Abstract
Primary retroperitoneal liposarcoma (PRPLS) is a rare malignancy with a high risk of recurrence and poor prognosis. To evaluate the prognostic value of the systemic inflammation response index (SIRI) in patients with PRPLS undergoing curative-intent surgical resection and to compare its predictive performance with other inflammatory markers. This retrospective study included 122 patients with pathologically confirmed PRPLS who underwent surgical resection at Peking University International Hospital between January 2021 and January 2024. Patients were stratified into high-SIRI and low-SIRI groups based on an optimal cutoff value determined by receiver operating characteristic (ROC) curve analysis. Clinical characteristics, laboratory parameters, and surgical outcomes were compared between groups. Recurrence-free survival (RFS) and locoregional recurrence-free survival (LRFS) were assessed using Kaplan–Meier survival curves and log-rank tests. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify independent prognostic factors. Post-hoc power analysis was conducted to ensure sample adequacy. SIRI demonstrated the highest prognostic accuracy among evaluated inflammatory markers, with an AUC of 0.831 (P < 0.001), outperforming NLR (AUC = 0.724), PLR (AUC = 0.730), and MLR (AUC = 0.747). Patients with high SIRI (≥ 1.95) had significantly shorter RFS and LRFS compared to those with low SIRI (P < 0.001 and P = 0.008, respectively). High SIRI was associated with larger tumor size, higher Ki-67 index, abnormal liver function markers, elevated CRP, and lower albumin levels. In multivariate Cox regression analysis, SIRI remained the only independent predictor of RFS (HR = 5.19, 95% CI: 2.356, P < 0.001). Preoperative SIRI is an independent and superior prognostic biomarker for recurrence in patients with PRPLS following surgical resection. Compared to conventional inflammatory indices, SIRI shows stronger predictive value for both RFS and LRFS, offering a simple and effective tool for risk stratification in clinical practice.
Similar content being viewed by others
Introduction
Liposarcoma, the most prevalent form of soft tissue sarcoma in adults1, can develop in various parts of the body. Typically, it originates in the extremities, followed by retroperitoneal and inguinal regions. The clinical features of liposarcomas often correspond to their pleomorphic histology, with larger lesions being more frequently observed in the retroperitoneum2. Diagnosing and treating liposarcomas presents challenges due to the absence of distinct clinical symptoms, the large size of the tumors, and the loose structure of the retroperitoneal space in adults. According to the World Health Organization (WHO) classification3, the major histological subtypes of primary retroperitoneal liposarcomas (PRPLS) include well-differentiated liposarcoma (WDLPS), dedifferentiated liposarcoma (DDLPS), myxoid liposarcoma, and pleomorphic liposarcoma. Among these, WDLPS and DDLPS are the most common in the retroperitoneal region and are characterized by amplification of the MDM2 and CDK4 genes located on chromosome 12q13-15, which aids in both diagnosis and prognostication4. Surgical resection is considered the primary treatment for PRPLS, whenever feasible5,6. Nonetheless, local recurrence is common, affecting 66% of patients7. A study of a large cohort of patients who underwent complete resections found a five-year overall survival (OS) rate of 54% for those with PRPLS8. Moreover, the tumor’s propensity for recurrence after resection remains a critical concern, leading to a considerable burden on patient survival and quality of life.
The prognostic assessment of PRL remains elusive, as conventional clinical and pathological factors, such as tumor size, histological grade, and mitotic index, have proven insufficient in accurately predicting outcomes9,10. Consequently, there is an urgent need to identify novel biomarkers that can provide more reliable prognostic information and aid in patient stratification. Systemic Inflammation Response Index (SIRI) has shown promise as a prognostic indicator in various malignancies, including gastrointestinal, breast, pancreatic and lung cancers11,12,13,14,15. SIRI’s ability to integrate multiple components of systemic inflammation makes it a compelling candidate for predicting cancer prognosis. Elevated levels of SIRI have been associated with poor outcomes, such as reduced OS and recurrence-free survival (RFS), in various cancers12,16. Specifically, SIRI reflects the interaction between the immune response and tumor progression, where an elevated inflammatory state may facilitate tumor growth, metastasis, and resistance to treatment17,18. Recent evidence has highlighted the prognostic value of systemic inflammation-based biomarkers in soft tissue sarcomas (STS), including liposarcoma. A retrospective study of 93 STS patients demonstrated that among several inflammatory and nutritional indices, the SIRI and albumin-to-globulin ratio (AGR) showed superior predictive performance for both overall survival (OS) and disease-free survival (DFS), with elevated SIRI independently associated with worse DFS (HR = 2.078, P = 0.034) and OS (HR = 3.729, P = 0.016)19. Similarly, in STS patients treated with trabectedin, elevated pretreatment SIRI was identified as an independent predictor of poor progression-free survival (PFS; P = 0.007) and OS (HR = 2.16, P = 0.006)20. These findings support the use of SIRI as a reliable, non-invasive biomarker for prognostic stratification in STS, particularly in liposarcoma. However, these findings were derived from heterogeneous STS populations or treatment-specific contexts, and few studies have specifically addressed the prognostic value of SIRI in PRPLS, a distinct and challenging STS subtype characterized by high recurrence rates and complex surgical management.
The present study aims to fill this gap by investigating the association between SIRI and the prognosis of PRPLS after surgical resection. By analyzing clinical data from 122 PRL patients, we aim to establish whether SIRI can serve as an independent prognostic factor for recurrence-free survival (RFS).
Methods
Participants enrollment
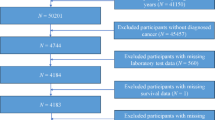
This study included patients diagnosed with PRPLS at Peking University International Hospital, between January 2021 and January 2024. Eligible participants met the following inclusion criteria: (1) a confirmed pathological diagnosis of PRPLS; (2) complete clinical data, including demographic information, imaging, laboratory tests, and pathological findings; (3) availability of follow-up data, including survival outcomes and recurrence status; and (4) patients who underwent surgical resection as the primary treatment. Exclusion criteria were as follows: (1) patients lost to follow-up; (2) patients who received any antitumor treatment, such as chemotherapy, radiotherapy, or targeted therapy, prior to surgery; (3) those with incomplete clinical or pathological data; (4) cases of recurrent retroperitoneal liposarcoma or secondary tumors; (5) patients with synchronous distant metastases at the time of initial diagnosis; and (6) patients with severe comorbidities or other malignancies that could significantly affect prognosis. A total of 122 patients who met the inclusion and exclusion criteria were ultimately enrolled in the study. This study was conducted in accordance with the Declaration of Helsinki and ethics approval was waived by the Ethics Committee of Peking University International Hospital due to retrospective nature and no intervention. Written informed consent was obtained from all participants.
To address concerns regarding sample adequacy, a post-hoc power analysis was performed based on the observed recurrence rates in the high SIRI group (42/50, 84.0%) and low SIRI group (20/72, 27.8%). Using a two-sided test with α = 0.05 and a group size ratio of 50:72, the calculated statistical power exceeded 99.9%. This indicates that the study was sufficiently powered to detect the observed differences in recurrence-free survival between the two SIRI strata.
Clinical Follow-Up
Patients were followed up via telephone interviews, outpatient records, hospitalization data, and imaging studies, including contrast-enhanced CT or MRI of the abdomen and pelvis. Routine laboratory tests, such as complete blood counts, liver and renal function panels, and inflammatory markers, were reviewed. Tumor recurrence, including both locoregional and distant events, was documented based on radiologic or histopathologic confirmation. Surgical margin status was recorded postoperatively and considered during follow-up evaluation, given its relevance to recurrence risk. Follow-up assessments were scheduled every three months for the first two postoperative years and every six months thereafter. The follow-up period ended in January 2024, with a median duration of 16 months. Two survival outcomes were analyzed. Recurrence-free survival (RFS) was defined as the interval from surgery to any tumor recurrence or last follow-up. Locoregional recurrence-free survival (LRFS) was defined as the time from surgery to recurrence confined to the retroperitoneum or last follow-up without such relapse. The Systemic Inflammation Response Index (SIRI) was calculated as (neutrophil count × monocyte count)/lymphocyte count21. Additional inflammatory indicators included the neutrophil-to-lymphocyte ratio (NLR = neutrophil/lymphocyte), platelet-to-lymphocyte ratio (PLR = platelet/lymphocyte), and monocyte-to-lymphocyte ratio (MLR = monocyte/lymphocyte).
Statistical analysis
Statistical analyses were conducted using SPSS 26.0 software. An ROC curve was plotted to determine the optimal cutoff value for SIRI, which was used for patient grouping. Categorical variables were presented as case numbers, and comparisons between groups were performed using the χ² test. Normality of continuous variables was assessed using the Shapiro–Wilk test. Variables including SIRI, BMI, platelet count, ALT, albumin, serum creatinine, and blood urea nitrogen showed no significant deviation from normality (P ≥ 0.05), and were presented as mean ± standard deviation and analyzed using student-t test. In contrast, variables such as tumor size, Ki-67, AST, TBIL, and CRP were not normally distributed (P < 0.05) and were summarized as medians with interquartile ranges (IQR) and analyzed using non-parametric tests. Survival curves were plotted using the Kaplan-Meier method, and intergroup comparisons were conducted with the log-rank test. Univariate Cox regression analysis was initially performed to identify variables significantly associated with recurrence-free survival (RFS). Variables that demonstrated statistical significance (P < 0.05) in the univariate analysis, including SIRI, tumor size, Ki-67 index, AST, ALT, albumin, and CRP, were subsequently included in the multivariate Cox proportional hazards regression model to assess independent prognostic factors for PRPLS. The stepwise forward likelihood ratio method was applied to construct the final multivariate model, and hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) were calculated. Multicollinearity was checked to ensure model robustness. P-value of < 0.05 was considered statistically significant.
Results
ROC analysis determining the optimal cutoff value for SIRI based on prognostic risk
To assess the prognostic value of SIRI and compare its discriminative ability with other systemic inflammatory markers, ROC curve analyses were performed (Fig. 1). As shown in Fig. 1A, SIRI demonstrated the highest predictive accuracy for recurrence, with an area under the curve (AUC) of 0.831 (P < 0.001), indicating strong discriminatory power. In comparison, the predictive performance of the NLR, PLR, and MLR was relatively lower. The AUCs for NLR (Fig. 1B), PLR (Fig. 1C), and MLR (Fig. 1D) were 0.724, 0.730, and 0.747, respectively (all P < 0.001). Although each of these markers exhibited statistically significant predictive ability, none achieved the same level of accuracy as SIRI. The Youden index and the corresponding optimal cutoff value were calculated. The results indicated that the optimal cutoff value for SIRI was 1.95. Patients were subsequently stratified into high-SIRI and low-SIRI groups based on this cutoff value. Specifically, the high-SIRI group (SIRI ≥ 1.95) included 50 patients, while the low-SIRI group (SIRI < 1.95) comprised 72 patients. Furthermore, the Kaplan-Meier (KM) survival analysis was performed to compare the difference of recurrence-free survival (RFS) between low and high SIRI groups (Fig. 2). The results demonstrated a significant difference in RFS between the two groups. Patients in the high-SIRI group exhibited markedly shorter RFS compared to those in the low-SIRI group, highlighting the prognostic value of SIRI in predicting recurrence in PRPLS. In addition, KM survival analysis was also conducted to compare the difference in locoregional recurrence-free survival (LRFS) between the low and high SIRI groups (Fig. 3). The results demonstrated a statistically significant difference in LRFS between the two groups (P = 0.008). Patients in the high-SIRI group exhibited markedly shorter LRFS compared to those in the low-SIRI group, underscoring the prognostic relevance of SIRI in predicting locoregional recurrence in patients with PRPLS following surgical resection.
ROC curve evaluating the prediction effects of SIRI, NLR, PLR, and MLR on the recurrence of primary retroperitoneal liposarcoma. (A) SIRI showed the highest predictive accuracy for recurrence, with an area under the curve (AUC) of 0.831 (P < 0.001). (B) The AUC for NLR was 0.724. (C) The AUC for PLR was 0.730. (D) The AUC for MLR was 0.747.
Baseline characteristics of patients with primary retroperitoneal liposarcoma stratified by SIRI
The baseline clinicopathological characteristics of patients in the low and high SIRI groups are summarized in Table 1. No significant differences were observed in sex distribution (P = 0.272), age (57.06 ± 4.45 vs. 57.96 ± 5.04 years, P = 0.298), BMI (23.53 ± 1.91 vs. 23.88 ± 2.10, P = 0.338), platelet count (240.77 ± 14.12 vs. 244.71 ± 14.61 × 10⁹/L, P = 0.138), serum creatinine (76.38 ± 5.10 vs. 76.90 ± 5.28 µmol/L, P = 0.589), or blood urea nitrogen (8.05 ± 1.49 vs. 8.12 ± 1.31 mmol/L, P = 0.783) between the two groups. In contrast, patients in the high SIRI group had significantly larger tumor sizes (21.95 [19.41, 24.87] cm vs. 16.95 [15.48, 18.58] cm, P < 0.001) and higher Ki-67 proliferation indices (24.73 [20.90, 27.12]% vs. 14.40 [12.22, 16.96]%, P < 0.001). Inflammatory and hepatic function parameters also showed significant differences. AST levels were elevated in the high SIRI group (23.01 [18.73, 25.65] U/L vs. 14.70 [12.68, 17.11] U/L, P < 0.001), as were ALT levels (30.26 ± 6.03 vs. 19.44 ± 4.72 U/L, P < 0.001) and TBIL levels (18.28 [14.52, 20.67] µmol/L vs. 12.15 [10.38, 14.13] µmol/L, P < 0.001). Conversely, albumin levels were significantly lower in the high SIRI group (31.23 ± 3.04 vs. 35.86 ± 2.83 g/L, P < 0.001). CRP levels were markedly elevated in the high SIRI group (62.17 [54.43, 66.71] mg/L vs. 17.39 [14.66, 22.19] mg/L, P < 0.001). No significant differences were observed in surgical margin status (P = 0.915), with comparable distributions of R0, R1, and R2 resections between the two groups. Similarly, the proportion of patients undergoing surrounding organ resection, including kidney, colon, or liver, did not differ significantly (P = 0.266). Histopathologic subtypes (WDLPS, DDLPS, and others; P = 0.941) and MDM2 expression status (P = 0.382) were also comparable between the two groups.
Uni- and multi-variate COX regression analysis identifying independent factors of primary retroperitoneal liposarcoma prognosis
The variables in baseline characteristics with statistical significance were next included in the univariate Cox regression analysis to evaluate their impact on prognosis of PRPLS (Table 2). The SIRI was a significant risk factor, with a hazard ratio (HR) of 8.069 (95% CI: 4.633–14.051, P < 0.001). Larger tumor size (HR: 1.187, 95% CI: 1.120–1.259, P < 0.001) and higher Ki-67 expression (HR: 1.07, 95% CI: 1.032–1.110, P < 0.001) were also significantly associated with reduced RFS. Liver function markers, including AST (HR: 1.09, 95% CI: 1.045–1.137, P < 0.001) and ALT (HR: 1.095, 95% CI: 1.059–1.132, P < 0.001), were significantly associated with worse prognosis, while higher albumin levels (HR: 0.903, 95% CI: 0.848–0.962, P = 0.001) demonstrated a protective effect. CRP, a systemic inflammation marker, was another significant predictor of reduced RFS (HR: 1.035, 95% CI: 1.023–1.047, P < 0.001). Then the multivariate COX regression analysis was implemented to determine the independent factors influencing the prognosis of PRPLS (Table 3). The results showed that only SIRI remains the independent risk factors (HR: 5.19, 95% CI: 2.356, P < 0.001) of the prognosis of PRPLS.
Discussion
PRPLS is a rare and aggressive soft tissue sarcoma, often characterized by large tumors in the retroperitoneum, making diagnosis and treatment challenging. Despite surgical resection being the primary treatment, recurrence rates remain high, and traditional prognostic factors have limited accuracy. The SIRI, a composite marker of systemic inflammation, has been identified as a promising prognostic tool in various cancers. However, its role in PRPLS remains underexplored. This study aimed to evaluate the prognostic value of the SIRI in patients with PRPLS. Our findings demonstrate that SIRI is significantly associated with RFS in PRPLS patients, independently predicting patient prognosis.
We identified that high SIRI (≥ 1.95) was associated with significantly shorter RFS compared to low SIRI (< 1.95) in patients with PRPLS. This result aligns with previous studies demonstrating the prognostic utility of SIRI in various malignancies, including gastrointestinal, breast, and lung cancers11,12,13,14,15. In these cancers, elevated SIRI levels were indicative of an unfavorable prognosis, often reflecting a hyper-inflammatory state that promotes tumor progression. Consistent with these reports, our study provides robust evidence supporting the use of SIRI as a reliable prognostic biomarker in PRPLS. Moreover, SIRI was identified as an independent risk factor for poor RFS in multivariate analysis, with a hazard ratio (HR) of 5.19, further underscoring its prognostic value.
The elevated SIRI observed in PRPLS patients may be driven by several biological mechanisms that link systemic inflammation to tumor progression and prognosis. SIRI integrates three peripheral blood parameters: NLR, PLR, and MLR, all of which reflect distinct aspects of the immune response in cancer. Each of these markers has been implicated in various aspects of tumor biology, such as inflammation, immune suppression, and metastatic potential22,23,24. Neutrophils play a central role in the tumor microenvironment by promoting inflammation25. In PRPLS, as in many other cancers, an elevated NLR may indicate an increased systemic inflammatory response, which can enhance tumor progression. Neutrophils secrete pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which can foster an immunosuppressive TME26,27. Additionally, neutrophils may support tumor metastasis by facilitating angiogenesis and promoting extracellular matrix remodeling through the release of matrix metalloproteinases (MMPs)28. A higher NLR in PRPLS patients might therefore reflect a tumor promoting inflammation, contributing to tumor growth, metastasis, and resistance to therapies, which could explain the association between high NLR and poor prognosis. On the other hand, lymphocytes, particularly T cells, are key players in anti-tumor immunity. A reduced lymphocyte count may signal immune dysfunction or suppression in PRPLS. This immunosuppression can be exacerbated by factors such as the recruitment of regulatory T cells (Tregs) and the secretion of immune-suppressive cytokines (e.g., IL-10)29. As lymphocyte count decreases and neutrophils increase, the immune response becomes less effective at controlling tumor growth, leading to a higher NLR and potentially worse outcomes.
Meanwhile, platelets have emerged as important players in cancer biology, influencing tumor progression in several ways. Elevated PLR in PRPLS may reflect a heightened systemic inflammatory state, which is often linked to increased platelet activation and aggregation. Tumor cells can directly interact with platelets, promoting tumor cell survival, proliferation, and metastasis30,31. For instance, platelets release growth factors such as platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β), which can stimulate tumor growth, angiogenesis, and immune evasion32,33. In addition, platelets can protect circulating tumor cells from immune detection and facilitate their homing to distant organs, increasing the risk of metastasis34. Moreover, Monocytes, which differentiate into macrophages in the tumor microenvironment, can have both pro-inflammatory and pro-tumorigenic effects35,36. In PRPLS, a high MLR may indicate increased monocyte recruitment to the tumor site, where they can polarize into tumor-associated macrophages (TAMs). TAMs, particularly those of the M2 phenotype, are known to support tumor growth and metastasis by secreting anti-inflammatory cytokines (e.g., IL-10) and growth factors (e.g., vascular endothelial growth factor [VEGF])37,38. They also help to remodel the extracellular matrix, promote angiogenesis, and suppress anti-tumor immune responses39,40. An elevated MLR could also indicate systemic inflammation that drives monocyte mobilization from the bone marrow, a process that is common in many cancers, including sarcomas. This systemic inflammation may contribute to immune suppression and tumor progression by enhancing monocyte infiltration into the tumor microenvironment, where they can further promote the cancerous phenotype. High MLR in PRPLS patients might therefore be a surrogate marker of an immunosuppressive TME, where monocytes and TAMs are recruited to support tumor progression.
Another key observation in this study was the association between high SIRI and impaired liver function, as evidenced by elevated levels of AST, ALT, and TBIL, along with lower Alb levels. This finding suggests that systemic inflammation in PRPLS may also reflect a dysfunction of vital organs, such as the liver. The liver plays a central role in regulating inflammatory responses through the production of acute-phase proteins, cytokine metabolism, and immune cell activation41. When its function is compromised, as often observed in patients with systemic inflammation or advanced cancer, the resulting metabolic and immunological imbalances can disrupt the body’s ability to control inflammation effectively42. This dysregulated immune state not only weakens anti-tumor immunity but also fosters a pro-inflammatory environment that supports tumor growth, angiogenesis, and metastasis, further exacerbating tumor progression. Meanwhile, elevated CRP levels in the high-SIRI group further support the notion that systemic inflammation is a driving force behind tumor aggressiveness. CRP, a well-established marker of systemic inflammation, has been linked to poor prognosis in several cancers, including sarcomas43,44. This association may reflect a complex interplay between the tumor and the host’s immune system, where chronic inflammation accelerates cancer progression by promoting angiogenesis, immune evasion, and metastatic spread. In addition, tumor size and Ki-67 expression were also significant prognostic factors in univariate analysis. Larger tumor size is a well-established poor prognostic factor in liposarcomas44, and Ki-67, a marker of cell proliferation, is frequently used to assess tumor aggressiveness and predict patient outcomes45. The fact that both of these markers were significantly associated with reduced RFS in our study further validates their relevance in assessing PRPLS prognosis.
The prognostic relevance of SIRI in PRPLS may be partially explained by tumor-specific biological mechanisms. PRPLS, particularly the well-differentiated and dedifferentiated subtypes, is characterized by the amplification of MDM2, a negative regulator of the p53 tumor suppressor pathway46. MDM2 amplification is known to promote tumor cell proliferation and evade apoptosis, contributing to aggressive clinical behavior47. Recent studies suggest that MDM2 overexpression may also induce a pro-inflammatory tumor microenvironment through modulation of cytokine secretion, which could further enhance systemic inflammation markers such as neutrophils and monocytes48. Moreover, the retroperitoneal anatomical compartment presents a unique immune milieu due to its relative lack of lymphatic drainage and limited immune surveillance, which may facilitate chronic inflammation and tumor immune evasion49. This localized immune tolerance may synergize with systemic inflammation to accelerate tumor progression. Inflammatory cells, particularly neutrophils and monocytes, can promote tumor growth via secretion of angiogenic factors (e.g., VEGF), proteolytic enzymes, and reactive oxygen species, while lymphopenia is often associated with impaired antitumor immune responses50. Therefore, a high SIRI value, integrating elevated neutrophils and monocytes with decreased lymphocytes, may reflect both the tumor’s intrinsic biological aggressiveness and a host immune environment that is permissive to progression. This interplay between tumor biology (e.g., MDM2-driven oncogenesis) and host systemic inflammatory status offers a plausible explanation for the independent prognostic value of SIRI in PRPLS. Further mechanistic investigations and translational studies are warranted to elucidate the bidirectional communication between the systemic immune response and the retroperitoneal tumor microenvironment in this unique disease entity.
Limitations
Our study highlights the prognostic value of SIRI in PRPLS, but several limitations warrant further investigation. First, while we established a significant association between high SIRI and poor RFS, the molecular mechanisms linking systemic inflammation to tumor progression remain unclear. Future studies should focus on identifying specific inflammatory mediators and immune cell interactions, particularly the roles of neutrophils, monocytes, and platelets in tumor behavior. Although PRPLS is a rare malignancy, the relatively small sample size (n = 122) may reduce the statistical power for detecting certain subgroup effects or confounder interactions. Multicenter, prospective studies with larger, more diverse cohorts are necessary to validate the prognostic utility of SIRI and strengthen external validity. Third, although we adjusted for multiple clinicopathological factors in our multivariate analysis, potential unmeasured or residual confounding cannot be excluded. In particular, systemic inflammation markers such as SIRI may be influenced by underlying comorbidities, including hepatic dysfunction, infections, or autoimmune disorders. For example, liver impairment can independently affect circulating neutrophil and monocyte counts, acting either as a mediator of systemic inflammation or a confounder of the association between SIRI and oncologic outcomes. Future studies should account for these clinical variables through more detailed biochemical assessments and stratified analyses. Finally, due to the retrospective nature and the recent treatment timeframe of the included cohort (2021–2024), the median follow-up period was limited to 16 months. While this may restrict the assessment of long-term recurrence, the majority of early recurrences, particularly in high-risk patients, typically occur within the first 12–24 months postoperatively. Therefore, the follow-up period remains meaningful for identifying early prognostic signals.
Conclusions
In conclusion, this study demonstrates that SIRI is a reliable prognostic biomarker for PRPLS after surgical resection, independently predicting recurrence-free survival. High SIRI correlates with aggressive tumor features, systemic inflammation, and impaired liver function, reflecting a complex interaction between the tumor and the immune system. These findings not only enhance our understanding of PRPLS prognosis but also provide important insights for clinical practice and future therapeutic strategies.
Data availability
The dataset generated and analysed during the current study is available from the corresponding author on reasonable request.
Abbreviations
- WHO:
-
World Health Organization
- OS:
-
overall survival
- SIRI:
-
Systemic Inflammation Response Index
- NLR:
-
neutrophil-to-lymphocyte ratio
- PLR:
-
platelet-to-lymphocyte ratio
- MLR:
-
monocyte-to-lymphocyte ratio
- RFS:
-
recurrence-free survival
- ROC:
-
receiver operating characteristic
References
Lee, A. T. J., Thway, K., Huang, P. H. & Jones, R. L. Clinical and molecular spectrum of liposarcoma. J. Clin. Oncol. 2, 151–159. https://doi.org/10.1200/jco.2017.74.9598 (2018).
Singer, S., Antonescu, C. R., Riedel, E. & Brennan, M. F. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann. Surg. 3 https://doi.org/10.1097/01.sla.0000086542.11899.38 (2003). 358 – 70; discussion 70 – 1.
Van De Rijn, M. & Fletcher, J. A. Genetics of soft tissue tumors. Annu. Rev. Pathol. https://doi.org/10.1146/annurev.pathol.1.110304.100052 (2006). 435 – 66.
Italiano, A. et al. HMGA2 is the partner of MDM2 in well-differentiated and dedifferentiated liposarcomas whereas CDK4 belongs to a distinct inconsistent amplicon. Int. J. Cancer. 10, 2233–2241. https://doi.org/10.1002/ijc.23380 (2008).
Schwarzbach, M. H. & Hohenberger, P. Current concepts in the management of retroperitoneal soft tissue sarcoma. Recent. Results Cancer Res. 301 – 19. https://doi.org/10.1007/978-3-540-77960-5_19 (2009).
Ferrario, T. & Karakousis, C. P. Retroperitoneal sarcomas: grade and survival. Arch. Surg. 3, 248–251. https://doi.org/10.1001/archsurg.138.3.248 (2003).
Raut, C. P. & Pisters, P. W. Retroperitoneal sarcomas: Combined-modality treatment approaches. J. Surg. Oncol. 1, 81–87. https://doi.org/10.1002/jso.20543 (2006).
Anaya, D. A. et al. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decision-making. Ann. Surg. 1, 137–142. https://doi.org/10.1097/SLA.0b013e3181928f2f (2009).
Wang, S., Han, X., Liu, S., Xu, G. & Li, J. Primary retroperitoneal liposarcoma: a rare case report. J. Int. Med. Res. 12, 3000605211063085. https://doi.org/10.1177/03000605211063085 (2021).
Osmanağaoğlu, M. A., Bozkaya, H., Ozeren, M. & Cobanoğlu, U. Primary retroperitoneal liposarcoma. Eur. J. Obstet. Gynecol. Reprod. Biol. https://doi.org/10.1016/s0301-2115(02)00484-0 (2003). 2: 228 – 30.
Chen, Y., Jin, M., Shao, Y. & Xu, G. Prognostic value of the systemic inflammation response index in patients with adenocarcinoma of the oesophagogastric junction: A propensity Score-Matched analysis. Dis. Markers. 4659048. https://doi.org/10.1155/2019/4659048 (2019).
Qi, Q. et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 14, 2158–2167. https://doi.org/10.1002/cncr.30057 (2016).
Cao, Y. et al. Levels of systemic inflammation response index are correlated with tumor-associated bacteria in colorectal cancer. Cell. Death Dis. 1, 69. https://doi.org/10.1038/s41419-023-05602-9 (2023).
Song, M. et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J. Cachexia Sarcopenia Muscle. 5, 2504–2514. https://doi.org/10.1002/jcsm.13032 (2022).
Zhu, M. et al. The systemic inflammation response index as an independent predictor of survival in breast cancer patients: A retrospective study. Front. Mol. Biosci. 856064. https://doi.org/10.3389/fmolb.2022.856064 (2022).
Ding, Y. et al. Predictive effect of the systemic inflammation response index (SIRI) on the efficacy and prognosis of neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. BMC Surg. 1, 89. https://doi.org/10.1186/s12893-024-02384-5 (2024).
Zhao, M. et al. Sarcopenia and systemic inflammation response index predict response to systemic therapy for hepatocellular carcinoma and are associated with immune cells. Front. Oncol. 854096. https://doi.org/10.3389/fonc.2022.854096 (2022).
Wang, P. et al. Systemic inflammation influences the prognosis of patients with radically resected non-small cell lung cancer and correlates with the immunosuppressive microenvironment. Int. J. Cancer. 4, 826–842. https://doi.org/10.1002/ijc.34547 (2023).
Yan, Y. et al. Prognostic value of inflammatory and nutritional indicators in Non-Metastatic soft tissue sarcomas. J. Inflamm. Res. https://doi.org/10.2147/jir.S501079 (2025)., 1941-50.
Imai, T. et al. Predicting trabectedin efficacy in soft tissue sarcoma: inflammatory biomarker analysis. Anticancer Res. 5, 2125–2132. https://doi.org/10.21873/anticanres.17018 (2024).
Tuzimek, A., Dziedzic, E. A., Beck, J. & Kochman, W. Correlations between acute coronary syndrome and novel inflammatory markers (Systemic Immune-Inflammation index, systemic inflammation response index, and aggregate index of systemic inflammation) in patients with and without diabetes or prediabetes. J. Inflamm. Res. 2623–2632. https://doi.org/10.2147/jir.S454117 (2024).
Templeton, A. J. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J. Natl. Cancer Inst. 6, dju124. https://doi.org/10.1093/jnci/dju124 (2014).
Liu, J. et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J. Clin. Lab. Anal. 8, e22964. https://doi.org/10.1002/jcla.22964 (2019).
Ma, X. et al. Nomogram based on Monocyte-to-Lymphocyte ratio to predict survival of unresectable esophageal squamous cell carcinoma who receive First-Line PD-1/PD-L1 inhibitors combined with chemotherapy. Curr. Oncol. 11, 8937–8954. https://doi.org/10.3390/curroncol29110702 (2022).
Giese, M. A., Hind, L. E. & Huttenlocher, A. Neutrophil plasticity in the tumor microenvironment. Blood 20, 2159–2167. https://doi.org/10.1182/blood-2018-11-844548 (2019).
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: N1 versus N2 TAN. Cancer Cell. 3, 183–194. https://doi.org/10.1016/j.ccr.2009.06.017 (2009).
Mantovani, A. The yin-yang of tumor-associated neutrophils. Cancer Cell. 3, 173–174. https://doi.org/10.1016/j.ccr.2009.08.014 (2009).
Ribatti, D. A double-edged sword in tumor angiogenesis and progression. Dual roles of mast cells, macrophages, and neutrophils. Pathol. Res. Pract. 154167. https://doi.org/10.1016/j.prp.2022.154167 (2022).
Zhao, L. et al. Impacts and mechanisms of metabolic reprogramming of tumor microenvironment for immunotherapy in gastric cancer. Cell. Death Dis. 4, 378. https://doi.org/10.1038/s41419-022-04821-w (2022).
Gareau, A. J. et al. Ticagrelor inhibits platelet-tumor cell interactions and metastasis in human and murine breast cancer. Clin. Exp. Metastasis. 1–2, 25–35. https://doi.org/10.1007/s10585-018-9874-1 (2018).
Schlesinger, M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 1, 125. https://doi.org/10.1186/s13045-018-0669-2 (2018).
Lu, M. et al. Platelets promote primary hepatocellular carcinoma metastasis through TGF-β1-mediated cancer cell autophagy. Cancer Lett. 217161. https://doi.org/10.1016/j.canlet.2024.217161 (2024).
Pan, S. et al. Platelet-derived PDGF promotes the invasion and metastasis of cholangiocarcinoma by upregulating MMP2/MMP9 expression and inducing EMT via the p38/MAPK signalling pathway. Am. J. Transl Res. 7, 3577–3595 (2020).
Ortiz-Otero, N., Marshall, J. R., Lash, B. W. & King, M. R. Platelet mediated TRAIL delivery for efficiently targeting Circulating tumor cells. Nanoscale Adv. 9, 3942–3953. https://doi.org/10.1039/d0na00271b (2020).
Qian, B. Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 1, 39–51. https://doi.org/10.1016/j.cell.2010.03.014 (2010).
Boutilier, A. J. & Elsawa, S. F. Macrophage polarization States in the tumor microenvironment. Int. J. Mol. Sci. 13 https://doi.org/10.3390/ijms22136995 (2021).
Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 11, 549–555. https://doi.org/10.1016/s1471-4906(02)02302-5 (2002).
Mantovani, A. & Locati, M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler. Thromb. Vasc Biol. 7, 1478–1483. https://doi.org/10.1161/atvbaha.113.300168 (2013).
Xu, Y. et al. TRAF2 promotes M2-polarized tumor-associated macrophage infiltration, angiogenesis and cancer progression by inhibiting autophagy in clear cell renal cell carcinoma. J. Exp. Clin. Cancer Res. 1, 159. https://doi.org/10.1186/s13046-023-02742-w (2023).
Basak, U. et al. Tumor-associated macrophages: an effective player of the tumor microenvironment. Front. Immunol. 1295257. https://doi.org/10.3389/fimmu.2023.1295257 (2023).
Ehlting, C., Wolf, S. D. & Bode, J. G. Acute-phase protein synthesis: a key feature of innate immune functions of the liver. Biol. Chem. 9, 1129–1145. https://doi.org/10.1515/hsz-2021-0209 (2021).
Robinson, M. W., Harmon, C. & O’farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 3, 267–276. https://doi.org/10.1038/cmi.2016.3 (2016).
Errani, C. et al. The Prognostic Value of the Serum Level of C-Reactive Protein for Survival of Children with Ewing’s Sarcoma. Cancers (Basel). 5 https://doi.org/10.3390/cancers15051573 (2023).
Wang, X., Liu, S., Zhao, X., Fang, E. & Zhao, X. The value of C-reactive protein as an independent prognostic indicator for disease-specific survival in patients with soft tissue sarcoma: A meta-analysis. PLoS One. 7, e0219215. https://doi.org/10.1371/journal.pone.0219215 (2019).
Menon, S. S., Guruvayoorappan, C., Sakthivel, K. M. & Rasmi, R. R. Ki-67 protein as a tumour proliferation marker. Clin. Chim. Acta. 39–45. https://doi.org/10.1016/j.cca.2019.01.011 (2019).
Somaiah, N. & Tap, W. MDM2-p53 in liposarcoma: the need for targeted therapies with novel mechanisms of action. Cancer Treat. Rev. 102668. https://doi.org/10.1016/j.ctrv.2023.102668 (2024).
Alaseem, A. M. Advancements in MDM2 inhibition: clinical and pre-clinical investigations of combination therapeutic regimens. Saudi Pharm. J. 10, 101790. https://doi.org/10.1016/j.jsps.2023.101790 (2023).
Zhou, L. et al. LincRNA-p21 knockdown reversed tumor-associated macrophages function by promoting MDM2 to antagonize* p53 activation and alleviate breast cancer development. Cancer Immunol. Immunother. 5, 835–846. https://doi.org/10.1007/s00262-020-02511-0 (2020).
Li, Y. et al. Tumor microenvironment promotes lymphatic metastasis of cervical cancer: its mechanisms and clinical implications. Front. Oncol. 1114042. https://doi.org/10.3389/fonc.2023.1114042 (2023).
Ozel, I. et al. The good, the bad, and the ugly: neutrophils, angiogenesis, and cancer. Cancers (Basel). 3 https://doi.org/10.3390/cancers14030536 (2022).
Funding
This research was supported by the Peking University International Hospital Research Fund (No. YN2021ZD04).
Author information
Authors and Affiliations
Contributions
Conceptualization: Chengli Miao.Data curation: Weida Chen and Wenqing Liu.Formal analysis: Weida Chen and Wenqing Liu.Investigation: Weida Chen and Wenqing Liu.Methodology: Weida Chen and Wenqing Liu.Resources: Chengli Miao.Software: Weida Chen and Wenqing Liu.Supervision: Chengli Miao.Validation: Weida Chen and Wenqing Liu.Visualization: Weida Chen and Wenqing Liu.Writing – original draft: Weida Chen and Wenqing Liu.Writing – review & editing: Chengli Miao.All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Peking University International Hospital. Written informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, W., Liu, W. & Miao, C. The association of the systemic inflammation response index with the prognosis of primary retroperitoneal liposarcoma after surgical resection. Sci Rep 15, 31884 (2025). https://doi.org/10.1038/s41598-025-17128-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17128-5