in children. When green space was additionally included in air pollution and climate factors, the composite exposure was associated with an AOR of 1.383 (95% CI: 1.325, 1.858).
Additionally, to assess the combined impact of climatic factors, TRAP, and green space on the risk of respiratory disease, each exposure variable was categorized into tertiles. The results showed that living in areas with limited green space, higher NO₂ levels, and elevated temperatures was associated with increased respiratory disease risk (Table S3). Sensitivity analysis indicated that our results were robust. Examination of the relationship between green space and TRAP, temperature, and respiratory diseases with buffers within 100, 200, and 300 m from residential areas yielded similar findings to the 500-m buffer (Table S4 and Table S5). Additionally, a continuous interaction term was included in analysis to investigate the interaction between climate factors, air pollution, and green spaces. The association was not significant, supporting exclusion of the interaction term (Table S6). Interaction analyses between SHS and environmental variables showed no significant effect modification on respiratory health (Table S7).
Discussion
Prenatal exposure to TRAP, including PM2.5, PMcoarse, and NO2, as well as climatic factors such as ambient temperature and humidity, was associated with an increased risk of respiratory diseases in children at six years of age. After stratifying the climate factors and air pollution and examining their combination for each tertile, it became apparent that combined exposure to TRAP and climate factors in the first trimester of pregnancy was associated with a higher risk of respiratory diseases in children at age 6 in the higher tertile. In addition, exposure to high tertiles of PM2.5 and temperature and to the low tertile of green space increased the risk of respiratory disease. As a result of complex exposure analysis of air pollution and climate factors, the impact on respiratory diseases was high, and the risk decreased when additional green space was included. Our study showed evidence for the impact of combined prenatal exposure to climate factors, air pollution, and green space on respiratory diseases in children.
This study demonstrates that prenatal exposure to TRAP is associated with increased odds of respiratory diseases in six-month-old infants. This aligns with the results of a study performed in Spain, which monitored 352 infants from birth to one year of age, indicating a positive association between wheezing and prenatal NO2 exposure27. Moreover, the association between asthma during preschool (0–5 years) and prenatal NO2 exposure was identified in a Canadian cohort of 58,306 children28. Specific maternal NO2 exposure, especially during the first trimester of pregnancy, was linked to a heightened risk of developing rhinitis, asthma, and eczema in infants29. A cohort study in the United States involving 708 children (0.3–2 years) documented that exposure to PM2.5 was associated with wheezing30, and a significant reduction in lung function was attributed to prenatal exposure to PM10 from road traffic31. Several recent reviews have underscored significant links between early-life exposure to TRAP and the onset of asthma in childhood8,32. Nevertheless, recent studies involving Chinese preschool children found no association between asthma and prenatal NO2 exposure33,34, a discrepancy that may stem from variations in the study population, exposure assessment, and outcome definitions.
The present study has delineated an association between respiratory diseases in infants aged six months and elevated mean ambient temperature during the first trimester of pregnancy. This relationship has been infrequently explored in previous research, with limited studies examining the influence of temperature during pregnancy on subsequent respiratory conditions in children. Our findings align with a cohort study conducted on 2,598 preschoolers in China, which indicated that high temperature exposure during the first trimester was correlated with asthma (AOR = 2.33; 95% CI: 1.11–4.90)35. Discussing the prenatal impact of temperature on infant respiratory health is challenging given the paucity of targeted research. However, existing studies focusing on short-term and postnatal effects of ambient temperature offer valuable insights. Several studies on the climatic risk factor for respiratory diseases, particularly asthma, have consistently shown that both high and low temperatures contribute to increased morbidity36,37. A time-series analysis from Hong Kong revealed a heightened risk of hospitalization for asthma when the average daily temperature rose above 27 °C during the hot season38. Moreover, an investigation in Brisbane, Australia, demonstrated that hot weather’s effects on children’s emergency room visits for asthma in children were immediate and significant37.
Despite these findings, several studies have not established a connection between short-term temperature exposure and asthma-related outcomes in several global locations, including Sweden39, Japan40, and China41. The current study adds to this discourse by identifying the prenatal effect of ambient temperature on respiratory diseases, providing evidence of temperature-triggered respiratory conditions.
We found that combined exposure to meteorological factors and TRAP during pregnancy increases respiratory disease in children. On cross-analyzing PM2.5 and average temperature into tertiles, diseases increased when both PM2.5 and average temperature were high. Additionally, combined exposure to climate factors and air pollution such as temperature, humidity, PM2.5, PMcoarse, and NO2 increased the risk of respiratory disease. Many studies have explored the effects of air pollution or climate factors on the disease, but there is not much research on the combined effects of factors on respiratory diseases. In particular, there is little research on the joint effects of environmental factors during pregnancy. A study by Yang et al. (2023)42 suggested that the interaction of PM10 and SO2 exposure during pregnancy with temperature and daily temperature changes was associated with the development of childhood pneumonia. A cohort study conducted in Changsha, China found that low and high temperatures during pregnancy each interacted with air pollution exposure during pregnancy to increase the impact on childhood asthma36. Our study provides further evidence for the impact of combined prenatal exposure to meteorological factors and air pollutants on respiratory disease in children.
The link between environmental pollution, such as climate factors and air pollution, and respiratory health can be understood through biological plausibility. Air pollution causes oxidative stress and airway inflammation, changes in lung endothelial cells, and leads to respiratory complications43. Prolonged exposure to heat can dilate blood vessels and activate thermoregulatory responses such as increased ventilation rate and lung volume. Such heat exposure has been shown to exacerbate airway hyperresponsiveness and augment oxidative stress within pulmonary tissues, thereby contributing to airway remodeling and the aggravation of asthma-related symptoms44. Furthermore, as the respiratory system serves as the primary route for the entry of airborne pollutants, heightened ventilation in hot environments may result in greater overall pollutant45,46. The immature respiratory system and limited adaptability of children in infancy may further enhance their vulnerability to air pollution and temperature changes47. The first trimester of pregnancy is a critical period for respiratory system development. During weeks 4 and 7 post-conception, primary lung buds form and branch, establishing the basic framework of the pulmonary lobular structure by the end of week five. By weeks 10–11, fetal breathing movements begin, facilitating further lung maturation. Environmental or physiological disturbances during this formative stage may result in long-lasting structural consequences48.
On the other hand, green environments have been found to play a role in regulating climate factors, reducing exposure to harmful pollutants, and lowering temperature or maintaining appropriate humidity42,49,50,51. This study showed that the impact of air pollution and temperature on respiratory diseases was higher in areas with less green space within a 500-m radius from residential areas. Even when analyzed using a complex exposure method, green space was found to play a protective role against respiratory disease risk related to air pollution and temperature. A study in Taipei found that when the green space such as green structures is low, the mortality rate of respiratory diseases such as pneumonia and chronic lower respiratory disease increases due to an increase in air pollutants, and when the ratio of green space is high, the mortality rate from diseases decreases14. A study of children under 5 years of age in Hanoi, Vietnam demonstrated that green spaces lower local temperatures, reducing the impact of heat on respiratory diseases52. These studies echo the findings in this study, which found that the size of green spaces affects respiratory disease due to exposure to air pollution and high temperature. However, a New York City birth cohort study showed conflicting results, suggesting that increased tree canopy coverage may increase the incidence of asthma or allergic diseases due to sources such as pollen53.
Green spaces can strengthen the immune system by releasing antibacterial organic compounds such as phytoncides54, and can alleviate oxidative stress by filtering out pollutants55,56. In a study of a group of lung disease patients in Japan, a significant decrease in inflammatory cytokines was observed in the forest-exposed group57. Surrounding green spaces may enhance local air quality through the absorption and filtration of pollutants, contribute to the moderation of microclimatic conditions by lowering ambient temperatures, and potentially mitigate the risk of respiratory diseases by modulating inflammatory processes58.
The key strength of this study resides in its design as a prospective birth cohort study initiated from early pregnancy. To the best of our knowledge, this represents the first study exploring the effect of exposure to a combination of climatic elements, air pollution, and green spaces on respiratory diseases. The synergistic exposure effects of climatic variables and air pollution were analyzed through a stratification analysis and quantile g-computation. Additional advantages include leveraging LUR and CMAQ models to objectively estimate exposure at the individual level and capture pollution variability at a granular scale.
Certain limitations should be noted for the interpretation of our findings. First, the diagnosis of respiratory diseases was contingent on interviews and answers to questionnaires completed by the primary caregiver, which might suffer self-reporting bias. Second, a joint effect analysis, employing the quantile g-computation model, enables consideration of the nonlinear correlation between exposure level and respiratory incidence and the effects of a combined exposure, but not the variability of each exposure or actual effects. Third, this study focused specifically on temperature and relative humidity as climate exposure variables. Nonetheless, these parameters, as well as air pollution, may be influenced by additional meteorological factors such as wind speed, solar radiation, precipitation, heatwaves, and cold spells. Therefore, future research should comprehensively account for these variables when evaluating climate-related risks of respiratory diseases in children. Fourth, because the study focused on three specific Korean cities (Seoul, Cheonan, and Ulsan), a careful analysis is required prior to extrapolating the results to other Korean urban areas. Fifth, owing to the spatial and temporal resolution of the available greenness data, residential green space exposure was evaluated for the entire pregnancy period rather than stratified by trimester. Future analyses will aim to improve exposure assessment by utilizing greenness data with higher temporal resolution. Finally, potential misclassification of exposure may exist, as our LUR and CMAQ models were estimated using residential addresses, not accounting for exposure when children were outside their areas of residence.
Conclusions
The findings from this study suggest that simultaneous exposure to TRAP, climate factors, and residential green areas during pregnancy has an association with respiratory diseases in infants. A reduced presence of green space in residential areas may amplify the relationship between exposure to air pollutants and climate factors and the incidence of respiratory diseases. Further research is imperative to elucidate the mechanism by which green space modulates the effects of respiratory diseases caused by the combined influence of climate and air pollution.
Data availability
The datasets generated or analysed during the current study are not publicly available due to ethical considerations for study participants, but are available from the corresponding author on reasonable request.
Abbreviations
- AOR:
-
adjusted odds ratio
- BMI:
-
body mass index
- CI:
-
confidence interval
- CMAQ:
-
community multiscale air quality
- GIS:
-
geographic information system
- LUR:
-
utilizing a land-use regression
- MOCEH:
-
Mothers and Children’s Environmental Health
- NO2 :
-
Nitrogen dioxide
- PM2.5 :
-
particulate matter with a diameter less than 2.5 μm
- PMcoarse:
-
particulate matter with a diameter of 2.5 to 10 μm
- SD:
-
standard deviation
- SHS:
-
secondhand smoke
- TRAP:
-
traffic-related air pollution
References
Goldizen, F. C., Sly, P. D. & Knibbs, L. D. Respiratory effects of air pollution on children. Pediatr. Pulmonol. 51, 94–108 (2016).
Johnson, N. M. et al. Air pollution and children’s health—a review of adverse effects associated with prenatal exposure from fine to ultrafine particulate matter. Environ. Health Prev. Med. 26, 72 (2021).
Sly, P. D. Adverse environmental exposure and respiratory health in children. Pediatr. Clin. North. Am. 68, 277–291 (2021).
Ginsberg, G. et al. Evaluation of child/adult Pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol. Sci. 66, 185–200 (2002).
Miller, M. D. et al. Differences between children and adults: implications for risk assessment at California EPA. Int. J. Toxicol. 21, 403–418 (2002).
Kim, D., Chen, Z., Zhou, L. F. & Huang, S. X. Air pollutants and early origins of respiratory diseases. Chronic Dis. Transl Med. 4, 75–94 (2018).
Schittny, J. C. Development of the lung. Cell. Tissue Res. 367, 427–444 (2017).
Khreis, H. et al. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ. Int. 100, 1–31 (2017).
Suhaimi, N. F., Jalaludin, J. & Roslan, N. I. S. Traffic-related air pollution (TRAP) in relation to respiratory symptoms and lung function of school-aged children in Kuala Lumpur. Int. J. Environ. Health Res. 34, 1384–1396 (2024).
Grigorieva, E. & Lukyanets, A. Combined effect of hot weather and outdoor air pollution on respiratory health: literature review. Atmosphere 12, 790 (2021).
Hazlehurst, M. F. et al. Maternal exposure to PM2.5 during pregnancy and asthma risk in early childhood: consideration of phases of fetal lung development. Environ. Epidemiol. 5, e130 (2021).
Kline, O. & Prunicki, M. Climate change impacts on children’s respiratory health. Curr. Opin. Pediatr. 35, 350–355 (2023).
Anderson, B. G. & Bell, M. L. Weather-related mortality: how heat, cold, and heat waves affect mortality in the united States. Epidemiology 20, 205–213 (2009).
Shen, Y. S. & Lung, S. C. C. Mediation pathways and effects of green structures on respiratory mortality via reducing air pollution. Sci. Rep. 7, 42854 (2017).
Tischer, C. et al. Urban green and grey space in relation to respiratory health in children. Eur. Respir J. 49, 1502112 (2017).
Zhang, J., Wang, Y., Feng, L., Hou, C. & Gu, Q. Effects of air pollution and green spaces on impaired lung function in children: a case-control study. Environ. Sci. Pollut Res. Int. 29, 11907–11919 (2022).
Wu, W. et al. The complex role of air pollution on the association between greenness and respiratory mortality: insight from a large cohort, 2009–2020. Sci. Total Environ. 899, 165588 (2023).
Cilluffo, G. et al. Associations of greenness, greyness and air pollution exposure with children’s health: a cross-sectional study in Southern Italy. Environ. Health. 17, 86 (2018).
Lambert, K. A. et al. Residential greenness and allergic respiratory diseases in children and adolescents: a systematic review and meta-analysis. Environ. Res. 159, 212–221 (2017).
Parmes, E. et al. Influence of residential land cover on childhood allergic and respiratory symptoms and diseases: evidence from 9 European cohorts. Environ. Res. 183, 108953 (2020).
Kim, B. M. et al. The mothers and children’s environmental health (MOCEH) study. Eur. J. Epidemiol. 24, 573–583 (2009).
Lamichhane, D. K. et al. Lung cancer risk and residential exposure to air pollution: a Korean population-based case-control study. Yonsei Med. J. 58, 1111–1118 (2017).
Lee, J. Y. et al. Land use regression model for assessing exposure and impacts of air pollutants in school children. J. Korean Soc. Atmos. Environ. 28, 571–580 (2012).
Bae, M., Kim, B. U., Kim, H. C. & Kim, S. A multiscale tiered approach to quantify contributions: a case study of PM2.5 in South Korea during 2010–2017. Atmosphere 11, 141 (2020).
Lee, J. Y. et al. Preventive effect of residential green space on infantile atopic dermatitis associated with prenatal air pollution exposure. Int J. Environ. Res. Public. Health 15, (2018).
Keil, A. P. et al. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect. 128, 47004 (2020).
Esplugues, A. et al. Outdoor, but not indoor, nitrogen dioxide exposure is associated with persistent cough during the first year of life. Sci. Total Environ. 409, 4667–4673 (2011).
Sbihi, H., Tamburic, L., Koehoorn, M. & Brauer, M. Perinatal air pollution exposure and development of asthma from birth to age 10 years. Eur. Respir J. 47, 1062–1071 (2016).
Deng, Q., Lu, C., Li, Y., Sundell, J. & Dan, N. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ. Res. 150, 119–127 (2016).
Chiu, Y. H., Coull, B. A., Cohen, S., Wooley, A. & Wright, R. J. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. Am. J. Respir Crit. Care Med. 186, 147–154 (2012).
Rice, M. B. & Mein, S. A. Prenatal air pollution and child lung function: the impossible search for a vulnerable trimester. Am. J. Respir Crit. Care Med. 202, 15–16 (2020).
Hehua, Z., Qing, C., Shanyan, G., Qijun, W. & Yuhong, Z. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: a systematic review. Environ. Res. 159, 519–530 (2017).
He, B. et al. The association of early-life exposure to air pollution with lung function at ~ 17.5 years in the children of 1997 Hong Kong Chinese birth cohort. Environ. Int. 123, 444–450 (2019).
Liu, W. et al. Associations of gestational and early life exposures to ambient air pollution with childhood respiratory diseases in shanghai, china: a retrospective cohort study. Environ. Int. 92–93, 284–293 (2016).
Lu, C. et al. Interaction of exposure to outdoor air pollution and temperature during pregnancy on childhood asthma: identifying specific windows of susceptibility. Build. Environ. 225, 109676 (2022).
Fitzgerald, E. F., Pantea, C. & Lin, S. Cold spells and the risk of hospitalization for asthma: new york, USA 1991–2006. Lung 192, 947–954 (2014).
Xu, Z. et al. The association between ambient temperature and childhood asthma: a systematic review. Int. J. Biometeorol. 62, 471–481 (2018).
Lam, H. C., Li, A. M., Chan, E. Y. & Goggins, W. B. 3rd The short-term association between asthma hospitalisations, ambient temperature, other meteorological factors and air pollutants in Hong kong: a time-series study. Thorax 71, 1097–1109 (2016).
Forsberg, B., Stjernberg, N., Falk, M., Lundbäck, B. & Wall, S. Air pollution levels, meteorological conditions and asthma symptoms. Eur. Respir J. 6, 1109–1115 (1993).
Abe, T. et al. The relationship of short-term air pollution and weather to ED visits for asthma in Japan. Am. J. Emerg. Med. 27, 153–159 (2009).
Zhang, J. et al. Air pollutants, climate, and the prevalence of pediatric asthma in urban areas of China. Biomed. Res. Int. 2935163 2016 (2016).
Yang, W. et al. Combined effect of preconceptional and prenatal exposure to air pollution and temperature on childhood pneumonia: a case-control study. Environ. Res. 216, 114806 (2023).
Wang, T. et al. Particulate matter disrupts human lung endothelial barrier integrity via ROS- and p38 MAPK-dependent pathways. Am. J. Respir Cell. Mol. Biol. 42, 442–449 (2010).
Lu, C. et al. Extreme temperatures exacerbated oxidative stress and airway inflammation in a mouse model of allergic asthma. Allergy 79, 1333–1335 (2024).
Gordon, C. J. Role of environmental stress in the physiological response to chemical toxicants. Environ. Res. 92, 1–7 (2003).
Qiu, H. et al. The burden of COPD morbidity attributable to the interaction between ambient air pollution and temperature in chengdu, China. Int J. Environ. Res. Public. Health 15, (2018).
Sheffield, P. E. & Landrigan, P. J. Global climate change and children’s health: threats and strategies for prevention. Environ. Health Perspect. 119, 291–298 (2011).
Nikolić, M. Z., Sun, D. & Rawlins, E. L. Human lung development: recent progress and new challenges. Development 145, dev163485 (2018).
Bowler, D. E., Buyung-Ali, L., Knight, T. M. & Pullin, A. S. Urban greening to cool towns and cities: a systematic review of the empirical evidence. Landsc. Urban Plan. 97, 147–155 (2010).
Jim, C. Y. & Chen, W. Y. Assessing the ecosystem service of air pollutant removal by urban trees in Guangzhou (China). J. Environ. Manage. 88, 665–676 (2008).
Shin, D. H. & Lee, K. S. Use of remote sensing and geographical information systems to estimate green space surface-temperature change as a result of urban expansion. Landsc. Ecol. Eng. 1, 169–176 (2005).
Nguyen, V. T. et al. The protective effect of green space on heat-related respiratory hospitalization among children under 5 years of age in hanoi, Vietnam. Environ. Sci. Pollut Res. 29, 74197–74207 (2022).
Lovasi, G. S. et al. Urban tree canopy and asthma, wheeze, rhinitis, and allergic sensitization to tree pollen in a new York City birth cohort. Environ. Health Perspect. 121, 494–500 (2013).
Kuo, M. How might contact with nature promote human health? Promising mechanisms and a possible central pathway. Front. Psychol. 6, 1093 (2015).
Chen, K. et al. Two-way effect modifications of air pollution and air temperature on total natural and cardiovascular mortality in eight European urban areas. Environ. Int. 116, 186–196 (2018).
Yeager, R. et al. Association between residential greenness and cardiovascular disease risk. J. Am. Heart Assoc. 7, e009117 (2018).
Jia, B. B. et al. Health effect of forest bathing trip on elderly patients with chronic obstructive pulmonary disease. Biomed. Environ. Sci. 29, 212–218 (2016).
Maas, J. et al. Morbidity is related to a green living environment. J. Epidemiol. Community Health. 63, 967–973 (2009).
Acknowledgements
This study was supported by the National Institute of Environmental Research (NIER), Ministry of Environment (MOE) of the Republic of Korea (NIER-2016–01-02–076) and the National Research Foundation of Korea (NRF-2020R1A5A2019210 and NRF- 2022R1I1A1A01068568).
Author information
Authors and Affiliations
Contributions
JYL, SL, and JHL planned and conceived the study. YH, HP, YK, MH, and EH contributed to the initial cohort data collection. SL, JO, WL, EK, and JYL conceived the research method and performed statistical analysis of the data. DL, SS, MP, and JYL were responsible for drafting the primary manuscript. JO, WL, DL, YH, HP, YK, MH, and EH performed the interpretation of study analysis results. JYL, SL, MP, and JHL revised and finalized the manuscript. All authors contributed to the completion of the manuscript and reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study received ethical approval from the Institutional Review Boards of Dankook University Hospital (IRB No. 2011-09-0340), Ulsan University Hospital (IRB No. 06–29), and Ewha Womans University (IRB No. 12-07B-15).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, J.Y., Lee, S., Lamichhane, D.K. et al. Combined effects of traffic-related air pollution, climate factors, and greenness on respiratory disease risk in infants. Sci Rep 15, 31250 (2025). https://doi.org/10.1038/s41598-025-16838-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16838-0