Abstract
This meta-analysis aimed to evaluate the effectiveness of repeated low-level red-light (RLRL) therapy compared to conventional myopia treatments to guide clinical application. A comprehensive literature search was conducted using PubMed, Embase, Web of Science, and Cochrane Library databases from their inception to December 2024. To quantify changes in axial length (AL), we computed weighted mean differences (WMDs) with 95% confidence intervals (CIs). Meta-regression and subgroup analyses were used to explore heterogeneity based on intervention duration and treatment type, while publication bias and result stability were assessed using Egger’s and Begg’s tests and sensitivity analysis, respectively. Seven studies, comprising one non-randomized controlled trial, two cohort studies, and four randomized controlled trials, were included, involving 691 pediatric participants (349 in the RLRL group and 342 in the control group receiving orthokeratology and atropine). Overall, AL progression in the RLRL group was significantly lower than in the control group (WMD=–0.24 mm, 95% CI [–0.33, − 0.14], P < 0.001, I2 = 93.5%), with benefits increasing over time. At the same time, we also found that the effect of RLRL adjunctive therapy (WMD=–0.35 mm, 95% CI = [–0.46, − 0.24]) in controlling axial length growth was better than that of monotherapy (WMD=–0.19 mm, 95% CI = [–0.28, − 0.10]). Moreover, RLRL demonstrated a notable enhancement in choroidal thickness, surpassing the effects observed with both orthokeratology (OK) lenses and 0.01% atropine treatments (WMD = 30.79 μm, 95% CI = [17.28, 44.30], I2 = 72.7%, P < 0.05). The reported data from the included studies demonstrated the absence of serious adverse events. RLRL therapy may slow myopia progression more effectively than current standard treatments, but further well-designed trials with longer follow-ups are needed to confirm its clinical value.
Similar content being viewed by others
Introduction
The rising prevalence of myopia, particularly high myopia, presents substantial public health challenges and economic repercussions on a global scale, with a pronounced impact on Asia1,2. High myopia is associated with serious ocular health issues, including glaucoma, cataracts, myopic choroidal neovascularization (CNV), and retinal detachment, which may result in permanent loss of vision and blindness3. In 2020, approximately 30% of the global population was affected by myopia, and projections suggest that this figure could rise to 50% by the year 20504. In China, several studies have reported a high prevalence of myopia among students, with 48.7–55.3% affected and an incidence rate of 85.0% among high school students5,6. Consequently, effective strategies for controlling myopia progression are crucial.
Currently, the main interventions for myopia management are orthokeratology (OK), low-concentration atropine, defocus-incorporated multiple-segment (DIMS) spectacles, multifocal soft contact lenses, and a variety of other less frequently utilized methods7. Recent literature suggests that pharmacological interventions, including low-concentration atropine, can significantly hinder the advancement of myopia in pediatric populations, especially in those predisposed to experiencing high myopia8. Additionally, optical interventions, including OK, multifocal soft contact lenses, and DIMS glasses, have demonstrated the potential to manage myopia progression without significant adverse effects9.
Recently, repeated low-level red light (RLRL) therapy has attracted attention as an emerging intervention to prevent and control myopia. The basic principle of this therapy is to use red light of specific wavelengths to stimulate eye tissues and promote metabolism and cellular function, which may help slow the progression of myopia10,11. Although the mechanisms underlying the control of myopia by RLRL remain unclear, numerous studies have confirmed that RLRL exposure can significantly slow the growth of spherical equivalent refraction (SER) and axial length (AL) in children with myopia12,13,14,15,16. Furthermore, the noninvasive and painless nature of RLRL therapy makes it a feasible intervention for myopia.
Although recent meta-analyses have compared various intervention methods for myopia control17,18 few studies have directly compared RLRL with other treatments. Additionally, the evaluation of the curative effects remains relatively limited. This study aimed to better understand the efficacy of RLRL through direct head-to-head comparisons, and provide a more comprehensive analysis of its effects on the choroid and its safety profile.
Subjects/materials and methods
Study design
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. All studies incorporated in this analysis complied with the Declaration of Helsinki, and the requirement for individual patient consent or ethical review was not applicable.
Literature search
We systematically searched relevant articles in PubMed, Embase, Web of Science, and the Cochrane Library from their establishment until December 2024. We also reviewed the references of the identified articles to ensure no relevant articles were overlooked. A literature search used the Medical Subject Headings (MeSH) terminology alongside free-text keywords. These included: “myopia” OR “nearsightedness” OR “myopias” OR “nearsightednesses,” “Repeated Low-Level Red-Light” OR “low-intensity laser” OR “RLRL” OR “Low-Level Red Light,” and “atropine” OR “orthokeratology lenses” OR “OK” OR “soft multifocal contact lens” OR “Defocus Incorporated Multiple Segments glasses” OR “spectacle lenses” OR “DIMS.”
Inclusion and exclusion criteria
The inclusion criteria encompassed: (1) all clinical studies that assessed the efficacy of RLRL therapy in comparison to conventional methods for controlling myopia; (2) participants were children and adolescents aged below 18 years diagnosed with myopia; (3) the intervention group was administered RLRL therapy, while the control group received conventional myopia control treatments, such as OK, atropine eye drops, DIMS, spectacle lenses or multifocal lenses; (4) studies that reported specific clinical outcomes related to myopia progression, such as changes in SER, AL, or visual acuity; (5) studies that provided follow-up data of at least three months to assess the effects of the interventions.
The exclusion criteria were as follows: (1) studies that were not original, including review articles, abstracts from conferences, expert analyses, and summaries; (2) studies that lacked complete data, making it impossible to extract essential information; (3) studies that did not involve children or adolescents with myopia; (4) studies that compared RLRL therapy with other nontraditional methods not classified as myopia controls; (5) research articles published in languages other than English or Chinese; and (6) research articles for which the full text was not accessible.
Data extraction and quality assessment
EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA) eliminated duplicate segments identified in the collected articles. Two independent evaluators examined the remaining articles’ titles, abstracts, and complete texts based on established inclusion and exclusion criteria. Finally, relevant information, including the first author’s name, year of publication, sample size, country of origin, study design, intervention duration, intervention details, baseline SER, and baseline AL, was independently extracted from the included studies. If needed, a third person made the final decision. All extracted data were collected during the treatment period. The articles with the most comprehensive data published by the same research group were selected. Continuous variables are typically summarized as means and standard deviations (SD). Without a directly specified standard deviation (SD), it was calculated using the RevMan calculator based on 95% confidence intervals (CIs) on the Cochrane website. Two reviewers independently assessed the quality of the studies included in the analysis. For randomized controlled trials (RCTs), we applied the “Cochrane Risk of Bias Tool.“19while for non-randomized trials and cohort studies, we utilized the “Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I)” framework20.
Data synthesis and analysis
This meta-analysis utilized Stata 15.0 software (Stata Corp LLC, College Station, TX, USA) and Review Manager 5.4 (The Cochrane Collaboration, London, UK). To assess changes in AL, we computed pooled weighted mean differences (WMDs) and 95% confidence intervals (CIs) for pertinent outcomes. A random-effects model was used in the meta-analysis when the I² statistic surpassed 50%. Furthermore, a meta-regression analysis was conducted to identify the possible sources of variability in the study findings. Subgroup analyses were performed using different methods (RLRL vs. OK, and RLRL vs. atropine) and therapy durations (1, 3, 6, and 12 months). To ensure robustness, a sensitivity analysis was conducted by methodically omitting each individual study. We generated a funnel plot and used Begg’s and Egger’s tests to examine possible publication bias.
Results
Search results
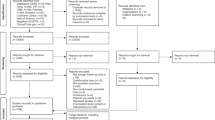
Sixty-two studies were identified from multiple databases, comprising 11 studies sourced from PubMed, 28 from Web of Science, 16 from the Cochrane Library, five from Embase, and two from other sources. After the removal of 23 duplicate publications, 39 studies remained. After evaluating the titles and abstracts, irrelevant articles were removed, and 11 full-text articles were assessed. After reading the full text, four studies were excluded (two studies had no AL data, and two may have been from the same population). Ultimately, seven studies (two cohort studies21,22four RCTs23,24,25,26and one non-RCT27 were included in this meta-analysis (Fig. 1).
Characteristics of studies included in Meta-analysis
Table 1 presents an overview of the fundamental characteristics of the seven studies included in this analysis. This meta-analysis included 691 patients. All studies included in this review, published between 2021 and 2024, were conducted in China. Five studies focused on the effects of RLRL versus OK lenses or 0.01% atropine (monotherapy group), whereas two studies focused on RLRL combined with OK versus OK alone (adjunctive therapy group). The treatment duration ranged from 3 to 24 months in all seven studies. All studies followed a uniform daily RLRL treatment protocol, which included two 3-min sessions each day, separated by at least 4 h. Four studies administered treatment seven days per week, one applied treatment more than 10 times per week, and one did not specify the treatment frequency.
Figure 2 illustrates the findings regarding the risk of bias from the RCTs included in the analysis. Supplementary Table S1 summarizes the risk of bias outcomes from non-RCTs and cohort studies.
Meta-regression analysis
We conducted a meta-regression analysis to determine how different study characteristics such as study design, treatment approaches, RLRL power, and intervention duration influence the overall findings of the meta-analysis. The results indicated that the intervention duration (P = 0.024) and RLRL power (P = 0.01) were the causes of heterogeneity.
Pooled effects of the efficacy results
Changes in AL
To provide larger samples for merged results, we combined RCTs, n-RCTs, and cohort studies because we found that the determination of the conclusions with no changes was performed by sensitivity analysis, excluding non-RCTs one by one (Supplementary Figure S1). Seven studies reported changes in the AL. The aggregated findings indicated that the changes in AL within the RLRL group were notably less pronounced than those seen in the group receiving conventional myopia treatments over the entire duration of treatment, spanning from the initial assessment to the concluding follow-up months of the myopic intervention (WMD = − 0.24, 95% CI = [–0.33, − 0.14], I2 = 93.5%, P < 0.05) (Fig. 3).
In the subgroup analysis, RLRL adjunctive therapy (WMD=–0.35 mm, 95% CI = [–0.46, − 0.24], P < 0.05) better controls axial length growth than monotherapy (WMD=–0.19 mm, 95% CI = [–0.28, − 0.10], P < 0.05) (Fig. 3).
In the subgroup analysis comparing different myopia treatments, the aggregated data indicated that the AL changes within the RLRL group were significantly less marked in comparison to both the OK group (WMD = − 0.15, 95% CI = [–0.27, − 0.04], I2 = 90.3%, P < 0.05) and the group treated with 0.01% atropine (WMD = − 0.25, 95% CI = [–0.29, − 0.20], I2 = 0.0%, P < 0.05) (Supplementary Figure S2). Therefore, RLRL demonstrated greater efficacy in controlling AL growth than 0.01% atropine and OK lenses.
In the subgroup analysis of RCTs (Xiong et al.23Chen et al.24Fu et al.25and Xiong et al.26, the aggregated data demonstrated that AL changes within the RLRL group were significantly diminished compared to those in the control group (WMD = − 0.22, 95% CI = [–0.30, − 0.14], I2 = 84.7%, P < 0.05) (Supplementary Figure S3).
In the subgroup analysis comparing intervention durations (1, 3, 6, and 12 months), the cumulative effect of RLRL compared to conventional myopia treatments increased with longer treatment durations. The WMDs were recorded as follows: 1 month, − 0.06 [–0.08, − 0.05]; 3 months, − 0.11 [–0.15, − 0.07]; 6 months, − 0.18 [–0.23, − 0.13]; and 12 months, the WMD reached − 0.32 [–0.39, − 0.25] (Fig. 4).
In the subgroup analysis, based on different RLRL power, upon comparison of the efficacy of RLRL with conventional treatment, the WMDs recorded were as follows: 0.35 mw, − 0.33 [–0.44, − 0.22]; 0.29 mw, − 0.29 [–0.36, − 0.22]; 2mw, − 0.12 [–0.17, − 0.07]; unkown, − 0.05 [–0.10, − 0.00] (Fig. 5).
Sensitivity analysis was conducted by systematically omitting each included study. The results (Supplementary Figure S4), which aligned with the initial analysis, revealed that individual studies had a minimal impact on the overall results, demonstrating a consistent overall effect size across the studies.
We assessed the existence of publication bias across the seven studies using a funnel plot (Supplementary Figure S5). Both Begg’s test (z = − 1.95, P = 0.07) and Egger’s test (t = − 2.5, P = 0.054) indicated an absence of significant publication bias in the seven studies.
Effectiveness
Table 2 outlines the efficacy outcomes observed in participants undergoing RLRL therapy compared with those undergoing conventional myopia treatments. In four of the seven studies, the results of RLRL, both alone and in combination with OK, were compared with those of conventional therapies such as OK or atropine. Most studies found that RLRL outperformed conventional therapies [AL increase of less than 0.10 mm/year: 86.2% vs. 14.3% (Xiong et al.26, 53.2% vs. 9.7% (Chen et al.24; AL increase of less than 0.30 mm/year: 85% vs. 56% in the first year, and 94% vs. 75% in the second year (Sun et al.21. However, Li et al.22 reported different results, showing that RLRL had a poorer effect [AL increase of less than 0.10 mm (90.0% vs. 93.3%)]. Nevertheless, the conclusions derived from this study were limited by the small sample size and relatively short follow-up period of three months.
Regression of AL
Six studies reported an obvious decrease in AL compared to the baseline AL after RLRL therapy (Table 2). Four studies assessed RLRL against conventional myopia control methods and discovered that RLRL exhibited a greater efficacy in reducing AL [AL shortening ≥ 0.05 mm: 55% vs. 3% at 1 month, and 42% vs. 2% at 12 months (Sun et al.21; 30.0% vs. 12.5% at 1 month, 42.9% vs. 12.5% at 3 months, 48.3% vs. 13.3% at 6 months, and 44.8% vs. 7.1% at 12 months (Xiong et al.23; 42.6% vs. 7.4% at 1 month, 50.0% vs. 10.0% at 3 months, 31.7% vs. 5.2% at 6 months, and 20.6% vs. 3.6% at 12 months (Chen et al.24; 62.22% vs. 9.52% at 6 months (Fu et al.25; 56.7% vs. 23.3% at 3 months (Li et al.22.
Changes in choroid thickness
Three of the seven studies compared the effects of RLRL therapy, OK lenses, and low-concentration atropine on choroidal thickness after myopia treatment. All three studies reported that RLRL significantly increased choroidal thickness, outperforming both OK lenses and low-concentration atropine (WMD = 30.79 μm, 95% CI = [17.28, 44.30], I2 = 72.7%, P < 0.05) (Fig. 6).
Adverse events
Three studies investigated the adverse events associated with RLRL and conventional therapies: Xiong et al.23 reported 26 cases, Chen et al.24 reported four cases, and Fu et al.25 reported five cases. Sun et al.21 did not report any adverse events (Table 3). Optical coherence tomography (OCT) revealed no discernible structural injury to the fundus. None of the studies reported significant adverse events.
Discussion
The incidence of myopia in children, particularly in those with high myopia, has increased in recent years. An increase of 1 diopter (D) in myopia significantly increases the likelihood of developing myopic maculopathy by 58%, increases the probability of retinal detachment by 30%, increases the chances of posterior subcapsular cataracts by 21%, and enhances the risk of open-angle glaucoma by 20%28. Consequently, research on methods for controlling myopia progression and reducing severe vision decline and its complications29. Various techniques have gained attention, including OK, atropine, DIMS, multifocal soft contact lenses, spectacle lenses, and innovative approaches such as RLRL12,30,31,32,33. A recent meta-analysis assessing the efficacy of 0.01% atropine indicated a weighted mean difference (WMD) of 0.20 D for SER and a reduction of 0.08 mm in AL per annum34. Another meta-analysis revealed that OK can decrease axial elongation by 0.15 mm yearly35. Contact lenses designed to modify peripheral defocus have been shown to decrease the average axial elongation by roughly 0.11 mm annually, while progressive addition spectacle lenses exhibit modest effects, with a refraction change of approximately 0.14 D and an AL change of − 0.04 mm annually36. Meanwhile, RLRL effectively controlled the mean SER change by 0.68 D per 6 months and reduced the average axial elongation to 0.35 mm per six months37. Despite these promising results, few systematic reviews have directly compared RLRL therapy to conventional myopia treatments. Therefore, this meta-analysis provides valuable insights into future research and clinical practice. Our findings demonstrate that RLRL may be more effective than conventional therapies in slowing myopia progression, with an effect size of WMD − 0.24 mm, and RLRL adjunctive therapy (WMD − 0.35 mm) was better than monotherapy (WMD − 0.19 mm). This not only indicates that the curative effects of RLRL in controlling axial length growth are better than those of OK lenses and 0.01% atropine but also that the combined auxiliary treatment can achieve an amplifying effect. This also suggests clinicians to consider RLRL for combined use with OK lenses.
Previous studies have shown that for each 1 mm increase in axial length, there is an approximate corresponding increase of 2.50-3.00 D in refractive progression38and AL change of more than 0.20 mm per year is considered a progressive condition39. Therefore, the intergroup differences in this study are roughly equivalent to a refractive change of 0.60–0.72 D, which may have clinical significance in the prevention and control of myopia in children and adolescents, particularly in delaying the long-term risk of high myopia and its retinal complications, such as maculopathy.
Furthermore, RLRL demonstrated greater effectiveness in promoting AL regression (AL shortening ≥ 0.05 mm) than conventional methods, with annual rates of 20.6–44.8%, compared to just 2–7.1%. However, this evidence has low reliability. In future, it is essential to conduct additional high-quality RCTs with larger sample sizes and longer follow-up durations.
The overall heterogeneity was high (I² = 93.5%), and the meta-regression analysis identified intervention duration as the source of heterogeneity. We performed a subgroup analysis based on the duration of therapy. Subtotal heterogeneity declined significantly. The findings showed that longer RLRL treatment durations led to greater cumulative AL (WMD) changes than those in the control group for up to 12 months. However, most of the study durations examined did not surpass the one-year timeframe. While only one study included a 2-year follow-up period, the other had a much shorter follow-up period of only three months. Although subgroup and sensitivity analyses were conducted to minimize heterogeneity, significant differences remained between the two groups. Baseline age and degree of baseline myopia can also affect heterogeneity.
Increasing evidence has shown that the choroid is vital for the regulation of eye growth and the progression of myopia. Numerous animal and human studies have demonstrated that choroidal thickness is reduced in myopic eyes40,41,42,43whereas myopia control methods lead to increased choroidal thickness44. The findings indicated that the subfoveal choroidal thickness (SFCT) exhibited a notable increase following orthokeratology (OK) intervention, which was further enhanced when administered in conjunction with 0.01% atropine. The weighted mean differences (WMDs) are 19.47 and 21.81, respectively44. Another meta-analysis found that the increase in choroidal thickness after 12 months was markedly more pronounced in the atropine-treated group than in the control group, with a WMD of 15.10 µm45. Additionally, He et al. found that the RLRL group experienced positive changes in macular choroidal thickness from baseline over 1 year, achieving a maximum increase of 9.09 μm at 12 months46. Studies have shown that choroidal thickness increases with changes in SER and AL after myopia control37,47. The specific pathophysiological mechanisms of myopia remain unclear, and the hypothesis that scleral hypoxia leads to scleral remodeling is currently widely accepted48,49,50. Previous studies have indicated that dopamine stimulates the release of nitric oxide (NO) from either the retina or choroid, leading to blood vessel dilation and increased blood flow, which enhances choroidal thickness, alleviates hypoxia, and inhibits ocular growth51,52. In our meta-analysis, RLRL significantly increased choroidal thickness, demonstrating a greater effect than OK lenses or low-concentration atropine, with a WMD of 30.79 μm.
Regarding safety, although several cases reported discomfort, the studies included in this meta-analysis did not indicate any vision-threatening occurrences following treatment or any structural damage identified in the photosensory layer through OCT imaging. With the increasing number of clinical studies on RLRL, particularly in China, its safety issues remain controversial. More experts have begun to focus on the safety issues of RLRL. A recent publication presented an atypical instance involving a 12-year-old female patient who exhibited bilateral disruption of the foveal ellipsoid zone and discontinuity of the interdigitation zone, as observed on OCT after five months of exposure to RLRL53. Moreover, a cohort study found that cone density in the paracentral fovea declined and other subtle retinal abnormalities occurred in some children after RLRL therapy 1 year later54. Although RLRL has a significant myopia control effect, some devices have a photodamaging effect. Clinicians should adopt vigilant, protocol-driven monitoring, especially in children, acknowledging its favorable risk profile in trials and the legitimate concerns arising from retinal imaging studies and photobiological modeling.
This study has some limitations. Robust RCTs that directly compare RLRL with the core alternatives are rare. Most studies have assessed RLRL alone, thus limiting definitive conclusions regarding its relative efficacy. The high heterogeneity observed across studies may reflect variations in study populations, intervention protocols, or outcome assessments. Although subgroup analysis was conducted, the analysis is insufficient due to the small number of studies included. The pooled effect estimates should be interpreted cautiously due to this substantial variability. Moreover, this obscures precise effect estimates and hinders the assessment of long-term sustainability (> 12–24 months). All the participants were predominantly Chinese children, severely limiting the generalizability to other ethnicities/regions. These factors increase the uncertainty in the meta-analytic estimates, constrain long-term applicability, and reduce evidence certainty. Future high-quality RCTs with direct comparisons, extended follow-ups, and diverse populations are critical.
In conclusion, our meta-analysis demonstrates that RLRL may be more effective than conventional myopia control methods in slowing axial growth, increasing choroidal thickness, and regressing AL without significant adverse events. However, evidence remains limited. More robust RCTs with prolonged follow-up durations are needed to enhance the recommendations for clinical practice.
Data availability
The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.
References
Baird, P. N. et al. Myopia. Nat. Rev. Dis. Primers. 6, 99. https://doi.org/10.1038/s41572-020-00231-4 (2020).
Bremond-Gignac, D. [Myopia in children]. Med. Sci. (Paris). 36, 763–768. https://doi.org/10.1051/medsci/2020131 (2020).
Theophanous, C. et al. Myopia prevalence and risk factors in children. Clin. Ophthalmol. 12, 1581–1587. https://doi.org/10.2147/opth.S164641 (2018).
Holden, B. A. et al. Global prevalence of myopia and high myopia and Temporal trends from 2000 through 2050. Ophthalmology 123, 1036–1042. https://doi.org/10.1016/j.ophtha.2016.01.006 (2016).
Zhao, L. et al. Prevalence and risk factors of myopia among children and adolescents in Hangzhou. Sci. Rep. 14, 24615. https://doi.org/10.1038/s41598-024-73388-7 (2024).
Wei, J. et al. Large-scale study in chengdu, china: the prevalence of myopia full-correction decreased with increasing myopia in adolescents. Heliyon 10, e31593. https://doi.org/10.1016/j.heliyon.2024.e31593 (2024).
Zaabaar, E., Asiamah, R., Kyei, S. & Ankamah, S. Myopia control strategies: A systematic review and meta-meta-analysis. Ophthalmic Physiol. Opt. 45, 160–176. https://doi.org/10.1111/opo.13417 (2025).
Sankaridurg, P. et al. IMI impact of myopia. Invest. Ophthalmol. Vis. Sci. 62 (2). https://doi.org/10.1167/iovs.62.5.2 (2021).
Shah, R., Vlasak, N. & Evans, B. J. W. High myopia: reviews of myopia control strategies and myopia complications. Ophthalmic Physiol. Opt. 44, 1248–1260. https://doi.org/10.1111/opo.13366 (2024).
Salzano, A. D. et al. Repeated Low-level Red-light therapy: the next wave in myopia management?? Optom. Vis. Sci. 100, 812–822. https://doi.org/10.1097/opx.0000000000002083 (2023).
Zhu, Q. et al. Repeated Low-Level Red-Light therapy for controlling onset and progression of Myopia-a review. Int. J. Med. Sci. 20, 1363–1376. https://doi.org/10.7150/ijms.85746 (2023).
Jiang, Y. et al. Effect of repeated Low-Level Red-Light therapy for myopia control in children: A multicenter randomized controlled trial. Ophthalmology 129, 509–519. https://doi.org/10.1016/j.ophtha.2021.11.023 (2022).
Dong, J., Zhu, Z., Xu, H. & He, M. Myopia control effect of repeated Low-Level Red-Light therapy in Chinese children: A randomized, Double-Blind, controlled clinical trial. Ophthalmology 130, 198–204. https://doi.org/10.1016/j.ophtha.2022.08.024 (2023).
Xu, Y. et al. Repeated Low-Level red light therapy for myopia control in high myopia children and adolescents: A randomized clinical trial. Ophthalmology 131, 1314–1323. https://doi.org/10.1016/j.ophtha.2024.05.023 (2024).
Liu, G. et al. Effectiveness of repeated low-level red light in myopia prevention and myopia control. Br. J. Ophthalmol. 108, 1299–1305. https://doi.org/10.1136/bjo-2023-324260 (2024).
Tian, L. et al. Six-month repeated irradiation of 650 Nm low-level red light reduces the risk of myopia in children: a randomized controlled trial. Int. Ophthalmol. 43, 3549–3558. https://doi.org/10.1007/s10792-023-02762-7 (2023).
Lawrenson, J. G. et al. Interventions for myopia control in children: a living systematic review and network meta-analysis. Cochrane Database Syst Rev 2, Cd014758, (2025). https://doi.org/10.1002/14651858.CD014758.pub3
Zheng, Z., Jiang, X., Chen, R. & Dong, L. Efficacy comparison of atropine, orthokeratology and repeated low-level red-light therapy for myopia control in children: a systematic review and network meta-analysis. Br. J. Ophthalmol. https://doi.org/10.1136/bjo-2025-327366 (2025).
Higgins, J. P. et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343, d5928. https://doi.org/10.1136/bmj.d5928 (2011).
Sterne, J. A. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj 355, i4919. https://doi.org/10.1136/bmj.i4919 (2016).
Sun, J. R., Du, Z. Q. & Wu, G. Y. Efficacy comparison of repeated low-level red-light therapy and orthokeratology lenses for myopia control. Optom. Vis. Sci. 101, 660–665. https://doi.org/10.1097/opx.0000000000002197 (2024).
Li, Z. et al. Efficacy of orthokeratology combined with lowlevel red light in the treatment of myopia in children. (2023). https://doi.org/10.21203/rs.3.rs-3457134/v1
Xiong, R. et al. Myopia control effect of repeated Low-Level Red-Light therapy combined with orthokeratology: a multicenter randomized controlled trial. Ophthalmology 131, 1304–1313. https://doi.org/10.1016/j.ophtha.2024.05.015 (2024).
Chen, Y. et al. Efficacy comparison of repeated Low-Level red light and Low-Dose Atropine for myopia control: A randomized controlled trial. Transl Vis. Sci. Technol. 11, 33. https://doi.org/10.1167/tvst.11.10.33 (2022).
Fu, A. et al. Repeated monochromatic low-level red-light versus 0.01% Atropine therapy for slowing myopia progression in children—a randomized controlled trial. (2024). https://doi.org/10.21203/rs.3.rs-4977250/v1
Xiong, F. et al. Orthokeratology and Low-Intensity laser therapy for slowing the progression of myopia in children. Biomed. Res. Int. 2021 (8915867). https://doi.org/10.1155/2021/8915867 (2021).
Yu, M. et al. Axial Length Shortening after Combined Repeated Low-Level Red-Light Therapy in Poor Responders of Orthokeratology in Myopic Children. Journal of Ophthalmology (2024). https://doi.org/10.1155/2024/4133686 (2024).
Bullimore, M. A. et al. The risks and benefits of myopia control. Ophthalmology 128, 1561–1579. https://doi.org/10.1016/j.ophtha.2021.04.032 (2021).
Walline, J. J. Myopia control: A review. Eye Contact Lens. 42, 3–8. https://doi.org/10.1097/icl.0000000000000207 (2016).
Lanca, C., Pang, C. P. & Grzybowski, A. Effectiveness of myopia control interventions: A systematic review of 12 randomized control trials published between 2019 and 2021. Front. Public. Health. 11, 1125000. https://doi.org/10.3389/fpubh.2023.1125000 (2023).
Logan, N. S. & Bullimore, M. A. Optical interventions for myopia control. Eye (Lond). 38, 455–463. https://doi.org/10.1038/s41433-023-02723-5 (2024).
Nucci, P. et al. A comparison of myopia control in European children and adolescents with defocus incorporated multiple segments (DIMS) spectacles, atropine, and combined dims/atropine. PLoS One. 18, e0281816. https://doi.org/10.1371/journal.pone.0281816 (2023).
Zhou, W. et al. Efficacy of different powers of Low-Level red light in children for myopia control. Ophthalmology 131, 48–57. https://doi.org/10.1016/j.ophtha.2023.08.020 (2024).
Sun, W. & Hasebe, S. Efficacy of 0.01% Atropine eye drops in controlling myopia progression and axial elongation in children: A Meta-analysis based on randomized controlled trials. Acta Med. Okayama. 76, 457–463. https://doi.org/10.18926/amo/63905 (2022).
Gong, Q. et al. Efficacy and adverse effects of Atropine in childhood myopia: A Meta-analysis. JAMA Ophthalmol. 135, 624–630. https://doi.org/10.1001/jamaophthalmol.2017.1091 (2017).
Huang, J. et al. Efficacy comparison of 16 interventions for myopia control in children: A network Meta-analysis. Ophthalmology 123, 697–708. https://doi.org/10.1016/j.ophtha.2015.11.010 (2016).
Tang, J. et al. Efficacy of repeated Low-Level Red-Light therapy for slowing the progression of childhood myopia: A systematic review and Meta-analysis. Am. J. Ophthalmol. 252, 153–163. https://doi.org/10.1016/j.ajo.2023.03.036 (2023).
Jiang, F., Wang, D., Yin, Q., He, M. & Li, Z. Longitudinal changes in axial length and spherical equivalent in children and adolescents with high myopia. Invest. Ophthalmol. Vis. Sci. 64, 6. https://doi.org/10.1167/iovs.64.12.6 (2023).
Chen, J. et al. Axial length changes in progressive and non-progressive myopic children in China. Graefes Arch. Clin. Exp. Ophthalmol. 261, 1493–1501. https://doi.org/10.1007/s00417-022-05901-5 (2023).
Lu, F. et al. Axial myopia induced by hyperopic defocus in Guinea pigs: A detailed assessment on susceptibility and recovery. Exp. Eye Res. 89, 101–108. https://doi.org/10.1016/j.exer.2009.02.019 (2009).
Howlett, M. H. & McFadden, S. A. Spectacle lens compensation in the pigmented Guinea pig. Vis. Res. 49, 219–227. https://doi.org/10.1016/j.visres.2008.10.008 (2009).
Jiang, L., Garcia, M. B., Hammond, D., Dahanayake, D. & Wildsoet, C. F. Strain-Dependent differences in sensitivity to Myopia-Inducing stimuli in Guinea pigs and role of choroid. Invest. Ophthalmol. Vis. Sci. 60, 1226–1233. https://doi.org/10.1167/iovs.18-25365 (2019).
Jin, P. et al. Retina 39, 1091–1099, doi:https://doi.org/10.1097/iae.0000000000002090 (2019).
Meng, Q. Y. et al. Choroidal thickness, myopia, and myopia control interventions in children: a Meta-analysis and systemic review. Int. J. Ophthalmol. 16, 453–464. https://doi.org/10.18240/ijo.2023.03.17 (2023).
Yang, Y., Wei, L., Wang, B. & Zheng, W. Effects of Atropine on choroidal thickness in myopic children: a meta-analysis. Front. Pharmacol. 15, 1440180. https://doi.org/10.3389/fphar.2024.1440180 (2024).
Xiong, R. et al. Longitudinal changes and predictive value of choroidal thickness for myopia control after repeated Low-Level Red-Light therapy. Ophthalmology 130, 286–296. https://doi.org/10.1016/j.ophtha.2022.10.002 (2023).
Zhang, R. et al. Associations between choroidal thickness and rate of axial elongation in orthokeratology lens users. Photodiagnosis Photodyn Ther. 51, 104450. https://doi.org/10.1016/j.pdpdt.2024.104450 (2024).
Wu, H. et al. Scleral hypoxia is a target for myopia control. Proc. Natl. Acad. Sci. U S A. 115, E7091–e7100. https://doi.org/10.1073/pnas.1721443115 (2018).
McBrien, N. A., Jobling, A. I. & Gentle, A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom. Vis. Sci. 86, E23–30. https://doi.org/10.1097/OPX.0b013e3181940669 (2009).
Hecht, I., Nitzan, I. & Safir, M. Myopia and systemic manifestation of tissue hyperlaxity: A population-based cross-sectional study. Clin. Exp. Ophthalmol. 53, 11–17. https://doi.org/10.1111/ceo.14450 (2025).
Nickla, D. L., Damyanova, P. & Lytle, G. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor L-NAME. Exp. Eye Res. 88, 1092–1099. https://doi.org/10.1016/j.exer.2009.01.012 (2009).
Nickla, D. L., Wilken, E., Lytle, G., Yom, S. & Mertz, J. Inhibiting the transient choroidal thickening response using the nitric oxide synthase inhibitor l-NAME prevents the ameliorative effects of visual experience on ocular growth in two different visual paradigms. Exp. Eye Res. 83, 456–464. https://doi.org/10.1016/j.exer.2006.01.029 (2006).
Liu, H., Yang, Y., Guo, J., Peng, J. & Zhao, P. Retinal damage after repeated Low-level Red-Light laser exposure. JAMA Ophthalmol. 141, 693–695. https://doi.org/10.1001/jamaophthalmol.2023.1548 (2023).
Liao, X. et al. Cone density changes after repeated Low-Level red light treatment in children with myopia. JAMA Ophthalmol. 143, 480–488. https://doi.org/10.1001/jamaophthalmol.2025.0835 (2025).
Acknowledgements
We thank all authors who were recruited for this study.
Author information
Authors and Affiliations
Contributions
L.P.L. designed the study. Y. S. H. and H. C. C. collected and analyzed the data. L.P.L. and Y. T. analyzed the data.L.P.L. wrote the paper. X. M. M. critically revised the manuscript. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, L.P., Hu, Y.S., Chen, H.C. et al. Repeated low-level red-light therapy vs. conventional treatments for myopic control in children: a systematic review and meta-analysis. Sci Rep 15, 30794 (2025). https://doi.org/10.1038/s41598-025-16868-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-16868-8