Abstract
Our study aimed to evaluate the survival impact of adjuvant radiotherapy (RT) following breast-conserving surgery (BCS) in elderly male patients with early-stage, low-risk breast cancer (node-negative, HR+), and to identify RT-benefiting subgroups using machine learning and causal inference approaches. We conducted a retrospective cohort study using Surveillance, Epidemiology, and End Results (SEER) database (2000–2021), including 360 patients after propensity score matching (PSM). Patients were grouped by RT and non-RT (NRT) status, and a 1:3 nearest neighbor PSM was applied. Overall survival (OS), relative survival (RS), standardized mortality ratio (SMR), and transformed Cox regression were used to estimate RT benefit. Additionally, machine learning models, including random forest, support vector machines and causal forest model, were applied for survival prediction and validation. In early-stage, low-risk male breast cancer (MBC) patients treated with BCS, adjuvant RT did not demonstrate a significant survival advantage over NRT. After PSM, 15-year OS, RS, and SMR were 31.8%, 15.2%, and 2.14 for RT versus 34.1%, 21.5%, and 2.25 for NRT (p = 0.36, 0.68, and 0.81, respectively). The cumulative incidence of breast cancer-related death (BCRD) and non-BCRD also showed no statistically significant differences between groups (p = 0.06 and 0.75). Machine learning models (Cox, GBM, and XGBoost) confirmed the limited contribution of RT to survival prediction, with the Cox model demonstrating the best discrimination (C-index = 0.713). While RT was associated with a lower risk of death within the first 10 years, its benefit diminished over time. Causal forest analysis revealed notable heterogeneity in treatment effects across subgroups. Patients who were younger, diagnosed earlier, or had stage I disease showed relatively higher estimated benefit from RT, while older patients or those with more recent diagnoses demonstrated attenuated benefit. In elderly, low-risk MBC patients treated with BCS, adjuvant RT was not associated with improved long-term survival. While our findings suggest that RT may be safely omitted in selected individuals, this decision should be made cautiously in the absence of recurrence data. Model-based analyses underscore the importance of tailoring treatment to patient-specific risk profiles. Prospective studies dedicated to MBC are needed to support individualized de-escalation strategies.
Similar content being viewed by others
Introduction
Breast cancer is common among women, with a lifetime risk of 1 in 8, while MBC remains rare, comprising only 1% of all breast cancer cases and a lifetime risk of approximately 1 in 1,000 for men1,2,3. Although MBC shares some genetic, environmental, and age-related risk factors with female breast cancer (FBC), it exhibits distinct differences in genetic mutations and biological characteristics4,5,6,7. MBC is often diagnosed at more advanced stages, leading to worse outcomes. This disparity may result from limited awareness of the disease and psychosocial factors that contribute to delayed diagnosis8,9,10,11,12,13. Consequently, treatments for MBC must address not only the biological characteristics of the disease but also the psychosocial challenges specific to male patients11. Despite this need, the differentiation between MBC and FBC is frequently overlooked, and treatment for MBC is often extrapolated from FBC guidelines or synthesized from broader literature reviews4. Moreover, the scarcity of robust treatment data for MBC is not simply due to its rarity.
Adjuvant RT is a cornerstone of breast cancer management. NCCN guidelines recommend either mastectomy or BCS with adjuvant RT for early-stage FBC patients14. However, in male patients, anatomical differences often result in mastectomy being the preferred surgical option, combined with axillary lymph node dissection or sentinel node biopsy, while adjuvant RT is typically omitted, even for early-stage cases1,15,16,17. For the limited number of early-stage MBC patients undergoing BCS, the role of postoperative RT remains debated. Some radiation oncologists argue that the limited breast tissue in men may lead to insufficient surgical margins, even for smaller tumors, thereby supporting the use of adjuvant RT to reduce risks associated with inadequate margins18,19.
In the treatment of early-stage FBC among elderly women, recent advances in surgical techniques and systemic therapies, including hormone therapy, have encouraged the de-escalation of treatment intensity20,21,22,23. Studies suggest that omitting RT for women over 65 with low-risk, hormone receptor-positive early-stage breast cancer does not adversely affect long-term OS or local control24,25. Considering the higher incidence of hormone receptor-positive tumors in MBC compared to FBC, emerging evidence suggests that tamoxifen maintenance therapy may provide significant benefit for early-stage MBC patients with hormone receptor-positive tumors and negative surgical margins following resection26,27. Nevertheless, the feasibility of omitting RT in favor of hormone therapy for elderly, low-risk MBC patients remain uncertain due to the lack of sufficient data.
Existing research on MBC primarily consists of small-scale retrospective studies, offering limited insights into the survival outcomes of omitting RT16,28,29. Large-scale studies using public databases often focus on absolute survival but fail to provide detailed analyses of net survival, disease-specific survival, and time-dependent survival following BCS3,30. To fill this gap, this study utilizes SEER database data to evaluate absolute survival, disease-specific survival, net survival, and time-dependent survival outcomes in MBC patients receiving adjuvant RT. This study aims to evaluate the survival impact of adjuvant RT in elderly MBC patients with low-risk features, and to identify subgroups that may benefit from RT using machine learning and causal inference methods.
Methods
Data source and patient selection
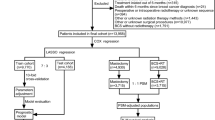
The study population was initially identified from the SEER-17 registries, consisting of 9,695 adult MBC cases diagnosed between 2000 and 2021. Patients were excluded based on the following criteria: (1) Breast cancer was not the first primary cancer; (2) Lack of positive histological confirmation; (3) Absence of BCS or unknown surgical status; (4) Not meeting the criteria for the early-stage low-risk group (defined as cases fulfilling all of the following: age > 65 years, stage T1-2N0M0, tumor size ≥ 3 cm, and hormone receptor positivity); (5) Unknown cause of death; (6) Unspecified RT method; (7) Incomplete follow-up data. After applying these inclusion and exclusion criteria, a total of 765 patients were included in the final analysis. The detailed selection process is illustrated in Fig. 1(A), and baseline patient characteristics were obtained from the SEER database.
Endpoint and definitions
Early-stage, low-risk MBC in elderly patients is defined as hormone receptor-positive breast cancer in men aged over 65 years, with stage T1 − 2N0M0 disease and tumor size ≥ 3 cm. The primary endpoint of the study is OS and disease specific survival. BCRD) was defined as patients dead from the disease. Non-BCRD was defined as patients dead from disease other than breast cancer. This classification was derived from the variables “SEER cause-specific death classification” and “SEER other cause of death classification” obtained from the SEER database. Patients with low-risk breast cancer were categorized into RT and NRT. Age was categorized into two groups (> 75 or 65–75 years) Race was categorized into three groups: white, black, and other/unknown. Year of diagnosis was classified as 2000–2004, 2005–2009, 2010–2014 and 2015–2021.
Statistical analysis
To address potential confounding factors and improve the accuracy of treatment effect estimates, we utilized a 1:3 nearest neighbor PSM method with a caliper set at 0.05. The covariates used for matching included race, age, year of diagnosis, marital status, tumor T stage, clinical stage, hormone receptor (ER and PR) status, HER2 status, and tumor size. To ensure proper covariate balance, control units without suitable matches were excluded from the analysis. The adequacy of matching was evaluated using standardized mean differences (SMDs), with values below 0.1 indicating an acceptable balance between the treatment and control groups. OS was calculated as the duration from diagnosis to death from any cause or the last follow-up date. The Kaplan-Meier method was employed to estimate OS, and group differences were analyzed using the log-rank test31. The cumulative incidence of mortality by cause was assessed through competing risks analysis using Fine-Gray’s test. To quantify the relative risk of BCRD and non-BCRD among treatment groups, sub-distribution hazard ratios (SHRs) were calculated. This approach evaluates the influence of covariates on the cumulative incidence function while accounting for competing risks, enabling direct comparisons of the cumulative incidence of BCRD while considering non-BCRD as a competing event. The SMR was calculated by comparing the observed mortality in MBC patients to the expected mortality in the general population. RS, on the other hand, was derived as the ratio of survival observed in cancer patients to the survival expected in a comparable general population cohort. To evaluate excess mortality linked to specific treatment groups, a transformed Cox regression model was used, incorporating expected mortality rates and accounting for population-specific risks. The life tables and death rates required for SMR and RS calculations were derived from U.S. population data. To analyze the relative proportions and hazard ratios of BCRD and non-BCRD across treatment groups, age was modeled as a continuous variable using spline functions. The optimal placement of spline knots was determined using the C-index and Akaike Information Criterion (AIC) to ensure model precision.
Least Absolute Shrinkage and Selection Operator (LASSO) was utilized for feature selection, identifying 11 key predictors of survival, including demographic, tumor, and treatment-related variables such as race, stage, grade, histology, primary site, laterality, treatment strategies, tumor size, year of diagnosis, marital status, and median household income. The dataset was then divided into training and validation sets at a 7:3 ratio. Three machine learning models were constructed to predict OS: Cox regression, gradient boosting machine (GBM), and Extreme Gradient Boosting (XGBoost). The performance of these models was assessed using the C-index, and receiver operating characteristic (ROC) curve. A Causal Forest model was constructed to estimate the conditional average treatment effect (CATE) of adjuvant RT on overall survival, conditioned on patient-level covariates. Additionally, categorical variables were compared using chi-square tests. All statistical analyses were conducted using R version 4.3.3 (R Foundation for Statistical Computing, Vienna, Austria), employing the tidycmprsk, survival, dplyr, mstate, cmprsk, survminer, riskRegression, cmprskcoxmsm, and splines packages. Data from the SEER database (SEER Research Data, 17 Registries, Nov 2023 Sub [2000–2021]) were retrieved using SEER*Stat software version 8.4.2.
Results
Baseline characteristics and treatment trend
We initially identified 9,695 MBC patients from the SEER-17 database as the study population. Following the application of exclusion criteria, 765 early-stage, low-risk patients were included, with 95 patients in the RT group and 670 in the NRT group. To mitigate potential selection bias and address the imbalance in sample sizes between the treatment and control groups, we conducted a 1:3 PSM. After PSM, a total of 360 patients were retained, comprising 93 in the RT group and 267 in the NRT group. Baseline characteristics before PSM are presented in Table 1, and the baseline characteristics after PSM are shown in Supplementary Table 1. The median age of the entire cohort before PSM was 75 years (range, 65–90), and 73 years (range, 65–90) after PSM. Stage I disease was observed in 68.4% and 70.8% of patients before and after PSM. After PSM, the distribution of characteristics between the treatment arms was well balanced (Fig. 1(B)).
The trend of treatment among early-stage MBC patients who underwent BCS is illustrated in Figure S1. The proportion of patients receiving RT remained consistently low, ranging from 10 to 25% throughout the study period, while the proportion of patients in the NRT group increased from 70% to nearly 90% by 2020. Notably, the RT group experienced minimal fluctuations, while the NRT group saw a steady rise, particularly after 2015. This trend suggests a shift in clinical practice towards more conservative treatment approaches, possibly influenced by the treatment strategies used in female cohorts, leading to the omission of adjuvant RT after BCS.
Absolute overall survival analysis
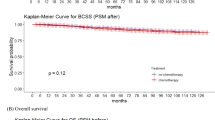
The median follow-up time for the entire cohort was 103 months, with 99 months for the RT group and 106 months for the NRT group. The OS of the groups was not continuously separated overtime both before and after PSM adjusted (Fig. 2). The unadjusted OS of 5-, 10-, 15-year were, respectively, 85.4%, 57.0% and 31.3% in RT group and 73.9%, 44.8% and 31.4% in NRT group (HR 0.78, 95% CI 0.55–1.10, p = 0.160; Fig. 2(A)). The PSM-adjusted OS of 5-, 10-, 15-year were, respectively, 86.8%, 57.9% and 31.8% in RT group and 75.4%, 43.7% and 34.1% in NRT group (HR 0.83, 95% CI 0.57–1.23, p = 0.360; Fig. 2(B)). To further minimize the impact of confounding factors, we performed a multivariable analysis based on Cox model. In this analysis, the use of RT had no significant effect on OS (HR 0.93, 95% CI 0.62–1.40; p = 0.73; Fig. 2(C)). Furthermore, age and stage were significantly associated with OS. Patients aged > 75 years had worse OS compared to those aged 65–75, both before and after PSM adjustment (65–75 vs. >75; 5-, 10-, and 15-year OS before PSM: 86.0%, 66.5%, and 49.7% vs. 62.4%, 21.5%, and 9.6%; HR 3.29, 95% CI 2.59–4.17; p < 0.0001 ; after PSM: 85.0%, 61.9%, and 49.7% vs. 67.6%, 24.2%, and 8.8%; HR 2.55, 95% CI 1.82–3.58; p < 0.0001; Figure S2(A–B)). Similarly, stage II disease was associated with poorer OS compared to stage I (Stage I vs. Stage II, before PSM: 76.6%, 49.7%, and 35.7% vs.72.7%, 38.6%, and 20.8%; HR 1.46, 95% CI 1.16–1.85, p = 0.0015; after PSM: 79.1%, 50.5%, and 38.5% vs. 76.9%, 37.7%, and 18.8%; HR 1.54, 95% CI 1.08–2.20, p = 0.016; Figure S2 (C–D)).
Kaplan-Meier curves for overall survival (OS) before and after propensity score matching (PSM) and multivariable adjustment based on Cox regression. (A) OS before PSM; (B) OS after PSM and (C) OS after balancing with PSM and adjusting for age, race, diagnosis year, hormone receptor status and marital status based on the Cox multivariable regression.
In disease specific analysis, RT showed limited impact on both BCRD and non-BCRD. The cumulative incidence of BCRD at 5, 10 and 15 years was 0%, 3.9%, and 6.9%, respectively, in the RT group, compared to 6.7%, 10.7%, and 12.8% in the NRT group (SHR 2.82, 95% CI 0.90–8.82, p = 0.06; Fig. 3(A)), before PSM adjustment. After PSM adjustment, the cumulative incidence remained similar, with rates of 0%, 3.9%, and 7.0% in the RT group, and 8.6%, 11.1%, and 12.4% in the NRT group (SHR 2.94, 95% CI 0.90–9.58, p = 0.06; Fig. 3(B)). The unadjusted cumulative incidence of non-BCRD at 5-, 10-, 15-year were, respectively, 14.6%, 39.1%, and 61.9% in RT group and 19.4%, 44.5%, and 55.8% in NRT group (SHR 1.04, 95% CI 0.74–1.44, p = 0.84; Fig. 3(C)), and 13.2%, 38.2%, and 61.2% in RT group and 16.0%, 45.2%, and 53.5% in NRT group after PSM adjusted (SHR 0.94, 95% CI 0.64–1.37, p = 0.75; Fig. 3(D)). Moreover, no significant difference in the cumulative incidence of BCRD was observed between the 65–75 and > 75 age groups, both before (65–75 vs. >75: 5.2%, 9.5%, and 13.0% vs. 6.5%, 10.2%, and 11.0%; SHR 0.94, 95% CI 0.56–1.58, p = 0.880; Figure S3A) and after PSM (6.2%, 10.0%, and 11.6% vs. 6.6%, 8.1%, and 9.7%; SHR 0.80, 95% CI 0.36–1.78, p = 0.660; Figure S3B), suggesting that age might not be a major determinant of BCRD. In contrast, patients aged > 75 showed a higher cumulative incidence of non-BCRD compared to those aged 65–75, both before (31.1%, 68.3%, and 79.4% vs. 8.8%, 24.0%, and 37.3%; SHR 3.65, 95% CI 2.81–4.73, p < 0.001; Figure S3C) and after PSM (25.8%, 67.7%, and 81.5% vs. 8.8%, 28.1%, and 38.7%; SHR 2.90, 95% CI 2.02–4.17, p < 0.001; Figure S3D), indicating a potential age-related vulnerability to non-cancer causes of death. With respect to disease stage, no statistically significant difference in BCRD was found between stage I and stage II patients, either before (stage I vs. stage II: 5.2%, 8.6%, and 10.7% vs. 7.1%, 12.6%, and 15.4%; SHR 1.40, 95% CI 0.83–2.38, p = 0.190; Figure S3E) or after PSM (stage I vs. stage II: 5.5%, 8.0%, and 10.3% vs. 8.7%, 12.9%, and 12.9%; SHR 1.39, 95% CI 0.63–3.09, p = 0.390; Figure S3F). For non-BCRD, the cumulative incidence was higher in stage II compared to stage I patients before PSM (stage I vs. stage II: 18.1%, 41.7%, and 53.6% vs. 20.2%, 48.8%, and 63.8%; SHR 1.34, 95% CI 1.04–1.73, p = 0.020; Figure S3G), but this difference was attenuated and became non-significant after PSM (stage I vs. stage II: 15.5%, 41.5%, and 51.3% vs. 14.4%, 49.4%, and 68.3%; SHR 1.40, 95% CI 0.96–2.04, p = 0.069; Figure S3H).
Cumulative incidence curves for breast cancer-related death (BCRD) and non-breast cancer-related death (non-BCRD) before and after PSM. (A) Cumulative incidence of BCRD before PSM. (B) Cumulative incidence of BCRD after PSM. (C) Cumulative incidence of non-BCRD before PSM. (D) Cumulative incidence of non-BCRD after PSM.
Net survival impact
To minimize the impact of background mortality on OS, we assessed the net survival benefit of RT in comparison to the general population. For patients with NRT, no significant differences were observed in SMR or RS compared to those who received RT. After PSM, the SMR of OS was 2.14 in the RT group and 2.25 in the NRT group (RR 0.95, 95% CI 0.65–1.40, p = 0.81; Figure S4(A)). Since the follow-up for the RT group did not extend to 20 years, the comparison focused on RS at 5, 10, and 15 years. During the observation period, RS remained stable in both the RT and NRT groups. After PSM, the 5-, 10-, and 15-year RS in the RT group were 77.5%, 46.6%, and 15.2%, respectively, compared to 71.3%, 45.5%, and 21.5% in the NRT group (HR 1.05, 95% CI 0.83–1.33, p = 0.68; Figure S4(B)).
To further explore the changes in dynamic RT-associated risk, we analyzed the relative risk (RR) at 5, 10, and 15 years after diagnosis. In the dynamic RR plot for BCRD (Figure S5(A)), the relative risk between the RT and NRT groups showed no significant increase in the RT group over time. At 5 years, the adjusted RR was 0.27 (95% CI, 0.06–1.18; p = 0.07), and at 10 years, the RR was 0.33 (95% CI, 0.10–1.10; p = 0.03). The risk remained relatively stable through 15 years, with an RR of 0.46 (95% CI, 0.39–1.26; p = 0.24). For non-BCRD (Figure S5(B)), the RT group demonstrated a stable relative risk in the early years, with an RR of 0.75 (95% CI, 0.37–1.52; p = 0.45) at 5 years and 0.70 (95% CI, 0.43–1.12; p = 0.35) at 10 years. However, by 15 years, there was an upward trend, with the adjusted RR reaching 1.20 (95% CI, 0.78–1.84; p = 0.68).
Time dependent survival evaluation
Based on the longitudinal assessment, the survival benefits of RT compared to NRT group changed rapidly within the first 10 years after diagnosis (Fig. 4). The hazard ratios for OS and RS changed over time, increasing from 0.658 to 0.572 at 1 year to 0.598 and 0.529 at 5 years, and further rising to 0.725 and 0.640 at 10 years, respectively. This finding underscores the necessity of a minimal 10-year follow-up to clearly identify the maximal survival differences and treatment benefits of RT compared to non-radiotherapy approaches, as demonstrated in the longitudinal data.
Using machine learning methods to predict survival
LASSO regression was applied for feature selection (Figure S6), identifying key predictors for OS (including stage, treatment group, Race, ER, PR, HER2 status, marital status, age, and year of diagnosis). Based on the selected variables, we developed three models: Cox model, GBM model, and XGBoost model. Model performance was evaluated using the C-index, time-dependent area under the receiver operating characteristic curve (AUC) at 3, 5, and 10 years. As shown in Table 2, the Cox model achieved the highest predictive accuracy (C-index: 0.713; 5-year AUC: 0.737; 10-year AUC: 0.707), outperforming GBM and XGBoost. The time-dependent ROC curves (Fig. 5) further illustrate this trend. Brier score analysis (Figure S7) indicated stable prediction error over time, supporting the robustness of the Cox model. Feature importance analysis (Figure S8) highlighted age as the most influential variable, followed by cancer stage and marital status, while the impact of RT on survival prediction remained relatively low. Partial dependence plots (Figure S9) provided additional insights into the effect of these predictors on survival.
Identification of RT-Benefiting subpopulations using causal forest analysis
To identify patients who may derive differential survival benefit from adjuvant RT, we constructed a causal fForest model using selected covariates mentioned before. The model was trained to estimate the CATE of RT at the individual level, enabling subgroup-specific effect estimation beyond average comparisons.
Variable importance ranking (Fig. 6A) identified age as the most influential predictor for treatment effect heterogeneity, followed by stage, marital status, and year of diagnosis. Partial dependence plots (Fig. 6B) demonstrated that CATEs varied across subgroups, with notably higher estimated effects observed in younger patients and those diagnosed in earlier calendar years. Stage also showed relevant contribution to heterogeneity. While stage I patients exhibited higher average CATEs, stage II patients had relatively lower estimates. Interaction analysis (Fig. 6C) revealed that the most influential interactions involved age, stage, and marital status, including combinations such as stage I × age and marital status × year of diagnosis. Although interactions involving HER2 status also ranked highly, a large proportion of patients were recorded as “HER2 record not available,” limiting the interpretability of these effects and warranting cautious interpretation.
Discussion
With the rising incidence of breast cancer, particularly among the aging population15,32, treatment de-intensification has become a focus for low-risk patients, especially those with hormone receptor-positive, low-grade tumors25,32,33. MBC remains rare, its increasing incidence highlights the need for tailored strategies34,35. While de-intensified treatments have shown promise in FBC, their applicability to MBC remains uncertain due to distinct biological and clinical differences. Further investigation is required to determine the feasibility and outcomes of less intensive treatments for MBC. Using SEER data, we comprehensively assessed the impact of adjuvant RT on survival in elderly (≥ 65 years), early-stage (T1 − 2N0M0), hormone receptor-positive MBC. Through multi-dimensional analyses—including OS, RS, SMR, and causal inference modeling—our findings suggest that RT may offer limited additional survival benefit in this low-risk population, particularly in the context of competing non-cancer-related mortality. These results support the need for individualized decision-making rather than routine RT omission or recommendation.
In our study, no significant differences in OS, RS, or BCRD were observed between the RT and NRT groups. After PSM and multivariable Cox regression adjustment, the survival curves remained closely aligned throughout the follow-up period. These findings were consistent across multiple endpoints and analyses, including SMR and RR, reinforcing the robustness of the results. Notably, while RT showed a modest early survival advantage in the first decade, this benefit diminished over time and was ultimately offset by rising non-cancer-related mortality in the elderly population. Such trends suggest that in select low-risk male patients, particularly those with competing mortality risks, the incremental survival benefit of RT may be limited. Nonetheless, the absence of a local recurrence endpoint—due to limitations of the SEER database— necessitates a cautious interpretation of RT’s clinical value, especially considering that the primary rationale for adjuvant RT after BCS is to reduce local recurrence and improve disease-free survival.
Our study observed a lower cumulative incidence of BCRD in the RT group compared to the NRT group, with a SHR of 2.94 (95% CI: 0.90–9.58; p = 0.06) after PSM. Although this finding did not reach statistical significance, the consistently lower absolute risks of BCRD at 5, 10, and 15 years in the RT group (0%, 3.9%, and 7.0%) versus the NRT group (8.6%, 11.1%, and 12.4%) may indicate a potential disease-specific benefit of RT. However, due to the limited number of BCRD events and wide confidence intervals, this trend should be interpreted cautiously and may warrant further investigation in larger or prospective cohorts. Subgroup analyses further clarified the influence of age and stage. While patients aged > 75 years exhibited significantly poorer OS compared to those aged 65–75, no significant difference in BCRD was observed between the two age groups. Competing risk models confirmed a markedly higher incidence of non-BCRD in older patients, with SHRs of 3.65 and 2.90 before and after PSM, respectively. These findings suggest that in elderly individuals, particularly those over 75, competing risks such as cardiovascular and other non-cancer-related mortality36,37,38, may diminish the long-term clinical benefit of RT. Additionally, patients with stage II disease demonstrated worse OS than those with stage I, yet BCRD did not differ significantly between the two stages. The initially higher risk of non-BCRD in stage II patients (SHR 1.34, p = 0.020) was attenuated after PSM (SHR 1.40, p = 0.069), implying that baseline imbalances may have contributed to the observed association. These findings indicate that although RT may provide limited disease-specific benefit, its absolute impact on OS in low-risk elderly MBC patients is likely offset by age-related competing risks. As suggested in prior studies6,33,36,37,39, individualized treatment strategies should account for both oncologic prognosis and non-cancer-related health risks when evaluating the necessity of RT in this population.
To enhance individualized survival prediction and identify patients who may derive differential benefit from RT, we employed machine learning approaches alongside traditional statistical models. Key prognostic variables were selected using LASSO regression, and three survival models—Cox regression, GBM, and XGBoost—were constructed. Among these, the Cox model demonstrated the best predictive performance with a C-index of 0.713 and the highest AUC at both 5 and 10 years, indicating stable and reliable discrimination. Feature importance analysis further revealed that age, stage, and marital status were the dominant predictors of survival, whereas RT itself may contribute minimally to survival prediction. These results align with our prior findings that non-cancer-related factors may influence long-term outcomes in this population.
Beyond survival prediction, we utilized a causal forest model to estimate the CATEs of RT and assess treatment effect heterogeneity across patient subgroups. The model revealed substantial variation in RT benefit, with age emerging as the most influential modifier. Higher estimated benefit from RT was observed in younger patients and those diagnosed in earlier years, while older individuals—particularly those over 75—and those with more recent diagnoses or stage II disease derived lower CATE estimates. On the contrary, a subset of stage I patients exhibited relatively higher estimated treatment effects, indicating that these patients might experience more noticeable survival gains from RT. However, this does not imply a complete lack of benefit in stage II patients; rather, their lower CATEs may reflect higher baseline risks of systemic failure or competing mortality, which attenuate the relative impact of local treatment. Additionally, interaction analysis identified combinations such as age × stage and marital status × year of diagnosis as key contributors to effect heterogeneity. While HER2 status also ranked among the top modifiers, its interpretability was limited due to missing values. Together, these results highlight that the benefit of adjuvant RT in elderly MBC patients is not uniform and support a more nuanced, personalized approach to treatment decision-making.
This study has several strengths. It leveraged a large, population-based, multi-institutional and multi-ethnic cohort, enabling robust and generalizable analysis of elderly MBC patients. Comprehensive statistical methods—including PSM, competing risk models, RS, and SMR—were applied to rigorously evaluate the impact of adjuvant RT on both cancer-related and non-cancer-related outcomes. Furthermore, we incorporated machine learning and causal inference approaches to enhance predictive precision and explore individualized treatment effects.
Despite its strengths, this study has several limitations. First, due to the rarity of MBC, data scarcity remains a fundamental barrier. Clinical management for MBC is often extrapolated from FBC studies, given the lack of MBC-specific prospective trials. Notably, many ongoing de-escalation trials40,41 exclude male participants, limiting their applicability. Our study also focused exclusively on patients aged ≥ 65 to align with elderly-focused FBC trials25,33. While this improves cohort homogeneity, it restricts the generalizability to younger patients—a limitation we are addressing in a parallel study. Second, the SEER database lacks crucial clinical variables including local recurrence, distant metastasis, endocrine therapy use, chemotherapy regimens, and RT technique. This not only limits assessment of the full benefit of RT—particularly for local control and disease-free survival—but also restricts evaluation of concurrent de-intensification strategies (e.g., omission of endocrine therapy), which are under investigation due to poor adherence in MBC42,43,44. Importantly, given the lack of recurrence data in SEER, we could not assess the impact of RT on local or distant control. As such, conclusions regarding the omission of RT should be interpreted with caution and cannot rely solely on survival outcomes. Third, the low rate of BCS in MBC (reported as 4–19.8% for T1N0 disease)4,45,46, inherently limits RT uptake and thus sample size. Moreover, although PSM adjusted for observed confounders, residual confounding from unmeasured variables cannot be excluded in this retrospective design.
In conclusion, our findings suggest that the omission of RT in selected elderly, low-risk MBC patients may not be associated with a significant detriment to long-term survival, this does not warrant a one-size-fits-all recommendation. Instead, our study highlights the growing need for individualized treatment strategies guided by patient characteristics, competing risks, and model-informed predictions. Prospective studies tailored specifically to MBC are urgently needed to inform de-escalation decisions with greater confidence.
Data availability
All data used in this study were obtained from the Surveillance, Epidemiology, and End Results (SEER) database, a publicly available cancer registry maintained by the National Cancer Institute. The SEER database provides comprehensive population-based cancer incidence and survival data, ensuring robust and generalizable findings. SEER database access and details are available at https://seer.cancer.gov/.
References
Lin, A. P., Huang, T. W. & Tam, K. W. Treatment of male breast cancer: meta-analysis of real-world evidence. Br. J. Surg. 108 (9), 1034–1042 (2021).
Fentiman, I. S., Fourquet, A. & Hortobagyi, G. N. Male breast cancer. Lancet 367 (9510), 595–604 (2006).
Vo, K. et al. Omission of adjuvant radiotherapy in low-risk elderly males with breast cancer. Breast Cancer. 31 (3), 485–495 (2024).
Ruddy, K. J. & Winer, E. P. Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship. Ann. Oncol. 24 (6), 1434–1443 (2013).
Gucalp, A. et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res. Treat. 173 (1), 37–48 (2019).
Giordano, S. H. Breast cancer in men. N Engl. J. Med. 378 (24), 2311–2320 (2018).
Anderson, W. F., Jatoi, I., Tse, J. & Rosenberg, P. S. Male breast cancer: a population-based comparison with female breast cancer. J. Clin. Oncol. 28 (2), 232–239 (2010).
Co, M., Lee, A. & Kwong, A. Delayed presentation, diagnosis, and psychosocial aspects of male breast cancer. Cancer Med. 9 (10), 3305–3309 (2020).
Thomas, E. Original Research: Men’s awareness and knowledge of male breast cancer. Am J. Nurs 110(10). (2010).
Culell, P. et al. Male breast cancer: a multicentric study. Breast J. 13 (2), 213–215 (2007).
Constantinou, N., Marshall, C. & Marshall, H. Discussion and optimization of the male breast cancer patient experience. J. Breast Imaging. 5 (3), 339–345 (2023).
Altiner, S. et al. Analysis of knowledge about male breast cancer among patients at tertiary medical center. Am. J. Mens Health. 17 (2), 15579883231165626 (2023).
Zeng, C. et al. Integrated bulk and single-cell transcriptomic analysis unveiled a novel cuproptosis-related lipid metabolism gene molecular pattern and a risk index for predicting prognosis and antitumor drug sensitivity in breast cancer. Discov Oncol. 16 (1), 318 (2025).
Gradishar, W. J. et al. Breast cancer, version 3.2024, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 22 (5), 331–357 (2024).
Chidambaram, A. et al. Male breast cancer: current scenario and future perspectives. Technol. Cancer Res. Treat. 23, 15330338241261836 (2024).
Rogowski, P. et al. Pattern of care of adjuvant radiotherapy in male breast cancer patients in clinical practice: an observational study. Strahlenther Onkol. 195 (4), 289–296 (2019).
Yadav, S. et al. Male breast cancer in the united states: treatment patterns and prognostic factors in the 21st century. Cancer 126 (1), 26–36 (2020).
Yu, E. et al. The impact of post-mastectomy radiation therapy on male breast cancer patients–a case series. Int. J. Radiat. Oncol. Biol. Phys. 82 (2), 696–700 (2012).
Stranzl, H. et al. Adjuvant radiotherapy in male breast cancer. Radiother Oncol. 53 (1), 29–35 (1999).
Mann, G. B. et al. Postoperative radiotherapy omission in selected patients with early breast cancer following preoperative breast MRI (PROSPECT): primary results of a prospective two-arm study. Lancet 403 (10423), 261–270 (2024).
Kouhen, F. Omitting radiotherapy in elderly breast cancer patients: valid strategy or illusory hope? Breast 72, 103598 (2023).
Haque, W., Verma, V., Butler, E. B. & Teh, B. S. Omission of radiotherapy in elderly women with early stage metaplastic breast cancer. Breast 38, 154–159 (2018).
Zeng, C. et al. Multi-cohort validation of a lipid metabolism and ferroptosis-associated index for prognosis and immunotherapy response prediction in hormone receptor-positive breast cancer. Int. J. Biol. Sci. 21 (9), 3968–3992 (2025).
Kunkler, I. H., Williams, L. J., Jack, W. J. L., Cameron, D. A. & Dixon, J. M. Breast-Conserving surgery with or without irradiation in early breast cancer. N Engl. J. Med. 388 (7), 585–594 (2023).
Kunkler, I. H., Williams, L. J., Jack, W. J. L., Cameron, D. A. & Dixon, J. M. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 16 (3), 266–273 (2015).
Slamon, D. et al. Ribociclib plus endocrine therapy in early breast cancer. N Engl. J. Med. 390 (12), 1080–1091 (2024).
Reinisch, M. et al. Efficacy of endocrine therapy for the treatment of breast cancer in men: results from the MALE phase 2 randomized clinical trial. JAMA Oncol. 7 (4), 565–572 (2021).
Arslan, U. Y. et al. Outcome of non-metastatic male breast cancer: 118 patients. Med. Oncol. 29 (2), 554–560 (2012).
Fouhi, M. E., Mesfioui, A. & Benider, A. Male breast cancer: a report of 25 cases. Pan Afr. Med. J. 37, 343 (2020).
Madden, N. A. et al. Radiotherapy and male breast cancer: A Population-based registry analysis. Am. J. Clin. Oncol. 39 (5), 458–462 (2016).
Xu, C. et al. Simultaneous integrated dose reduction intensity-modulated radiotherapy improves survival in patients with locally advanced non-small cell lung cancer by reducing cardiac irradiation exposure. Discov Oncol. 16 (1), 300 (2025).
Biganzoli, L. et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European society of breast cancer specialists (EUSOMA) and the international society of geriatric oncology (SIOG). Lancet Oncol. 22 (7), e327–e340 (2021).
Hughes, K. S. et al. Lumpectomy plus Tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J. Clin. Oncol. 31 (19), 2382–2387 (2013).
Reddington, R. et al. Incidence of male breast cancer in Scotland over a twenty-five-year period (1992–2017). Eur. J. Surg. Oncol. 46 (8), 1546–1550 (2020).
Mukherjee, A. G. et al. The incidence of male breast cancer: from fiction to reality - correspondence. Int. J. Surg. 109 (9), 2855–2858 (2023).
Jørgensen, T. L., Hallas, J., Friis, S. & Herrstedt, J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br. J. Cancer. 106 (7), 1353–1360 (2012).
de Glas, N. A. et al. Performing survival analyses in the presence of competing risks: A clinical example in older breast cancer patients. J Natl. Cancer Inst 108(5). (2016).
Zeng, C. et al. Investigating cardiovascular diseases related to endocrine therapy in hormone receptor-positive early breast cancer: insights from a nationwide real-world study. Cardiooncology 11 (1), 35 (2025).
Yang, P. et al. Immediate risk of non-cancer deaths after a cancer diagnosis. BMC Cancer. 21 (1), 963 (2021).
Meattini, I. et al. Exclusive endocrine therapy or partial breast irradiation for women aged ≥ 70 years with luminal A-like early stage breast cancer (NCT04134598 - EUROPA): proof of concept of a randomized controlled trial comparing health related quality of life by patient reported outcome measures. J. Geriatr. Oncol. 12 (2), 182–189 (2021).
Leone, J. P. et al. Locoregional treatment and overall survival of men with T1a,b,cN0M0 breast cancer: A population-based study. Eur J. Cancer 71. (2017).
Demissie, S., Silliman, R. A. & Lash, T. L. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J. Clin. Oncol. 19 (2), 322–328 (2001).
Pemmaraju, N., Munsell, M. F., Hortobagyi, G. N. & Giordano, S. H. Retrospective review of male breast cancer patients: analysis of tamoxifen-related side-effects. Ann. Oncol. 23 (6), 1471–1474 (2012).
Venigalla, S. et al. Use and effectiveness of adjuvant endocrine therapy for hormone Receptor-Positive breast cancer in men. JAMA Oncol. 4 (10), e181114 (2018).
Chichura, A. et al. Male breast cancer patient and surgeon experience: the male WhySurg study. Ann. Surg. Oncol. 29 (10), 6115–6131 (2022).
Elmi, M. et al. Evolving surgical treatment decisions for male breast cancer: an analysis of the National surgical quality improvement program (NSQIP) database. Breast Cancer Res. Treat. 171 (2), 427–434 (2018).
Funding
Medical Education Collaborative Innovation Fund of Jiangsu University (No. JDY2023017), the funds of Changzhou Sci &Tech Program (CJ20230007), and the Changzhou Health Care Youth Talent Training Project (CZQM2023028).
Author information
Authors and Affiliations
Contributions
Chang Xu & Cheng Zeng: Conceptualization, Formal analysis, Statistical analysis, Writing - Original Draft;Qi Zhu&Yue Wang: Supervision, Validation, Writing - Review & Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This study is based on data from the Surveillance, Epidemiology, and End Results (SEER) public database. Since the SEER database contains de-identified patient information, no ethical approval or informed consent was required for this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, C., Zeng, C., Zhu, Q. et al. Survival impact of adjuvant radiotherapy in early stage low risk elderly male breast cancer patients treated with breast conserving surgery. Sci Rep 15, 31108 (2025). https://doi.org/10.1038/s41598-025-17083-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17083-1