Abstract
In current study, a simple hydrothermal approach was used to synthesis of BiFeO3 nanocomposites on reduced graphene oxide (rGO/BiFeO3) under visible LED light (Vis LED) for photocatalytic degradation of amoxicillin. X-ray diffraction (XRD), field emission scanning electron microscope (FE-SEM), and energy dispersive X-ray (EDX) techniques were applied for characterization of the structural, optical, and surface morphological properties of the synthesized catalysts. The effect of different operating parameters such as pHs (3–9), amoxicillin concentrations (5–30 mg/L), catalyst concentrations (0.5–3 g/L) in different reaction times were investigated. The findings revealed that a 1 g/L rGO/BiFeO3 nanocomposite with an initial pH of 5 and amoxicillin concentration of 5 mg/L could achieve a removal of 80% after 30 min under visible LED light. TOC and COD analyses were achieved to explore the amoxicillin mineralization rate in optimum conditions. The results indicated that 42.78% and 51.12% of TOC and COD were removed after 60 min contact time, respectively. The results also indicated that as the ionic strength increased, the amoxicillin removal rate slightly declined. The degradation of amoxicillin was pseudo-first-order kinetics. The identification of intermediates and final products led to the conclusion that enhanced degradation of amoxicillin during rGO/BiFeO₃ photocatalysis was achieved through β-lactam ring cleavage, and hydroxylation. Also, rGO/BiFeO3 catalyst exhibited excellent recyclability and durability.
Similar content being viewed by others
Introduction
Among emerging pollutants (EPs), pharmaceutical compounds have attracted significant research interest due to (i) limited data on their environmental behavior and impacts, including those of their by-products, and (ii) growing demand, which raises the risk of their release into surface and groundwater1. These compounds originate from various sources, such as municipal wastewater treatment plants, hospitals and medical centers, pharmaceutical manufacturing facilities, and livestock farms1,2. Antibiotics, intended for both human and veterinary applications, represent as one of the most significant categories of pharmaceuticals. Antibiotics, widely used in human and veterinary applications, are a major category of pharmaceuticals. Their partial metabolism results in unmetabolized forms entering wastewater treatment systems, while aquaculture applications may lead to direct discharge into natural water bodies. Owing to their high water solubility (with log Kow ≤ 1) and low biodegradability, often pass through conventional wastewater treatment processes and ultimately accumulate in natural aquatic environments2. Amoxicillin (AMX), a widely used β-lactam antimicrobial drug, has received approval from the U.S. Food and Drug Administration (FDA) for application in primary care settings3. AMX is frequently detected in environmental samples due to its widespread use and continuous emission. Conventional wastewater treatment methods, such as activated carbon adsorption, electrocoagulation, and biological processes, are often ineffective due to AMX’s high toxicity4. Today, advanced oxidation processes (AOPs) including photochemical and thermal (non-photochemical) methods have emerged as promising alternatives for antibiotics treatment. In these processes, reactive species such as superoxide anion radicals (•O2‾) and hydroxyl radicals (•OH) improve the mineralization of organic pollutants to H2O and CO2, without producing harmful secondary by-products5. Among the major AOPs techniques, photocatalytic technology utilizing semiconductor taking advantage of renewable solar energy is regarded as one of the most promising approaches to overcome energy and environmental challenges6. Several main advantages of photocatalytic technology including complete mineralization of non-biodegradable organic pollutants without generating secondary pollution, operation under ambient temperature and pressure and low operating cost led to its widespread application in the wastewater treatment7.
Nowadays, a diversity of semiconductors such as TiO2 and ZnO have been broadly studied for photocatalytic degradation of days and other persistent organic pollutants8,9. Our previous studies have represented the kinetic and mechanistic aspects of this photocatalytic techniques for the removal of organic pollutants10. However, the practical application of these photocatalysts were hindered by several technical challenges. Primarily, their wide band gap structure meant that only the UV portion of the solar spectrum could be absorbed. Additionally, the rapid recombination of electron-hole pairs (e−– h+) reduced the availability of charge carriers on the catalyst surface for redox reactions8,11,12. To overcome these limitations, a wide range of photocatalysts, such as sulfides, nitrides, and complex oxides, have been developed to extend their optical response into the visible light spectrum, enabling the potential practical utilization of natural solar energy13. In contract, ternary metal oxides offer significant advantages over binary metal oxides like TiO₂ or ZnO, including narrower band gaps (e.g., 2.2 eV for BFO) that enhance visible-light absorption and promote efficient electron-hole pair separation12,14.
Their complex perovskite structures enable tunable electronic properties, resulting in superior photocatalytic efficiency. For example, Kamo et al. (2024)15 reported that magnesium-ion modified ternary zinc-tin-oxide nanoparticles exhibit strong visible-light-driven photocatalytic and antibacterial activity due to an optimized band gap.
In this context, semiconductor nanoheterostructures have emerged as a promising approach to enhance photoconversion efficiency by improving charge separation and extending light absorption16.
Among these, bismuth-based oxide photocatalysts-particularly bismuth ferrite (BiFeO₃, or BFO), which possesses a distorted perovskite rhombohedral structure-have attracted significant attention for the degradation of organic pollutants. This is largely due to their narrow band gap of 2.2 eV, high chemical stability, efficient visible-light harvesting, and low cost17,18. Some literatures have confirmed the photocatalytic activity of BFO materials under visible light irradiation degrading organic compound17,18,19. However, there are several disadvantages for BFO used as photocatalyst. For example, pure BFO exhibits limited catalytic capability for degrading stable pollutants due to its high electron-hole recombination rate, which diminishes the availability of charge carriers for redox reactions17. Additionally, its moderate band gap (2.2 eV), while enabling visible-light absorption, may not generate sufficient reactive oxygen species to effectively break down complex pollutants. Impurities such as Bi₂Fe₄O₉ or Bi₄₆Fe₂O₇₂, often formed during conventional synthesis, further reduce its photocatalytic efficiency by acting as recombination centers or limiting active surface sites20,21. To overcome the previously mentioned challenges related to photocatalytic activity, Pt doped BiFeO3 nanoparticle22BiFeO3/TiO2 core shell nanocomposite23AgCl/Ag/BiFeO3 conjugated nanocomposite24have been employed to increase the photodegradation efficiency of organic pollutants under visible light irradiation.
Recently, the addition of graphene oxide (GO) and specially, reduced graphene oxide (rGO) as a carbonaceous electrical double-layer capacitor type materials have been attracting an increasing interest to improve the photocatalytic performance due to their large specific surface area, non-toxicity, and excellent charge carriers mobility25,26,27,28,29.
Reduced graphene oxide employed as an exceptional electron acceptor, effectively suppressing the recombination of electron-hole pairs. Additionally, it adsorbs significant amounts of contaminants on the catalyst surface, positioning it as an ideal co-catalyst for use alongside semiconductor photocatalysts26,28.
Subsequently, some composite photocatalysts related to rGO such as TiO2–rGO28rGO/ZnIn2S429, rGO-AgO, and rGO-ZnO30rGO-SnO231 nanocomposites were also synthesized and used for the photodegradation of dyes and persistent organic pollutants. However, the photocatalytic activities of the composites were yet not too high. To achieve more efficient performance, the development of novel photocatalytic composites with advanced properties and improved efficiency is essential.
It is expected that the combination of rGO with BFO significantly enhance the suppression of photogenerated electron-hole pair recombination and boost photoelectrical performance, outperforming the individual use of either rGO with BFO. Most recently, Ghorbani et al. have successfully synthesized rGO/BiFeO3 nanocomposites by a deposition–precipitation method for removing methyl orange under visible light irradiation32. However, the photocatalytic activities of prepared nanocomposites were improved, the organic dye was not completely degraded under visible light irradiation and the mechanism responsible for enhancing visible light performance was not clearly described. The photocatalytic activity of catalysts can also be adjusted by tuning their properties with respect to light absorption mechanism, electron/energy transfer processes, and excited-state evolution in photocatalytic cycles33. The advancement of photodegradation processes by light-emitting diode (LED) lamps has garnered significant attention due to its environmentally friendly nature, energy efficiency, long spectral purity, extended lifespan, and suitability for photoreactor system designs34,35. Importantly, LED lamps generate very little heat and enabling reactions to occur at room temperature without requiring any cooling water36. These findings are consistent with recent progress in semiconductor nanoheterostructures for photoconversion applications, where heterojunction design is employed to improve charge separation and enhance photocatalytic activity under visible light irradiation37.
According literatures, no research has been carried on the removal of AMX using rGO/BFO heterojunction photocatalyst under Vis LED light irradiation in aqueous solutions. Hence, in this study the rGO/BFO nanocomposite was synthesized with hydrothermal method. The prepared nanocomposite showed higher efficiency in degradation of AMX under Vis LED light irradiation in the short contact time. Also, the effect of operational parameters, radical scavengers, ion strength and the possible AMX photodegradation mechanism were investigated.
Materials and methods
Chemicals and reagents
The chemicals used included amoxicillin, bismuth nitrate pentahydrate (Bi(NO3)3⋅5H2O; ≥98.0%), iron nitrate nonahydrate (Fe(NO3)3·9H2O; ≥98.0%), sodium nitrate (NaNO3), sodium hydroxide (NaOH; ≥98.0%), Hydrochloric acid) HCl; 37%), potassium permanganate (K2MnO₄), hydrogen peroxide (H2O2), potassium hydroxide (KOH), ascorbic acid (C6H8O6), sulfuric acid (H2SO4), all of which were procured from Merck, Germany. In addition, tert-butyl alcohol (TBA), ethylene diamine tetra acetic acid (EDTA), and benzoquinone (BQ) were used as scavengers for hydroxyl radicals, holes and superoxide anions, respectively. Solutions were prepared using double-distilled Milli-Q water. The starting GO powder was bought from Sigma A. with 10 mm lateral size, and > 99.5% purity.
GO and rGO synthesis
Graphene oxide was synthesized using the modified Hummers’ method38. In this procedure, 166 mmol of natural flake graphite with a mean size of 60 mesh and purity of 98% were mixed well with 12 mmol NaNO3 and H2SO4 (98%) as oxidizer and kept in an ice bath (0–5 °C). To accelerate the oxidation process, 38 mmol of K2MnO₄ was added as an oxidizing agent, and the reaction temperature was maintained below 15 °C. The mixture was gradually diluted with the slow addition of deionized (DI) water and stirred for 2 h. It was then heated in a reflux system at 98 °C for 10–15 min. Finally, H2O2 (30%) was added and stirred for 1 h to eliminate the excess K2MnO₄, after which the mixture was left undisturbed for 4 h. The resulting mixture was repeatedly washed by centrifugation with HCl (10%) to eliminate impurities, followed by several washes with deionized (DI) water. It was then dried at 60 °C for over 6 h to obtain GO powder38. To synthesize rGO from GO, 5 mmol of dried GO powder was placed in an empty beaker. The beaker was covered with aluminum foil perforated with multiple small holes and heated on a hot plate at 350 °C for 10 min under a fume hood. The resulting black rGO powder was subsequently collected from the beaker39.
rGO/BFO synthesis
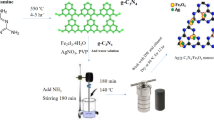
To prepare the rGO/BFO nanocomposite, 1 mmol of Bi(NO₃)₃·5 H₂O and 1 mmol of Fe(NO₃)₃·9 H₂O were dissolved separately in 50 mL of DI water and stirred for 4 h. The pH of the resulting solution was then adjusted to 10 by the dropwise addition of 1 M KOH solution. A predetermined amount of rGO was added to the mixed metal solution and stirred for 1 h to form the composite precursor. The resulting mixture was then transferred into a Teflon-lined stainless steel autoclave and heated in an oven at various reaction temperatures for 6 h. After the hydrothermal reduction process, the synthesized samples were thoroughly washed multiple times with DI water and ethanol, then dried at 100 °C for 10 h to obtain the desired nanocomposites38.
Characterization and analytical methods
The crystallographic structure of the catalysts was analyzed using X-ray diffraction (XRD, Rigaku Ultima IV) with “Cu-kα” radiation (λ = 1.54 Å, 45 kV, 40 mA) at 25 °C over a 2θ range of 10–80°. The surface and morphology of the catalysts were determined using field emission scanning electron microscopy (FE-SEM), (FEI Nova NanoSEM 450), as well as the size of the synthesized nanoparticles. Additionally, energy dispersive X-ray spectroscopy (EDX, BRUKER XFlash 6|10) with elemental mapping was employed to identify the elemental composition and to assess the distribution and density of elements on the surface and near-surface regions of the composite. The AMX concentration was analyzed using a UV–visible spectrophotometer (HACH DR-5000, US) at maximum absorption (λmax; 230 nm). The intermediates and AMX by products during photodegradation of AMX were identified using liquid chromatograph-mass spectrometer (LC-MS), (Waters Alliance 2695 HPLC-Micromass Quattro micro API Mass Spectrometer, US). A chromatographic column T3-C18 column (3 μm, 2.1 × 100 mm) was used for liquid chromatography. Also, the pH of solutions was determined using a Hach pH meter (HQ430D, USA). The chemical oxygen demand (COD) was determined using the closed reflux colorimetric analysis (COD Reactor AL38, Germany), in accordance with the Standard Methods for the Examination of Water and Wastewater40. Also, the mineralization efficiency of the process was evaluated by measuring the total organic carbon (TOC) content using a TOC analyzer (Vario TOC Cube, Elementar, Germany). The intensity and spectrum of the LED lamp light were measured using a spectroradiometer (Sekonic C7000, Japan).
The photocatalytic activity of the process, in terms of AMX photodegradation, was determined using Eq. (1), as follows:
where Co is initial concentration of AMX (mg/L) and Ct represents the concentration after photodegradation at t minute.
The kinetic studies of AMX photodegradation by photocatalysis systems and various experimental factors were conducted using Eq. (2), as follows:
where Co and Ct are the AMX amounts at 0 and t time (min), respectively, and kapp (min−1) shows the first-order rate constant. Plotting Ln (C/Co) versus the processing time creates a straight line in the pseudo-first-order equation, in which kapp is the plot slope.
Experimental procedure and photocatalytic reactor
In the present study, experiments were condcted in a lab-scale, batch mode visible LED photoreactor, consisting of a 120 mL open cylindrical glass vessel and two 50 W LED lamps (Cree LED Chip Wisconsin US), positioned 5 cm from each side of the vessel (Fig. 1). The broadband excitation light emitted by the LED lamps ranged from approximately 420 to 700 nm, with a peak wavelength at 454 nm and an intensity of 46 mW/cm². To minimize light loss and water evaporation, the photoreactor was enclosed with a reflective mirror cover. Additionally, two fans were installed on either side of the photoreactor to dissipate heat from the light source and reduce heat transfer to the sample. A magnetic stirrer placed under the photoreactor ensured uniform exposure by maintaining continuous mixing, with the stirring speed set at 200 rpm.
For experiments, 0.1 g rGO/BFO was dispersed in 100 mL AMX solution with an initial concentration of 50 mg/L in a glass vessel. The mixed solution had been magnetically stirred in the dark for 30 min prior to visible light irradiation for achieving adsorption/desorption equilibrium of the catalyst surface and eliminate the effects of adsorption during the reaction33.
The initial pH of the AMX solutions was adjusted within the range of 3–11 using 0.1 M NaOH and 0.1 M HCl with a pH meter. To evaluate the effects of rGO/BFO dosages and initial AMX concentrations on AMX photodegradation, rGO/BFO and AMX concentrations were adjusted within the ranges of 0.5–1 g/L and 5–30 mg/L, respectively. At specified intervals during visible light irradiation (5, 10, 15, and 30 min), 5 mL aliquots of the reaction solution were collected and centrifuged at 10,000 rpm for 20 min. The supernatant was subsequently filtered through 0.22 μm membranes (Millipore Co.) to obtain a clear solution for spectrophotometric analysis. To evaluate the stability and reusability of the rGO/BFO nanocomposite under optimal photocatalytic conditions, the efficiency of the recycled catalyst was investigated. At the end of each cycle, the recovered sample which centrifuged at 10,000 rpm for 20 min, rinsed twice with ethanol and double-distilled water, and dried at 80 °C. After that, the recycled catalyst was re-suspended in a fresh AMX solution. In addition, 1 mM TBA, 1 mM EDTA, and 1 mM BQ were used as scavengers for hydroxyl radicals, holes, and superoxide anions, respectively41. Finally, the effects of the different chloride ion (Cl−) concentration as a common inorganic substance was examined on the photodegradation performance42.
Results and discussion
Characterization of nanocomposites
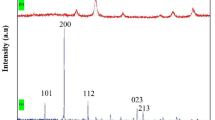
Figure 2 presents the XRD patterns of the synthesized photocatalysts, revealing information about their crystalline structures. All samples exhibited distinct diffraction peaks, indicating strong crystallinity of the materials. Figure 2a presents the XRD pattern of graphene oxide (GO) synthesized via the modified Hummer’s method, displaying a sharp diffraction peak at 2θ = 11.95°, corresponding to an interlayer spacing of ~ 7.4 Å, indicative of oxygen-functionalized graphene layers25,43. The reduction of graphene oxide (GO) to reduced graphene oxide (rGO) leads to structural changes, which can be observed by comparing their X-ray diffraction (XRD) patterns which shown in Fig. 2b. For rGO, the characteristic peak at 2θ = 26.55° corresponds to the (002) diffraction plane. Besides the intense (002) peak, other low-intensity peaks associated with the (101), (004), (111), (116), diffraction planes were also observed in the XRD plot. After the thermal treatment of GO, the sharp diffraction peak shifts to 2θ = 26.55° (interlayer spacing ~ 3.35 Å, JCPDS No. 75-1621), confirming the removal of oxygen-containing groups and restoration of a graphitic structure43. Furthermore, XRD results of the mesoporous BFO revealed that the perovskite structure with rhombohedral symmetry of polycrystalline BFO nanocrystals was formed. The XRD patterns of BFO and rGO/BFO nanoparticles are given in Fig. 2b. The XRD patterns of BFO and rGO/BFO displayed characteristic peaks of the rhombohedral perovskite structure (JCPDS No. 86-1518), with no detectable impurity phases such as Bi₂Fe₄O₉ or Bi₄₆Fe₂O₇₂. Also, it was clearly observed that despite the increase in BFO content, all synthesized samples displayed a similar crystal composition with rhombohedral symmetry. As the BFO contents were integrated into GO, the intensity of diffraction peaks associated with the perovskite phase of BFO decreased compared to that of pure mesoporous BFO confirming the composite formation. Overall, XRD analysis confirmed the successful intercalation of BFO nanoparticles within the GO nanosheets44.
FESEM was utilized to study the surface morphology of GO and rGO to observe the change in corrugation. Figure 2c shows the sheet-like structure of GO. This image demonstrated that the GO material consists of stacked sheets with a smooth surface resembling a wrinkled structure, which is a typical characteristic of nanosheet structures. Figure 2d shows the ultra-thin graphene obtained from the reduction of GO. FE-SEM images demonstrate that rGO with a thickness of less than 40 to 160 nanometrs can be synthesised by this process, and also that the well-separated platelets have a close connection with each other. Figure 2e and f illustrates the highly wrinkled structure of pure BFO and rGO/BFO nanocomposite. By observing Fig. 2f, it can be concluded that the size of BFO nanoparticles and rGO in the rGO/BFO nanocomposite has significantly decreased compared to their pure form. This indicates that the incorporation of graphene has reduced the particle size of the composites, and BFO nanoparticles are uniformly distributed among the rGO nanosheets45. The EDX analysis shown in Fig. 2g reveals the main chemical elements that constitute the catalysts, including bismuth (Bi), iron (Fe), carbon (C), and oxygen (O). Also, this analysis reveals that Bi, O, C, and Fe, respectively, constituted 31.8%, 17.75%, 36.75%, and 14.42% of the rGO/BFO nanostructure. Therefore, the EDX spectral observations demonstrated no other elements or impurities in the purity of the synthesized samples.
AMX photodegradation experiments
Effects of initial pH
The pH of solutions is a crucial factor in the degradation of organic pollutants, as it influences both the size of the formed aggregate particles and the surface charge of the photocatalyst. The generation of charged radicals during photocatalytic oxidation is also strongly influenced by the pH of the sample46. To evaluate the effects of the initial solution pH on the rGO/BFO photocatalyst process for AMX degradation (catalyst: 1 g/L, AMX: 20 mg/L) in different contact times, samples were produced within the pH ranges from 3 to 9. Figure 3 presents the effects of different initial pH on the AMX removal efficiency. Accordingly, the removal rate initially improved and then decreased by an increased sample pH from 3 to 9, while the initial pH of 5 led to the maximum removal rate (71.53%). They are effective due to the different ionization stages of AMX, attributed to the presence of functional groups such as carboxyl (pKa = 2.68), phenol (pKa = 8.94), and amine (pKa = 7.49), which cause this pollutant to exhibit different behaviors at various pH levels47. In general, the pKa value of amoxicillin is 2.75. For this reason, it is usually present in molecular form in acidic solutions. In this condition, due to the presence of H⁺ions, H radicals are formed, which react with the available oxygen to produce •HO2. These radicals ultimately generate OH radicals, which are highly reactive and have a high oxidative potential48. Based on the literature, the reduction process in acidic pH should be favored as it results in the protonation of functional groups that attract bromate ions. However, it is noted for slightly acidic pH, as under very high acidic conditions, the electrons are lost and may produce reactive oxygen species that cause the oxidation of AMX again49.
Effects of catalyst dosage
The effects of catalyst dosage in the ranges from 0.5 to 1 g/L at the initial solution pH of 5.0 and the AMX concentration of 20 mg/L on rGO/BFO/Vis LED process efficiency were investigated. Increasing the catalyst dosage from 0.5 g/L to 1 g/L enhanced AMX removal efficiency from 49.61 to 71.53% within 30 min (Fig. 4). As is shown in Fig. 4, the removal efficiency of AMX at 0.5 g/L of the catalyst was 49.61%, while 1 g/L of the catalyst substantially enhanced AMX removal (71.53%). However, increasing the catalyst dosage negatively affected the catalytic performance during the 30 min contact time. In this condition, the removal efficiency of AMX decreased to 56.38% at a dosage of 3 g/L. This can be explained by noting that increasing the catalyst dosage to a specific value enhances the formation of hydroxyl radicals and expands the active surface area of the photocatalysts, thereby promoting the degradation process. However, at higher concentrations of rGO/BFO catalysts in the solution, excessive aggregation occurs, leading to increased turbidity and light scattering which reduces the light absorption and limits the availability of active surface sites for hydroxyl radical generation50.
Effects of initial AMX concentration
The performance of the rGO/BFO/Vis LED process was assessed by varying initial AMX concentrations from 5 to 30 mg/L under optimal pH and catalyst dosage conditions in different contact times. As shown in Fig. 5, the experimental results indicate that the AMX degradation rate decreased with increasing initial AMX concentration. Accordingly, the degradation rate was approximately 80.16% at the AMX concentration of 5 mg/L, while it declined to 68.94% and 60.37% at concentrations of 20 mg/L and 30 mg/L, respectively. It can be explained that at higher pollutant concentrations, photoactivity is often limited by the fixed amount of photogenerated species produced from a constant photocatalyst dosage. Since the generation of hydroxyl radicals remains steady at a given catalyst dosage, the ratio of OH radicals to AMX molecules decreases as the concentration of AMX increases. This lower ratio becomes insufficient for effective AMX degradation at higher concentrations. Furthermore, higher AMX concentrations led to the formation of higher intermediate compounds, which competed with AMX for degradation by hydroxyl radicals51. These results are consistent with the findings of studies conducted by references52 and53 on the photodegradation of various organic compounds.
AMX degradation in different processes
To accurately evaluate the performance of visible LED irradiation and different catalyst systems on the degradation rate of AMX, the pollutant sample was examined both with and without LED exposure and pure catalysts (Initial AMX = 5 mg/L, Catalyst dosage = 1 g/L, pH = 5.0). Figure 6, shows AMX degradation in various systems, including sole Vis LED, BFO/dark, rGO/dark, BFO/Vis LED, rGO/Vis LED, rGO/BFO/dark and rGO/BFO/Vis LED. According to the findings, sole Vis LED irradiation could hardly lead to the significant degradation of AMX and only removed 3.04% of AMX. Indeed, the generated energy by the Vis LED light emissions was insufficient to break the bonds of the AMX molecules, which is consistent with the previous findings in this regard54.
Visible LED irradiation alone resulted in minimal AMX removal (3.04%), as the energy was insufficient to break AMX molecular bonds. This is because the energy generated by the visible LED light was insufficient to break the bonds within AMX molecules, consistent with previous studies on this subject54. In aqueous solution, AMX can undergo photodegradation by LED through two ways: (i) direct photolysis, as demonstrated in Eqs. (3–11), as follows:
And (ii) the free radical reactions in the solution are presented in the following Eqs. (4–11)55,56,57:
As can be seen in Fig. 6, AMX adsorption by rGO/BFO was higher compared to BFO and rGO. No significant adsorption was observed under dark and static conditions. However, the combination of pure catalysts with visible LED irradiation significantly improved AMX removal, achieving 46.09% with BFO/Vis LED and 34.95% with rGO/Vis LED. This result can be attributed to the activation of BFO and rGO under visible LED irradiation. In photocatalysis, light energy is crucial, as semiconductor photocatalysts absorb photons with energy equal to or greater than their band gaps, leading to the generation of electron-hole pairs in the conduction and valence bands. Subsequently, the generated electron–hole (e⁻/h⁺) pairs can react with H₂O and O₂ on the catalyst surface to produce superoxide and hydroxyl radicals, which contribute to AMX degradation, as illustrated in the following Eqs. (12–16)38:
Beyond bandgap engineering and the effective coupling between BFO and rGO, the enhanced photocatalytic performance can also be attributed to the increased interaction between rGO and the pollutant. Additionally, the suppression of electron–hole recombination plays a crucial role in improving photocatalytic efficiency. In rGO/BFO nanocomposites, the photogenerated electrons are efficiently transferred to the rGO sheets. This effective electron migration from BFO nanoparticles to the rGO surface inhibits electron–hole recombination, thereby enhancing the photocatalytic activity38.
The photocatalytic properties of BFO and other related materials were previously studied for various pollutants degradation under visible light. The effect of several conditions such as the light source, pH of solution, initial organic concentration and dosage of catalyst may influence the efficiency of the photocatalyst. Table 1 show the percentage of degradation of BFO and doped BFO materials on organic matter with the effect of change in time, concentration and the source of light. For instance, Farhadi et al. (2020) reported that BiFeO₃/rGO achieved an approximate removal efficiency of 70% for acetaminophen (10 mg/L) under visible light (xenon lamp) in 120 min18. Similarly, Ghorbani et al. (2022) documented an approximate 85% removal efficiency for methyl orange (20 mg/L) in 180 min using BiFeO₃/rGO under visible light irradiation32. These findings highlight the positive impact of rGO incorporation on enhancing interfacial charge transfer and photocatalytic activity. In comparison with other photocatalysts, such as TiO₂/rGO, which exhibited an approximate 65% removal efficiency for methylene blue (10 mg/L) in 120 min (Wang et al., 2013), BFO-based nanocomposites demonstrate superior performance, particularly at shorter reaction times28. Additionally, the Ag/AgCl/BiFeO₃ nanocomposite achieved an impressive removal efficiency of approximately 90% for rhodamine B (10 mg/L) in 60 min (Wang et al., 2016)24. Furthermore, BiFeO₃/ZrO₂ exhibited an approximate 75% removal efficiency for tetracycline (20 mg/L) under white LED irradiation in 120 min (Hu et al., 2019)57. However, in the present study, the rGO/BFO nanocomposite demonstrated outstanding photocatalytic activity for the degradation of AMX (5 mg/L) under visible LED irradiation, achieving a removal efficiency of 80.16% within 30 min. This performance surpasses that of several comparable photocatalysts reported in the literature.
Effects of chloride ion
Certain inorganic ions have been reported to influence photocatalytic efficiency in municipal and industrial wastewater treatment by acting as free radical scavengers. In the current study to establish a theoretical basis for practical applications, the effect of chloride ions (Cl⁻), a common inorganic anion present in wastewater, has been investigated42. Chloride ions at concentrations of 1, 5, and 10 mM were added to the solution, while all other process conditions were kept constant (catalyst dosage = 1 g/L, AMX = 5 mg/L, pH = 5.0). As shown in Fig. 7, the AMX removal rate in the absence of chloride ions (control) was 80.16%. However, the addition of 1, 5, and 10 mM Cl⁻ reduced the removal efficiency to 70.32%, 62.21%, and 54.29%, respectively. The presence of chloride ions partially inhibited AMX removal efficiency, with the degree of inhibition increasing with higher chloride concentrations. This effect may be attributed to interactions such as hydrogen bonding and Van der Waals forces, through which chloride ions trap reactive species (e.g., photogenerated hydroxyl radicals and holes) on the photocatalyst surface, thereby reducing its photoactivity58. The chloride ion reactions are shown in the following Eqs. (25–27)59:
Cl‾ + •OH → •Cl + OH‾.
Cl‾ + h+ → •Cl.
Cl‾ + •Cl → •Cl‾2.
However, some studies suggest that AMX degradation was not affected by the chloride anion, implying that Cl‾ in the oxidation system of AMX does not convert into active chlorine products60.
Effects of radical scavengers
To further investigate the photocatalytic mechanism of the rGO/BFO/Vis LED process in AMX degradation, the involvement of reactive species was examined. Following a standardized protocol, tert-butyl alcohol (TBA; 1 mM), ethylenediaminetetraacetic acid (EDTA; 1 mM), and benzoquinone (BQ; 1 mM) were added to the AMX solution as selective scavengers for hydroxyl radicals (•OH), holes (h⁺), and superoxide anions (•O₂⁻), respectively. The experimental findings demonstrate that the inhibitory effects manifested in three distinct reaction pathways, each exhibiting varying degrees of inhibition. This observation aligns with the presence of three active species participating in the photocatalytic system, suggesting a multi-mechanistic interaction governing the process. As shown in Fig. 8, the addition of BQ (scavenger of •O2‾) has a minimal impact on the AMX decomposition rate during the photodegradation process. In contrast, the addition of EDTA and TBA markedly suppresses the photocatalytic reaction, decreasing the AMX degradation efficiency from 80.16 to 19.5% and 14%, respectively. These findings demonstrated that the primary mechanism involved •OH− driven reactions, confirming the critical role of OH radicals in the photodegradation of AMX via the rGO/BFO/Vis LED process. Moreover, the inhibition of hole formation would minimal the production of hydroxyl radicals, thereby decreasing AMX removal rate61,62. However, numerous studies have identified superoxide radicals (•O₂⁻) as the dominant reactive species driving the photocatalytic degradation of organic pollutants in aqueous solutions50,55.
Reusability and stability of rGO/BFO catalyst
Catalyst reusability and stability are widely recognized as critical factors for practical applications. To evaluate the reusability of the rGO/BFO nanocomposite in AMX photodegradation, three consecutive recycling experiments were performed under identical conditions. As shown in Fig. 9, the catalyst’s performance slightly decreased from 80 to 73% and then to 62% over three consecutive cycles. Nevertheless, maintaining an AMX removal efficiency above 62% demonstrates that the rGO/BFO nanocomposite remains stable and effective for AMX degradation through at least three reuse cycles. Additionally, the slight deactivation of the photocatalyst may be due to catalyst loss and incomplete recovery during each cycle50. Moreover, the catalyst surface could become partially blocked by residual contaminants and intermediates that were not fully removed55,58. To address catalyst loss, the recovered rGO/BiFeO₃ mass was quantified gravimetrically after each cycle, revealing cumulative losses of 4.2%, 7.8%, and 11.3% after the first, second, and third cycles, respectively, attributed to incomplete recovery during centrifugation and minor adhesion to reactor surfaces.
Mineralization
Beyond the decomposition of organic pollutants, effective treatment requires significant mineralization to convert pollutants into CO₂ and H₂O, ensuring the process is a viable option for wastewater treatment58. In this regard, TOC and COD were determined in order to evaluate the mineralization efficiency of AMX by the rGO/BFO/Vis LED process under optimized conditions (initial AMX concentration = 5 mg/L, catalyst dosage = 1 g/L, initial pH = 5.0, reaction time = 30 min). As shown in Fig. 10, the efficiency of TOC and COD removal rate was 30.7% and 38.61%, respectively, while the rate of AMX removal reached 80.16% under the same experimental conditions. During the photocatalytic process, some AMX molecules are converted into CO₂ and H₂O, while others break down into intermediate by-products. These intermediates are more resistant to degradation than the original AMX molecules, resulting in a mineralization efficiency that is significantly lower than the overall AMX removal rate under the same conditions. Figure 10, also shows that extending the irradiation time to 60 min increased TOC and COD removal efficiencies to 42.78% and 51.12%, respectively. These results confirm that the duration of visible light exposure plays a crucial role in achieving complete AMX mineralization under appropriate operating conditions. Similarly, studies57 and63 reported that longer visible light irradiation enhances the mineralization rate in photocatalytic processes.
Intermediates and possible degradation pathways
The photocatalytic mechanism of AMX degradation can be explained by considering the valence and conduction band potentials of the rGO/BFO nanocomposite alongside free radical trapping experiments. Upon illumination, photo-generated electrons and holes are produced in the conduction and valence bands of rGO/BFO, respectively, as described in Eq. (18). The presence of GO nanosheets facilitates the transfer of photogenerated electrons from the rGO/BFO conduction band to the GO surface, enhancing the separation of electrons and holes as shown in Eq. (19). The holes generated in the valence band can directly degrade AMX molecules and also produce •OH radicals by reacting with surface hydroxyl groups and adsorbed water molecules, as shown in Eq. (20). This is possible because the redox potential of OH⁻/•OH (1.89 eV) is lower than the valence band hole potentials of BFO (2.1 eV) and rGO/BFO (2.2 eV). Additionally, on the rGO surface, adsorbed oxygen reacts with photogenerated electrons to form superoxide anion radicals, as described in Eq. (21)2,32,57,63.
Consequently, successive reactions with superoxide anion and hydroxyl radicals result in the degradation of AMX molecules, as illustrated in Eqs. (22) and (23), respectively. It has been concluded that the highly oxidative •OH radicals play a more significant role in breaking down AMX compared to superoxide anion radicals. This is due to the conduction band edge potential of rGO/BFO (+ 0.19 eV) being more positive than the redox potential of O₂/•O₂⁻ (− 0.33 eV), which limits the generation of superoxide radicals32.
The schematic diagram of photocatalytic AMX degradation mechanism for BFO and rGO/BFO nanocomposites shown in Fig. 11.
In order to suggest the degradation pathway of amoxicillin in the rGO/BFO/Vis LED process, intermediates were identified through LC-MS analysis. A total of 15 by-products resulting from the degradation of amoxicillin in this process were detected, the characteristics of which are presented in Table 2. Based on these observations, as well as data published in similar studies3,64,65,66 two possible degradation pathways for amoxicillin are proposed, as shown in Fig. 12. The first proposed pathway for the degradation of amoxicillin involves the cleavage of the four-membered beta-lactam ring, leading to the formation of the penicilloic acid isomer (A1) and subsequent derivatives from A2 to A9. However, the formation of penicilloic acid may also result from the hydrolysis process. The second pathway is the hydroxylation process, during which a hydroxyl group is added to the structure of amoxicillin. The addition of a hydroxyl group is the most important degradation mechanism for antibiotic compounds due to the presence of a benzene ring in their structure. This process results in the formation of products such as A10 to A15 and eventually leads to the production of carbon dioxide and water during mineralization. Oxidation of the methyl group in the thiazolidine ring has been detected in products A10 to A11. A12 is the result of carboxyl group removal from isomer A11. The cleavage of the bond between nitrogen and the amine group and the carbonyl group has been observed in product A14. The A15 isomer could result from the removal of CO from the beta-lactam ring. Subsequently, the decarboxylation reaction, which involves the removal of the –COOH group from the compound’s structure, has been observed in this pathway, leading to the formation of the A11 isomer.
Conclusion
An efficient rGO/BFO heterostructure photocatalyst was successfully synthesized using a hydrothermal method. Comparative studies on the degradation of AMX reveal that the rGO/BFO photocatalyst under visible LED irradiation (rGO/BFO/Vis LED) exhibits superior efficiency compared to rGO/BFO without light, BFO under visible LED, rGO under visible LED, BFO in the dark, rGO in the dark, and the visible LED alone. Experimental results showed that a catalyst dosage of 1 g/L, a pH of 5.0, and an AMX concentration of 5 mg/L were the optimal conditions for achieving over 80% degradation of AMX within 30 min of irradiation. Under these optimal conditions, the TOC and COD removal rates reached 30.7% and 38.61%, respectively, after 60 min of irradiation. Kinetic studies revealed that the degradation of AMX in various processes followed pseudo-first-order kinetics. Additionally, the rGO/BFO nanocomposite showed good reusability and stability under optimal conditions. This study demonstrates that the rGO/BFO heterojunction is an effective approach for developing high-efficiency photocatalysts and offers valuable insights into photocatalytic processes.
Data availability
The data of this study is available for any person after publication. For any questions about the data from this work contact the corresponding.
References
Qutob, M. et al. A review of radical and non-radical degradation of amoxicillin by using different oxidation process systems. Environ. Res. 214, 113833 (2022).
Yang, S., Liu, X., He, S., Jia, C. & Zhong, H. Amoxicillin degradation in persulfate activation system induced by concrete-based hydrotalcites: efficiency, mechanism, and degradation pathway. J. Mol. Liq. 394, 123688 (2024).
Ashraf Ali, M. & Maafa, I. M. Photodegradation of amoxicillin in aqueous systems: A review. Int. J. Mol. Sci. 25 (17), 9575 (2024).
Liu, H. et al. Study on photocatalytic degradation of amoxicillin in wastewater by Bi2WO6/nano-ZnO. Opt. Mater. 123, 111835 (2022).
Kanakaraju, D., Glass, B. D. & Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 219, 189–207 (2018).
Guo, X. et al. Effective visible-light excited charge separation in all-solid-state ag bridged BiVO4/ZnIn2S4 core-shell structure Z-scheme nanocomposites for boosting photocatalytic organics degradation. J. Alloys Compd. 887, 161389 (2021).
Chong, M. N., Jin, B., Chow, C. W. & Saint, C. Recent developments in photocatalytic water treatment technology: a review. Water Res. 44 (10), 2997–3027 (2010).
Cuadra, J. G. et al. Functional properties of transparent ZnO thin films synthetized by using spray pyrolysis for environmental and biomedical applications. Ceram. Int. 49 (20), 32779–32788 (2023).
Cuadra, G. et al. Multifunctional silver-coated transparent TiO2 thin films for photocatalytic and antimicrobial applications. Appl. Surf. Sci. 617, 156519 (2023).
Seid-Mohammadi, A., Asgari, G. & Chavoshi, S. Photocatalytic synergistic degradation of 2,4-dichlorophenol using ultraviolet LED light/zno hybrid oxidation process. Desalin. Water Treat. 274, 115–113 (2022).
Li, X. et al. Challenges of photocatalysis and their coping strategies. Chem. Catal. 2 (6), 1315–1345 (2022).
Basith, M. A., Ahsan, R., Zarin, I. & Jalil, M. A. Enhanced photocatalytic dye degradation and hydrogen production ability of Bi25FeO40-rGO nanocomposite and mechanism insight. Sci. Rep. 8, 11090 (2018).
Conesa, J. C. Sulfide-based photocatalysts using visible light, with special focus on In2S3, SnS2 and ZnIn2S4. Catalysts 12 (1), 40–46 (2021).
Preetha. Promoting photocatalytic interaction of Boron doped reduced graphene oxide supported BiFeO3 nanocomposite for visible-light-induced organic pollutant degradation. J. Alloys Comd. 904, 164038 (2022).
Kamo, A., Sonmezoglu, O. A. & Sonmezoglu, S. Ternary, zinc–tin-oxide nanoparticles modified by magnesium ions as a visible-light-active photocatalyst with highly strong antibacterial activity. Nanoscale Adv. 6, 6008–6018 (2024).
Fang, M. J. & Tsao, C. W. Semiconductor nanoheterostructures for photoconversion applications. J. Phys. D: Appl. Phys. 53 (14), 143001 (2020).
Huo, Y., Jin, Y. & Zhang, Y. Citric acid assisted solvothermal synthesis of BiFeO3 microspheres with high visible-light photocatalytic activity. J. Mol. Catal. Chem. 331 (1–2), 15–20 (2010).
Farhadi, A. R. K., Rahemi, N., Allahyari, S. & Tasbihi, M. Metal-doped perovskite BiFeO3/rGO nanocomposites towards the degradation of acetaminophen in aqueous phase using plasma-photocatalytic hybrid technology. J. Taiwan. Inst. Chem. Eng. 120, 77–92 (2021).
Verma, M. et al. Efficient and rapid sunlight-driven photocatalytic degradation of methylene blue dye using multiferroic BiFeO3 nanoparticles. J. Sol-Gel Sci. Technol. 113, 356–373 (2025).
Mehrdadian, E., Sheibani, S. & Ataie, A. Outstanding photocatalytic activity of a mechano-thermally synthesized Z-scheme BiFeO3- Fe2O3 heterostructure. J. Alloys Compd. 997, 174900 (2024).
Verma, M. et al. Efficient and rapid sunlight-driven photocatalytic degradation of methylene blue dye using multiferroic BiFeO3 nanoparticles. J. Sol-Gel Sci. Technol. 113. 113, 356–373 (2025).
Niu, F. et al. Synthesis of Pt/BiFeO3 heterostructured photocatalysts for highly efficient visible-light photocatalytic performances. Sol Energy Mater. Sol Cells. 143, 386–396 (2015).
Liu, Y. L. & Wu, J. M. Synergistically catalytic activities of BiFeO3/TiO2 core-shell nanocomposites for degradation of organic dye molecule through piezophototronic effect. Nano Energy. 56, 74–81 (2019).
Wang, L., Niu, C. G., Wang, Y., Wang, Y. & Zeng, G. M. The synthesis of Ag/AgCl/BiFeO3 photocatalyst with enhanced visible photocatalytic activity. Ceram. Int. 42 (16), 18605–18611 (2016).
Si, Y. et al. Enhanced visible light driven photocatalytic behavior of BiFeO3/reduced graphene oxide composites. Nanomaterials 8 (7), 526 (2018).
Lakhera, S. K. et al. Design of a highly efficient ternary AgI/rGO/BiVO4 nanocomposite and its direct solar light induced photocatalytic activity. Appl. Surf. Sci. 487, 1289–1300 (2019).
Kamo, A., Ozcan, A., Sonmezoglu, O. & Sonmezoglu, S. 10 Understanding antibacterial disinfection mechanisms of oxide-based photocatalytic materials. In Nanocomposite and Nanohybrid Materials: Processing and Applications (eds Verma, R. et al.) 195–222 (De Gruyter, 2024).
Wang, P. et al. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B. 132, 452–459 (2013).
Chen, Y. et al. Reduction degree of reduced graphene oxide (RGO) dependence of photocatalytic hydrogen evolution performance over RGO/ZnIn2S4 nanocomposites. Catal. Sci. Technol. 3 (7), 1712–1717 (2013).
Elbasuney, S., El-Sayyad, G. S., Tantawy, H. & Hashem, A. H. Retracted article: promising antimicrobial and antibiofilm activities of reduced graphene oxide-metal oxide (RGO-NiO, RGO-AgO, and RGO-ZnO) nanocomposites. RSC Adv. 11 (42), 25961–25975 (2021).
Feng, Q., Li, X. & Wang, J. Percolation effect of reduced graphene oxide (rGO) on ammonia sensing of rGO-SnO2 composite based sensor. Sens. Actuators B Chem. 243, 1115–1126 (2017).
Ghorbani, M., Sheibani, S., Abdizadeh, H. & Golobostanfard, M. R. Modified BiFeO3/rGO nanocomposite by controlled synthesis to enhance adsorption and visible-light photocatalytic activity. J. Mater. Res. Technol. 22, 1250–1267 (2023).
Ahmadi, Y. & Kim, K. H. Modification strategies for visible-light photocatalysts and their performance-enhancing effects on photocatalytic degradation of volatile organic compounds. Renew. Sust Energ. Rev. 189, 113948 (2024).
Seidmohammadi, A., Amiri, R., Faradmal, J. & Lili, M. Asgari. Gh. UVA-LED assisted persulfate/nzvi and hydrogen peroxide/nzvi for degrading 4-chlorophenol in aqueous solutions. Korean J. Chem. Eng. 35, 694–701 (2018).
Bayat, R., Derakhshi, P., Rahimi, R., Safekordi, A. A. & Rabbani, M. A. Magnetic ZnFe2O4/ZnO/perlite nanocomposite for photocatalytic degradation of organic pollutants under LED visible light irradiation. Sol State Sci. 89, 167–171 (2019).
Gao, X. M., Fu, F. & Li, W. H. Photocatalytic degradation of phenol over Cu loading BiVO4 metal composite oxides under visible light irradiation. Phys. B. 412, 26–31 (2013).
Pu, Y. C., Chou, H. Y., Kuo, W. S., Wei, K. H. & Hsu, Y. J. Interfacial charge carrier dynamics of cuprous oxide-reduced graphene oxide (Cu2O-rGO) nanoheterostructures and their related visible-light-driven photocatalysis. Appl. Catal. B-Environ. 204, 21–32 (2017).
Jalil, A., Karthikeyan, S., Selvapandiyan, M. & Sankar, A. Investigation of structural, magnetic and photocatalytic properties of Gd doped bismuth ferrite-reduced graphene oxide nanocomposites. Post graduate dissertations. Bangladesh University of Engineering and Technology.
Karthikeyan, S., Selvapandiyan, M. & Sankar, A. Electrochemical performance of reduced graphene oxide (rGO) decorated lanthanum oxide (La2O3) composite nanostructure as asymmetric supercapacitors. Inorg. Chem. Commun. 139, 109331 (2022).
WEF. Standard Methods for the Examination of Water and Wastewater 31 Edn (APHA, 2005).
Dehdar, A., Asgari, G., Leili, M., Madrakian, T. & Seid-Mohammadi, A. Step-scheme BiVO4/WO3 heterojunction photocatalyst under visible LED light irradiation removing 4-chlorophenol in aqueous solutions. J. Environ. Manage. 297, 113338 (2021).
Li, K. et al. Boosting photocatalytic Chlorophenols remediation with addition of sulfite and mechanism investigation by in-situ drifts. J. Hazard. Mater. 398, 123007 (2020).
Alam, S. N., Sharma, N. & Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 6 (01), 152–161 (2017).
Kadi, M. W., Mohamed, R. M. & Ismail, A. A. Facile synthesis of mesoporous BiFeO3/graphene nanocomposites as highly photoactive under visible light. Opt. Mater. 104, 109842 (2020).
Abdel-Aal, S. K., Beskrovnyi, A. I., Ionov, A. M., Mozhchil, R. N. & Abdel-Rahman, A. S. Structure investigation by neutron diffraction and Xray diffraction of graphene nanocomposite CuO–rGO prepared by low cost method. Phys. Status Solidi A. 218 (12), 2100138 (2021).
Zangeneh, H., Zinatizadeh, A., Habibi, M., Akia, M. & Isa, M. H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 26, 1–36 (2015).
Kanakaraju, D., Kockle, J., Motti, C. A., Glass, B. D. & Oelgemöller, M. Titanium dioxide/zeolite integrated photocatalytic adsorbents for the degradation of amoxicillin. Appl. Catal. B-Environ. 166, 45–55 (2015).
Sayadi, M., Chamanehpour, E. & Fahoul, N. Recent advances and future outlook for treatment of pharmaceutical from water: an overview. Int. J. Environ. Sci. Technol. 20 (3), 3437–3454 (2023).
Kumar, A. et al. Highly visible active ag2CrO4/Ag/BiFeO3@ RGO nano-junction for photoreduction of CO2 and photocatalytic removal of Ciprofloxacin and bromate ions: the triggering effect of ag and RGO. Chem. Eng. J. 370, 148 – 65(2019).
Malathi, A., Vasanthakumar, V., Arunachalam, P., Madhavan, J. & Ghanem, M. A. A low cost additive-free facile synthesis of BiFeWO6/BiVO4 nanocomposite with enhanced visible-light induced photocatalytic activity. J. Colloid Interface Sci. 506, 553–563 (2017).
Payan, A., Fattahi, M. & Roozbehani, B. Synthesis, characterization and evaluations of TiO2 nanostructures prepared from different Titania precursors for photocatalytic degradation of 4-chlorophenol in aqueous solution. J. Environ. Health Sci. Eng. 16, 41–54 (2018).
Seid-Mohammadi, A., Asgarai, G., Ghorbanian, Z. & Dargahi, A. The removal of cephalexin antibiotic in aqueous solutions by ultrasonic waves/hydrogen peroxide/nickel oxide nanoparticles (US/H2O2/NiO) hybrid process. Sep. Sci. Technol. 55 (8), 1558–1568 (2020).
Bazrafshan, E. et al. Photocatalytic degradation of catechol using ZnO nanoparticles as catalyst: optimizing the experimental parameters using the Box-Behnken statistical methodology and kinetic studies. Microchem J. 147, 643–653 (2019).
Gao, B., Wang, J., Dou, M., Xu, C. & Huang, X. Enhanced photocatalytic removal of amoxicillin with Ag/TiO2/mesoporous gC3N4 under visible light: property and mechanistic studies. Environ. Sci. Pollut Res. 27, 7025–7039 (2020).
Dobaradaran, S. et al. Catalytic decomposition of 2-chlorophenol using an ultrasonic-assisted Fe3O4–TiO2@ MWCNT system: influence factors, pathway and mechanism study. J. Colloid Interface Sci. 512, 172–189 (2018).
Chiu, Y-H., Chang, T. M., Chen, C. Y., Sone, M. & Hsu, Y. J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 9 (5), 430 (2019).
Hu, L., Hu, H., Lu, W., Lu, Y. & Wang, S. Novel composite BiFeO3/ZrO2 and its high photocatalytic performance under white LED visible-light irradiation. Mater. Res. Bull. 120, 110605 (2019).
Li, S. et al. Fabrication of vessel–like biochar–based heterojunction photocatalyst Bi2S3/BiOBr/BC for diclofenac removal under visible LED light irradiation: mechanistic investigation and intermediates analysis. J. Hazard. Mater. 391, 121407 (2020).
Krivec, M. et al. The nature of chlorine-inhibition of photocatalytic degradation of dichloroacetic acid in a TiO2-based microreactor. Phys. Chem. Chem. Phys. 16 (28), 14867–14873 (2014).
Lam, S. M., Sin, J. C., Abdullah, A. Z. & Mohamed, A. R. Sunlight responsive WO3/ZnO nanorods for photocatalytic degradation and mineralization of chlorinated phenoxyacetic acid herbicides in water. J. Colloid Interface Sci. 450, 34–44 (2015).
Li, F. et al. Hydrothermal synthesis of graphene grafted titania/titanate nanosheets for photocatalytic degradation of 4-chlorophenol: solar-light-driven photocatalytic activity and computational chemistry analysis. Chem. Eng. J. 331, 685–694 (2018).
Song, H. et al. Synthesis of Fe-doped WO3 nanostructures with high visible-light-driven photocatalytic activities. Appl. Catal. B-Environ. 166, 112–120 (2015).
Jiang, L. et al. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant. Appl. Catal. B-Environ. 227, 376–385 (2018).
Trovo, A., Pupo Nogueira, R., Aguera, F., Fernandez-Alba, A., Malato, S. & A. R. & Degradation of the antibiotic amoxicillin by photo-Fenton processes, chemical and toxicological assessment. Water Res. 45, 1394–1402 (2011).
Fawzy, A., Mahanna, H. & Mossad, M. Efective photocatalytic degradation of amoxicillin using MIL–53(Al)/ZnO composite. Environ. Sci. Pollut Res. 29, 68532–68546 (2022).
Behnami, A., Aghayani, E., Zoroufchi, B. K., Sattari, M. & Pourakbar, M. Comparing the efficacy of various methods for sulfate radical generation for antibiotics degradation in synthetic wastewater: degradation mechanism, kinetics study, and toxicity assessment. RSC Adv. 12, 14945 (2022).
Acknowledgements
Financial assistance for this study project is provided from Hamadan University of Medical Sciences (Grant No. 140203091662).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mersa, L., Seid-mohammadi, A., Samarghandi, M.R. et al. Photocatalytic degradation of amoxicillin in aqueous solutions using rGO/BiFeO3 nanocomposites in the presence of LED light irradiation. Sci Rep 15, 34879 (2025). https://doi.org/10.1038/s41598-025-17033-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17033-x