Abstract
Urban expansion is a global driver of wildlife decline, with cities encroaching into forested areas, leading among other things to habitat loss and fragmentation. This can result in remnant forest patches becoming isolated within anthropogenic mosaics, trapping wildlife unable to move beyond the urban areas. The city of Moyobamba, capital of Peru’s San Martin region, population has increased rapidly since 1970 and is currently estimated at ~ 90,000 people. Moyobamba’s urban forest patches are home to the endemic and Critically Endangered San Martin titi monkey (Plecturocebus oenanthe). We conducted an 18-month survey of primates in 23 urban forest patches to determine the presence of P. oenanthe, evaluate the patch-specific variables influencing its presence, and estimate its population size and density in the remaining green areas of Moyobamba city. Plecturocebus oenanthe was present in 17 patches and was never detected outside of patches. Using triangulation we estimate 26 groups of P. oenanthe in Moyobamba, with an average occupied-patch density of 26.9/km2. The number of groups per patch was positively correlated with patch area (p = 0.023) and distance between patches (p = 0.0004), whereas presence and density were not correlated with any other landscape metrics, patch size, or habitat structure. Our results demonstrate that urban forests can support important populations of primates, particularly endemic and threatened species. Therefore, remnant forest patches and green corridors should be prioritized in urban planning strategies to ensure long-term wildlife persistence in rapidly developing landscapes.
Similar content being viewed by others
Introduction
Globally, rapid urban expansion has led to reductions in natural habitats, and in some cases left remnant populations of wildlife persisting in urban environments, and in other cases wild species have colonized or recolonized urban areas, adapting to exploit novel resources1,2. Rapid rates of land conversion have led to an increase in negative interactions between humans and wildlife, such as crop foraging, hunting, anthropozoonotic disease transmission, electrocution on power lines, and automobile accidents3,4. And in urban environments this can also cause problems such as garbage-foraging, attack, and disease spread1,5,6,7. Some primates can adapt, at least in the short term, to anthropogenic habitat disturbance, surviving in peri-urban and urban environments8,9,10,11,12,13. Adaptation to anthropogenic landscapes can lead to changes in primate behavior and ecology14,15. Where provisioning occurs, either intentionally (e.g. food provisioning) or unintentionally (e.g. rubbish bins), primates are at risk of developing health problems due to nutritional deficiencies or excesses, and heightened risk of negative interactions with humans or domesticated animals, leading to bites, scratches or other injuries16. The persistence of primates in urban landscapes can in some cases provide benefits for local human populations, i.e. through the generation of income from tourism17. Research has only recently begun focusing on urban primates in South America, but in other regions some primates have thrived in anthropogenic landscapes for centuries, for example, bonnet and rhesus macaques in India11.
Peru holds the second largest area of Amazonian forest but has been losing this at a rate of > 100,000 ha annually, due to industrial development, agricultural expansion and urban growth18. The northern Amazonian region of San Martin is the most deforested in Peru19. Massive land conversion has been driven by the opening of the Carretera Marginal de la Selva starting in the 1970s. From 2001 to 2024, San Martín lost over 766 kha of tree cover, equivalent to a 16% decrease in just over two decades, mostly in primary forest, with 29 kha, or just 0.6%, of forest gain over the same period20. The region’s lowlands and flat valley floors have been the most affected. Most land cover occurs as an agriculture mixed landscape, with cattle ranching and rice, cacao and coffee production. Larger areas of remaining forests in the region are now limited to the steeper slopes of the Andean foothills less suited to extensive commercial agriculture, but which are increasingly being affected. Deforestation rates have increased alongside human populations, with a 10% increase between 2007 and 201721. Urban growth influences deforestation in neighboring rural areas, and population growth via birth or migration to urban areas results in sprawling urbanization22. The urban population of San Martin nearly doubled between 2000 and 2015, with the most significant increase in the region’s two largest cities, Tarapoto and Moyobamba23. San Martin is also one of the most primate-diverse regions of Peru with ˜20 species, four of which are endemic to Peru, and some sites with as many as seven species living in sympatry in remaining forest areas in relative proximity to towns and cities24.
The San Martin titi monkey (Plecturocebus oenanthe) is endemic to northern San Martin, Peru, primarily at elevations between ~ 200 and 1,000 m above sea level (a.s.l.)25,26,27. The species has a very restricted distribution in the Alto Mayo and western Huallaga river valleys, with an estimated maximum Extent of Occurrence (EOO) of ~ 12,000 km², of which at least 50% has been totally lost. Most remaining habitat is severely fragmented19. There are no reliable estimates of the number of P. oenanthe remaining in the wild, but the IUCN Red list classifies P. oenanthe as Critically Endangered based on an estimated population declined of ≥ 80% in the past 25 years due to habitat loss, fragmentation, and hunting28. Due to these high levels of anthropogenic habitat loss and forest fragmentation P. oenanthe is now found in many remnant forests, inhabiting patches as small as 2 ha29, and even in stands of just a dozen trees. Population density estimates of P. oenanthe vary widely between areas. In relatively large areas of forest, densities of 4-6.5 groups per km² have been found30, whereas in areas of small fragmented forests patches, much higher densities of 35–38 groups per km² have been found31.
We aimed to determine the presence of the Critically Endangered P. oenanthe, evaluate which patch-specific variables may affect its presence, and estimate its population size and density in the remaining green areas of Moyobamba city. This was done to help promote the conservation of the remaining forests in the city and to evaluate the ability of urban forests to support the persistence of threatened primate species.
Results
Population analysis
We detected two species of primate during surveys. Leotocebus leucogenys was detected in all patches surveyed, whereas P. oenanthe was only detected in 17 patches. We calculated a 95% site detection probability from 2 (1.7) visits for P. oenanthe (Supplemental Table 1). We calculated landscape level density for P. oenanthe, taking into account all 23 patches in our survey, and occupied-patch density by removing seven patches in which P. oenanthe was never detected, and one where after 10 visits we only detected a single individual on two occasions, thus representing a very low probability of contributing to the population. Titi monkeys were not detected on 11 out of 156 survey days. No calls were recorded before 06:05 h or continued later than 09:44 h. Average bout duration was 6 min 28 s (SD 7 min 51 s).
We tested the effects of the environmental variables for observations for which data were available: 1,451 records of temperature, cloudiness, and rainfall; 1,433 ambient noise records; and 992 humidity measurements. There was a negative relationship between call frequency and temperature (E = −2.460, SE = 0.772, p = 0.002, considering both day and patch, and E = −2.548, SE = 0.955, p = 0.008, and considering only considering day), as well as call frequency and ambient noise (Sum Sq = 12.82, Mean Sq = 12.823, F = 15.471, p = 0.000113; considering day and patch, and Sum Sq = 14.01, Mean Sq = 14.009, F = 14.323, p = 0.000221, only considering day). No relationship was found with cloudiness and call frequency (Supplemental Table 2). Model evaluation with non-parametric alternatives supported these findings (Supplemental Table 2).
The total area surveyed in Moyobamba was 147 ha, and mean area size was 13.4 (Min 1.5, Max, 34.72, Table 1). The total effective listening area covered was 125.9 ha, mean per patch 20.1 (Min 2.9, Max 34, Supplemental Table 3). No observer bias was found in daily detection rates (X² = 84, p = 0.301). Our dataset included 1,453 observations. Of these, 580 could be triangulated. To this we added 129 visual observations, and 192 reliable single observer detections, giving a total of 709 and 901 detections, respectively, which were plotted. As there was no significant difference between these data sets (W = 16, p = 0.8182), we chose to include reliable single observer detections in analyses. Using the Sigloc package we obtained 580 independent locations, which after cleaning was reduced to 345. This resulted in landscape density of 18.9 groups/km2 and 64.83 individuals km2 and an occupied-patch density of 26.18 groups/km2, and 90.03 individuals/km2. Individual area results are given in Table 1.
Habitat analysis
All habitat analyses included the 22 patches from our surveys for which we had data. Normality tests showed that detections per day, temperature, and density were not normally distributed (Supplemental Table 4). Several environmental variables were found to be correlated, including cloudiness and temperature (X² = 94.159, p < 0.0001), humidity and cloudiness (X² = 67.553, p < 0.0001), humidity and rainfall (X² = 44.158, p < 0.0001) humidity and temperature (z = −19.795, p < 0.0001) (Supplemental Table 5). Habitat characterization measurements were taken on a total of 989 trees. We found correlation among all the habitat variables (Supplemental Table 6). We built final models using temperature, cloudiness, and ambient noise as environmental variables, and DBH and tree height as habitat variables. The average habitat characteristics were: nearest neighbor distance between trees, 4.67 m; tree height, 9.66 m; trunk circumference, 80.4 cm; crown diameter, 5.77 m; DBH, 25.22 cm; basal area, 62.41 cm²; and crown coverage, 33.3 m². As these were all positively correlated, we chose to use DBH and height as variables in subsequent analysis. No significant differences were found between the 22 patches. Averages for individual patches are given in Table 1.
Using FRAGSTATS, overall class metrics were NP of 47, a mean ENN of 34.30 m, COHESION of 98.68, and an AI of 95.1. Full results are given in supplemental Table 7. The number of titi monkey groups per patch was positively correlated with patch area (E = −1.301, SE = 0.364, p = 0.023), while presence and densities were not (E = −0.101, SE = 0.038, p = 0.055 and E = 3.552, SE = 5.405, p = 0.511, respectively). We crosschecked the results of the GLM with non-parametric equivalents, which confirmed the results (Supplemental Table 8). Titi monkey presence and group density were not found to be related to the patch metrics (Supplemental Table 9). The number of groups per patch was positively related to CONTIG (E = 1.17E + 02, SE = 8.148, p = 0.000452), but not PROX or ENN (E = −1.43E-04, SE = 7.93E-04, p = 0.864 and E = −1.27E-01, SE = 6.97E-02, p = 0.128, respectively), and non-parametric alternatives confirmed the results (Supplemental Table 9). In addition, non-parametric results showed a relationship between titi monkey presence and CONTIG (X2 = 5.4, p = 0.02014). GLMs for forest composition showed no effects on the number of groups, group density, or species presence, but model assumptions could not be fully met and goodness of fit was low (Supplemental Table 10). Non-parametric tests showed weak relationships between tree height and the number of groups, group density, and presence (respectively: z = 2.140, p = 0.032; z = 2.022, p = 0.043; X2 = 5.4, p = 0.020, respectively).
Discussion
Continuing forest loss for farming and urban growth is threatening the persistence of the Critically Endangered Plecturocebus oenanthe and the integrity of remaining habitat in San Martin32. With increasing human population growth and further fragmentation and encroachment on forests globally, urban and peri-urban habitats are of increasing importance and can provide refuge for this and other species2. This highlights the importance of reducing potential conflict with local human populations. Based on occupied-patch density we estimated the presence of 30 groups of P. oenanthe in Moyobamba. Assuming true absences where we did not detect the species, means the city has potential to hold a larger population of the species. We did not investigate the direct reasons for absence of the species from these areas in this study, although recent historic anthropogenic causes such as hunting, predation by domestic animals, and disease transfer commonly effect the species in the area33,34,35. It could also be that there were no P. oenanthe present at the time of patch isolation and the species has not yet been able to successfully recolonize these areas. Conversely, L. leucogenys was detected in all patches and residents reported often seeing them crossing non-forest urban areas terrestrially or via non-natural substrates such as across power lines and roof-tops. The use of non-forest areas was never observed or reported by local residents for P. oenanthe. The ability to use artificial substrates allows for migration and recolonization between patches in urban areas, apparently restricting P. oenanthe to remaining natural habitat. Thus, once extirpated, it is unlikely that the species will subsequently be able to recolonize a fragment without assistance. The ability of P. oenanthe to recolonize areas has been recorded previously, but this was after assisted regeneration of secondary forest, where the animals could access the area across natural substrates36.
We expected to find a relationship between patch metrics and P. oenanthe presence and densities, but none of the patch habitat variables we compared in the survey appear to influence group density. This could be explained by the high aggregation index we obtained. Even though the patches we surveyed are separated from larger rural forest areas, the urban patches are still close in relation to each other. We did find that the number of groups per patch was positively correlated with high contiguity between patches, suggesting that they can move between patches where partial connectivity is maintained with natural aerial substrates such as overhanging branches and vines across paths and roads, or through gardens, possibly allowing for the recolonization of areas from which they had been extirpated, or for the colonization of new areas if habitat becomes available. The creation of green corridors connecting fragments could be included in city planning, as has been recommended for the pied tamarin in Manaus, Brazil, which increases inter-patch mobility reducing the need for artificial substrate use10,37.
In previous studies on the species, Plecturocebus oenanthe’s presence and density has been shown to be at least in part determined by habitat type. A preference for edge and border forest has been suggested by several authors24,26,38, with much lower densities found in interior forests when compared to adjacent forest edge, but in another study lower densities were found in secondary as compared to primary forests30. The small fragment sizes and similarities in habitat characteristics across patches in Moyobamba make similar comparisons impossible, however these characteristics and our density estimates, which fall in the central range of previous estimates for the species, suggest that these patches hold suitable remnant forest habitat. Access to a particular food resource could be a limiting factor in the species’ persistence/presence in isolated patches. Further study identifying possible differences in tree species composition may highlight important differences between habitats where P. oenanthe were detected or not in Moyobamba. Fruit generally makes up at least half of the species dietary intake29, although this has been found to be as low as 39% in one previous study in a forest patch on the outskirts of the Moyobamba39. Primary forest areas have been shown to be favored over disturbed and edge forest in other titi monkeys40,41. Van Kuijk et al.30 suggested that the higher densities of P. oenanthe they found in border areas between secondary and primary forest may be attributed to the loss of outlying habitat, forcing groups to migrate, thus artificially inflating densities in border areas. Although our study took place in urban forests, the habitat metrics we collected are similar to those from studies in nearby rural areas36,42, suggesting that remaining habitat in the city has maintained structural similarity to its original state. Natural forest edge, for example river banks, have been suggested as preferred habitat for P. oenanthe24, although other studies have found no specific habitat preference26. Artificially inflated densities and short-term persistence in secondary and edge forest could mask the effects of anthropogenic disturbance, with increased new growth and edge effects in disturbed habitats mimicking conditions of natural regrowth and populations carrying an extinction debt, persisting for the short term until effects such as resource scarcity or inbreeding become sufficiently pronounced43.
Population density estimates for P. oenanthe vary widely with habitat type and size. In rural patches, higher densities of 35–38 groups/km2, have been reported29, and conversely, at extensive forest sites, much lower densities than we found, of between 12 and 49.5 individuals/km2, have been reported, in interior and edge forests, respectively30. This variation is mirrored across the genus, with estimates of between 6.4 and 9.5 individuals/km2 for Callicebus personatus in the Atlantic forest of Brazil44 and up to 80–100 individuals/km2 of C. nigrifrons, in montane Atlantic forest in Brazil45. The omnivorous diet, small body size and monogamous social system of titi monkeys may allow for a level of adaptability to conditions in different habitat types, with individual characteristics and pressures at each site determining densities. Although this adaptability alone may not be enough to ensure long term persistence46,47.
Urban and peri-urban environments can increase stress factors for wildlife, including the increased ambient noise from traffic, industry and music or loudspeakers48. This may be particularly important in highly vocal species, where noise pollution can impact communication, territorial defense and mating49. We found that P. oenanthe call frequency was negatively correlated with ambient noise. Changes in vocalization-related behavior appear to correspond with stress in some primates. Gómez-Espinosa et al.50 found that male mantled howler monkeys Alouatta palliata vocalized more often and were more vigilant in the presence of high-sound pressure level anthropogenic noise (e.g. aerial traffic). At the same site, increased intensity of anthropogenic noise resulted in increased fecal glucocorticoid metabolites and behavioral stress responses, suggesting a relationship between calling frequency and stress levels51. Behaviorally, red howler monkeys in Ecuador were found to avoid vocalizing near a lake with heavy motorboat traffic52. Increased incidences of anthropogenic noise pollution has also been found to affect vocalization frequency of the Mexican mantled howler monkey (Alouatta palliata) and black-fronted titi monkeys (Callicebus nigrifrons)50,53. A previous study found P. oenanthe to be absent from areas with high levels of auditory disturbance, such as chainsaws and gunshots30. Ambient noise could be a factor in the persistence and recolonization of patches in Moyobamba by P. oenanthe, with areas with greater noise being avoided and audio disturbance impeding migration, mating, and territorial defense. As L. leucogenys do not share the use of long distance vocalizations, ambient noise could have a less pronounced effect. However, more frequent vocalizing and changes in vocalizations have been observed in black-tufted marmosets (Callithrix penicillata) in proximity to mining, with higher levels of anthropogenic noise disturbance54. Although our listening points were positioned to ensure coverage within the critical listening distance for titi monkeys, in some cases the relatively large distance between them meant that we may have failed to detect some distant calls due to ambient noise. Further research is needed on species’ persistence and welfare within human-dominated environments. For example, what is the relationship between persistence and levels of ambient noise in and around forest and urban fragments? What insights can a similar examination of fecal glucocorticoid metabolites provide into stress and welfare?
Primate species distributions and behavior are partially determined by climate and can be affected by changes in weather, both over the short term, and by changes in long term weather patterns30. Increased rainfall has been shown to alter the activity budget of P. oenanthe, specifically reducing time spent resting and vocalizing30,31. Similar findings have been shown for northern buff-cheeked crested gibbons (Nomascus annamensis), where increased wind speed reduced frequency of vocalizations55. We found that higher temperatures resulted in less calling. Various studies have investigated the relationship between ambient temperature and calling behavior. Some primates have been found to vocalize more often as the temperature increases, for example indris and chimpanzees56,57. Smaller patches, and urban environments, with greater relative areas of edge forest have higher temperatures58, all of which are set to be compounded by predicted climate change and global heating. Climate change is also predicted to lead to temperature increases and changes in rainfall frequency, negatively affecting Amazonian primates in conjunction with shifting habitat conditions and anthropogenic forest loss, with previous studies highlighting up to ~ 99% of current P. oenanthe habitat lost under business-as-usual models19,59.
Fragmentation increases access for hunters and domestic predators, and when it occurs in close proximity to humans and domesticated animals, it can lead to increased likelihood of anthropozoonotic disease transfer or death or injury from power lines or road traffic accidents3,4. These factors can lead to the extirpation of primates from remaining habitat. Instances of P. oenanthe being hunted for food and as pets have been recorded, and opportunistic hunting still occasionally occurs within Moyobamba26,29. Our regular contact with local residents and government authorities suggests that little conflict exists with P. oenanthe due to positive perceptions of the species and lack of incidences of opportunistic foraging in houses or shops. On the other hand, L. leucogenys is known to enter residences and cause damage, which occasionally leads to retaliatory actions. This suggests that in addition to negative interactions and local perceptions, a species’ ability to use non-habitat areas within cities, and to recolonize patches, are important considerations in designing management and mitigation strategies. The use of un-occupied patches, in combination with additional connectivity, could be used for reintroductions/releases of P. oenanthe individuals seized by the authorities from the illegal wildlife trade, where the species is commonly recorded34,35. If done properly this could both supplement the urban population of the species and provide an ethical solution to re-homing captive animals rather than in rescue centers60.
Current conservation initiatives for P. oenanthe and urban habitat in Moyobamba focus on public education through workshops, fetes, murals, and exhibitions to increase local knowledge on the species and to generate empathy and pride. Education campaigns also aim to reduce opportunistic hunting and capture of wildlife and reduce contamination of patches which are often used as illegal tipping sites. Local businesses are beginning to use the species’ presence to promote tourism, taking birdwatchers and other tourists to see them, thus providing an economic interest in the species’ persistence. Most of these projects are run by local and national NGOs and government agencies. The district municipality is promoting the conservation of these remaining patches through the creation of locally managed protected areas (Ordenanza Municipal No 607-MPC, 2024), to stem encroachment of surrounding houses and evict land invaders/traffickers. Encroachment of buildings onto these patches is of particular worry to local government and residents as many of the patches are on steep gullies and provide the city with natural drainage. Construction and infilling often causes subsidence and flooding where invasions have occurred. The municipality has also begun reforestation within the patches, but thus far no plans have been made to increase connectivity between them with either artificial or natural substrates (or natural substitutes such as hemp rope).
Many questions remain as to what allows species to persist, and even thrive, in an urban environment. Species’ intrinsic traits, such as dietary flexibility, level of terrestriality, and gregariousness all likely play a role2, whereas other traits, such as body size and social structure are less clear. In our study, the two primates surviving in Moyobamba are both small species (0.35–1 kg), whereas in Africa and Asia it is the much larger baboons, langurs, macaques and vervet monkeys, which are often found in urban settings2,11,61. Landscape characteristics will also play a role, with matrix quality, permeability and travel/migration routes determining the ability to persist and recolonize areas. Finally, individual, local and cultural human tolerance of primates is a very important factor, even in cases where this leads to the creation or exacerbation of conflict, through provisioning for example2,11. In Brazil, capuchin monkeys have been observed to modify activity budgets to find energy-rich foods, which are often human-provided62. Many primate species that have historically coexisted with humans in urban landscapes are increasingly considered to be ‘pests’. Rhesus macaques, for example, are venerated in India, but at the same time conflict and persecution of the species are increasing due to negative encounters11,63. Species’ survival and coexistence with people is the result of a dynamic balance between species biology, landscapes, and human culture. Coexistence exists in varied forms, and there will be no one size fits all solution across species and locations11. A closer examination of the traits and conditions that allow some primate species to persist could help in developing effective strategies that benefit species like P. oenanthe. Although urban environments are less than ideal, ensuring coexistence is necessary given current realities of habitat loss and urban expansion.
With urban expansion continuing globally, and remaining forests being degraded and fragmented, the importance of investigating responses of threatened species to urban and peri-urban habitats cannot be understated. Between 1993 and 2007 the population of San Martin increased by 300%21, attributed to incentivized settlement programs and the improvement of road networks - a pattern repeated across the tropics. Conservation action is needed to protect fragmented and urban wildlife populations. Stricter enforcement of anti-trafficking laws, along with conservation education, is needed to reduce pressure on species living in anthropized environments. Maintenance of remaining habitat can help ensure species persistence, while recuperation of habitat and reconnection of fragmented areas can help populations recover64. Plecturocebus oenanthe is not found in any regional or national government-protected areas, and only receives habitat protection from local community and private initiatives28. As a species with a very restricted range in one of the most deforested parts of Peru, fully understanding which processes led to the species’ extirpation or persistence in Moyobamba’s remaining forest patches could be key for designing and implementing strategies for recolonization of available habitat within the city and beyond. Similarly, this understanding will be of benefit to and be benefited by management experience for other species coexisting with humans in urban settings.
Methods
Study site
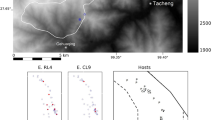
The city of Moyobamba is the capital of San Martin region in northern Peru (76°58’25.438"W, 6°2’17.772"S), and lies on the eastern foothills of the Andes at an elevation of 860 m.a.s.l. (Fig. 1). Founded in 1540, the city remained fairly isolated and inaccessible until the construction of the PE-5 N highway (Carretera Fernando Belaúnde Terry) at the end of last century. Since the opening of the highway the city has grown substantially, with a current population of over 50,000 (2017 census), and it now covers an urban area of approximately 10 km². The city includes many green spaces of highly disturbed primary and secondary forest vegetation, of varying sizes, which have been constantly reduced in area due to the city’s growth outwards and the increased concentration of construction (Fig. 2). Two species of primate, both endemic to Peru, have been confirmed to persist within the city limits: the Andean saddle-back tamarin (Leontocebus leucogenys) and the San Martin titi monkey (Plecturocebus oenanthe). Historically primate diversity would have been much higher, with up to seven sympatric primate species documented in larger forests nearby24.
Map of the city of Moyobamba showing urban forest patches surveyed during this study, with listening areas used in triangulation marked and areas used for density calculations highlighted. Map made by S. Shanee in ArcMAP 10.1 (https://www.esri.com/en-us/arcgis/geospatial-platform/overview).
Satellite image time series showing urban growth between 1970 (left), 2005 (center) and 2021 (right). Figure made by S. Shanee using images downloaded from Google Earth Pro (https://earth.google.com/web/, Image sources: U.S. Geological Survey, 1970; © 2025 Maxar technologies, 2005; and © 2025 CNES/Airbus).
Field surveys
Through prior identification of ‘green spaces’ within and surrounding the city, using satellite images in Google Earth, we identified sites for surveys (Fig. 1). We made preliminary visits to all patches, carrying out ad hoc searches for titi monkeys, and asking local residents if they knew whether primates were present, and if so, which species. Patches were defined as units of remnant primary or secondary forests, physically separated from other patches by open space at ground level, buildings, and/or roads/paths. In some cases, patches still maintained partial functional connectivity through areas of overhanging canopy, powerlines, or other aerial infrastructure. Partial connectivity was assessed visually on the ground, but not quantified. In these cases, we divided the larger areas into sections as forest patch listening areas based on ground level separation/connection and to ensure the best possible coverage (Supplemental Fig. 1), with listening points within the critical hearing distance. We then re-combined them again for density analyses (Table 1).
We used triangulation of vocalizations to estimate the density of P. oenanthe, as it is a highly vocal species. For triangulation we established two listening points on opposite sides of each patch. Listening points were separated by an average of ~ 240 m (min 120 m, max 410 m). Seven pairs of listening points were over 250 m apart, but we ensured that at least one listening point was within the critical hearing distance of 250 m of all parts of each patch, based on studies of P. moloch65. As much as possible, listening points were selected with clear coverage across patches, avoiding physical barriers which could block the sound of titi monkey song during triangulation.
Triangulation took place between February 2020 and June 2021; no triangulation took place between the 13th March and 29th May 2021 due to COVID restrictions. We collected data between 6:00 and 9:30 am, or until the last vocalization was heard, alternating between patches and ensuring that neighboring patches were never sampled on consecutive days. During surveys we recorded the compass bearing and the estimated distance to each vocalization from both listening points. We noted the start and end time of each vocal bout, to help differentiate between calls from different groups. We used an estimated travel distance of 150 m over 5 min from maximum daily travel estimate of 1400 m to distinguish calls from the same group in two different locations based on time and distance between events36,66. We recorded P. oenanthe group size and composition opportunistically upon visual encounters. We also noted weather conditions (rain, cloud, temperature, humidity). In order to compare whether detection or vocalization rates varied depending on anthropogenic audio disturbance, we also noted ambient noise levels per day. We surveyed each patch between 5 and 15 times. The mean number of days between surveys was 67 (min 1, max 385, SE 5), surveys took place during every month except august due to limited availability of personnel. If a patch was surveyed 5 times without detecting the species’ presence we discontinued surveys and treated this as an absence. We used Presence program V2.15.1867 to estimate detection probability (P) we ran two pre-defined single season models, with constant probability and survey-specific probability, with no covariables. We repeated this with data for the first 5 visits only, and then for all site visits (Supplemental Table 1). From this we estimated the number of site visits needed to confidently detect (95%) the species using:
Where Nmin is the estimated minimum number of site visits needed to confidently detect the species and p is the averaged detection probability across models.
Habitat characterization
We used point quarter sampling to characterize habitat in each of the sampled patches. We placed habitat sampling points at listening points when located in the interior of the patches (Four were located on the border due to limitations of access), and within each patch, ensuring that they were minimum 50 m apart to ensure no trees were sampled twice at subsequent points. In each quadrant we sampled the nearest tree to the point, and its nearest neighbor within the same quadrant, thus giving up to 142 sample points and eight samples per point68. We included all trees of DBH (diameter at breast height, ~ 150 cm) ≥ 10 cm. For each tree sampled we recorded DBH, height, crown spread (average of the maximum and minimum extensions on perpendicular axes), and distance to nearest tree. We did not identify the tree species sampled. If no trees of DBH ≥ 10 cm were present < 30 m from the point we did not sample vegetation in that quadrant.
Preparatory mapping
We made a two-class (patches and matrix) raster of Moyobamba, and polygons of the patch borders by drawing around visible green spaces based on satellite images, and ground truthed these with handheld GPS units. Effective listening distance was calculated as a circle of diameter 250 m around each listening point. These were then merged and clipped to within the bounds of the patch polygons. We used this final area as sample coverage from which to estimate densities. All mapping and geographic analyses were made in ArcMap 10.7.169 except where stated.
Data analysis
All statistical analyses were performed in R v.4.0.370, unless stated below. All data were tested to check for errors and outliers, removing any incomplete or erroneous records which could not be corrected post-hoc (i.e. incorrectly noted bearings, typos in data sheets, missing information) and detections falling outside estimated sample area. To check for observer bias we tested the average number of calls reported per day by each observer. We also tested for correlations among the reported values for independent environmental variables (temperature, humidity, cloud cover, rain, ambient noise) using Kendall rank correlation coefficient and chi-squared tests.
We mapped call locations using the Sigloc package in R v.3.5.071, intersecting bearings and distances of recorded calls from each listening point. Triangulated points were based on comparisons of call time and duration, treating calls heard > 5 min apart as independent events36. We then added visual observations to the maps of triangulated data. We also included some locations detected from only one listening point when the detection was within the effective listening area of that point and clearly discernible. We calculated the number of groups detected and estimated group density for the data with and without single observer detections, and tested for differences between them (Mann-Whitney U). Detection locations were overlaid on the effective sample areas to remove any remaining outliers.
We estimated the density per patch using the formula:
where n is the average number of groups heard per patch during the survey days, E is total effective listening area72. The correction factor p(m) was calculated using the formula:
where m is the number of survey days for patch X, p(1) is the probability of singing for each day calculated for each patch, given by the average number of groups heard per day/the highest number of groups heard per day.
We calculated individual density based on the mean group size for each patch, based on the number of individuals counted during visual detections. We did not calculate individual densities from sightings as not all counts were complete and, as a monogamous species, group and individual densities will be highly correlated.
We compared titi monkey presence and densities between patches based on habitat, patch, and matrix characteristics using FRAGSTATS73. We carried out our analysis using the default ‘no sampling’ settings to calculate patch metrics of contiguity (CONTIG), proximity (PROX), and Euclidean nearest neighbor distances (ENN). For classes, we calculated the number of patches (NP), mean Euclidean nearest neighbor (ENN_MN) distance, cohesion index (COHESION), and aggregation index (AI). We employed an eight-cell rule for neighborhood size. CONTIG gives the connectedness within the analyzed patch as a positive value between 0 (low contiguity) and 1 (high contiguity). PROX is and index of the presence of similar patches close to the analyzed patch. Higher values indicate the presence of a higher number and closer proximity of similar patches, and that there is greater contiguity between these patches73. ENN indicates the distance to the nearest neighboring patch of the same type. NP is the number of patches of the corresponding patch type. Low COHESION values indicate that the patch is subdivided and less physically connected while higher values, up to 100, represent a less fragmented landscape. Aggregation index indicates aggregation of patch types, with lower values indicating disaggregation (higher values indicate greater aggregation)73.
Before testing any statistical model, we assessed the normality of the continuous variables (Shapiro-Wilk) and transformed the variable where necessary. We constructed three generalized linear models (GLMs) to investigate the influence of patch size on group density, number of groups per patch, and titi monkey presence in a given patch (family-Gamma, Gamma, Binomial, respectively). We tested group, rather than individual, density, and number of groups per patch, as we didn’t complete group counts for the majority of observations, and as pair living primates, there is little variation in group size. We tested four GLMs to assess the influence of the patch metrics obtained from FRAGSTAT on area: group density; number of groups; and presence (family-Gaussian, Gaussian, Gaussian, Binomial). We followed the same methodology to examine the influence of forest composition on group density, number of groups per patch, and titi monkey presence in a given patch, constructing three more GLMs (family-Gaussian, Gaussian, Binomial). We considered the mean of each of the chosen metrics (DBH and Height) collected for the composition of each forest patch as independent variables. To test the assumptions of the GLMs, we performed Durbin-Watson tests for autocorrelation, and visually inspected the distribution of residuals, using Q-Q plots and plotting the response variable with the residuals.
We examined the influence of environmental variables on the timing and frequency of vocalizations, using the number of calls recorded each day from each listening point. We tested the effect of temperature on the number of calls collected, by performing two GLM models (both family-Gaussian). When necessary, we normalized variables using a cube root transformation. First, we compared the mean temperature and total number of calls each day in each patch. Second, we compared the mean temperature and number of calls each day, without considering patch. To examine the effect of ambient noise and weather/cloud cover on vocalizations, we used two way ANOVAs, firstly modelling the number of calls detected each day for each patch with cloud cover and noise (Binomial) and then number of calls each day without considering patch. We tested the assumptions of homoscedasticity with a Bartlet test.
As the goodness of fit for some models was low, and for others sample sizes were small, we also repeated all models with non-parametric equivalents. We used Kruskal-Wallis tests as alternatives to the ANOVA models. Because presence was binomial, we also used Kruskal-Wallis tests for models where presence was the dependent variable. For all other models, we used Kendall rank correlation coefficient.
Data availability
No datasets were generated or analysed during the current study.
References
Nurul Huda Aslari, E., Badrul Munir, M. Z. & Norlinda, M. D. Waste exploitation and nuisance behaviour of Macaca fascicularis (long-tailed macaque) in an urban environment. Mamm. Study. 49, 229–238. https://doi.org/10.3106/ms2023-0057 (2024).
Thatcher, H. R., Downs, C. T. & Koyama, N. F. in Primates in Anthropogenic Landscapes: Exploring Primate Behavioural Flexibility Across Human Contexts (eds Tracie McKinney, Siân Waters, & Michelle A. Rodrigues) 121–137Springer International Publishing, (2023).
Hockings, K. J. in The Int. Encyclopedia Primatology (ed Fuentes, A.), 1–8 John Wiley & Sons (2016).
Humle, T. & Hill, C. M. in In an Introduction To Primate Conservation. 219–240 (eds Wich, S. A. & Marshall, A. J.) (Oxford University Press, 2016).
Lousa, T. C., Reis, C. M., Vasquez, M. R. & Mendes, C. and F. D. Disputes over feeding resources provoke agonistic interactions between capuchins and humans in Brasília National Park, Brazil. Anthrozoös 37, 1051–1065, (2024). https://doi.org/10.1080/08927936.2024.2412407
Mansell, N. L. & McKinney, T. Interactions between humans and Panamanian white-faced capuchin monkeys (Cebus imitator). Int. J. Primatol. 42, 548–562. https://doi.org/10.1007/s10764-021-00218-2 (2021).
Suwanpakdee, S. et al. Potential zoonotic infections transmitted by free-ranging macaques in human–monkey conflict areas in Thailand. Zoonoses Public. Health. 72, 349–358. https://doi.org/10.1111/zph.13211 (2025).
Bicca-Marques, J. C. in The Int. Encyclopedia Primatology(ed Fuentes, A.) 1–5 John Wiley & Sons (2016).
Gordo, M., Calleia, F. O., Vasconcelos, S. A., Leite, J. J. F. & Ferrari, S. F. in Primates in Fragments: Complexity and Resilience (eds Laura K. Marsh & Colin A. Chapman) 357–370Springer New York, (2013).
Lindshield, S. M. in In Ethnoprimatology: Primate Conservation in the 21st Century. 351–369 (eds Waller, M. T.) (Springer International Publishing, 2016).
Radhakrishna, S., Anand, S. & Sengupta, A. Differing trajectories: Understanding coexistence through the human–macaque interface in India. Int. J. Primatol. https://doi.org/10.1007/s10764-025-00485-3 (2025).
Rodrigo, C. P. et al. The urban monkeys program: A survey of Alouatta clamitans in the south of Aorto Alegre and its influence on land use policy between 1997 and 2007. Primate Conservation 11–19, (2010). https://doi.org/10.1896/052.025.0103 (2010).
Sinha, A. & Vijayakrishnan, S. in The Int. Encyclopedia Primatology(ed Fuentes, A.) 1–5 John Wiley & Sons (2017).
Hansen, M. F., Kaburu, S. S. K., Morrow, K. S. & Maréchal, L. In Primates in Anthropogenic Landscapes: Exploring Primate Behavioural Flexibility across Human Contexts183–201 (eds T. McKinney, T & Waters, S.) (Springer International Publishing, 2023).
Riley, C. M. et al. in In Ethnoprimatology: Primate Conservation in the 21st Century. 283–300 (eds Waller, M. T.) (Springer International Publishing, 2016).
Pontzer, H. The provisioned primate: patterns of obesity across lemurs, monkeys, apes and humans. Philosophical Trans. Royal Soc. B: Biol. Sci. 378, 20220218. https://doi.org/10.1098/rstb.2022.0218 (2023).
Franquesa-Soler, M., Spaan, D., Hernández-Jaramillo, A. & Andresen, E. Citizen’s perceptions of urban black howler monkeys (Alouatta pigra) in the City of Palenque (Mexico): A case study to aid policy decisions. Int. J. Primatol. 44, 357–376. https://doi.org/10.1007/s10764-022-00339-2 (2023).
Bosques, P. Programa Nacional De Conservación De Bosques Para La Mitigación Del Cambio Climatico (Lima, 2015).
Shanee, S. et al. Threat analysis of forest fragmentation and degradation for Peruvian primates. Diversity 15, 276 (2023).
Watch, G. F. World Resources Institute open data porta, (2020). http://data.globalforestwatch.org/datasets/mining-concessions
INEI & San Martin Resultados Definitivos Tomo 1 Características de la Población. 1352 (Instituto Nacional de Estadística e Informática, Lima, Peru, (2018).
Pauchard, A. & Barbosa, O. in In Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities: A Global Assessment. 589–608 (eds Elmqvist, T.) (Springer Netherlands, 2013).
CEIC. Peru Population: Urban: San Martín, < (2023). https://www.ceicdata.com/en/peru/population-by-region/population-urban-san-martn
Shanee, S., Allgas, N., Ocampo-Carvajal, C. & Shanee, N. A high-diversity primate community in a mid-elevation flooded forest, the Jungla de Los Monos community reserve, Peru. Primates https://doi.org/10.1007/s10329-020-00833-2 (2020).
Aquino, R. & Encarnación, F. Los primates Del Peru. Primate Rep. 40, 1–130 (1994).
Bóveda-Penalba, A., Vermeer, J., Rodrigo, F. & Guerra-Vásquez, F. Preliminary report on the distribution of (Callicebus oenanthe) on the Eastern feet of the Andes. Int. J. Primatol. 30, 467–480. https://doi.org/10.1007/s10764-009-9353-2 (2009).
Shanee, S., Tello-Alvarado, J. C. & Vermeer, J. Boveda-Penalba, A. J. GIS risk assessment and GAP analysis for the Andean Titi monkey (Callicebus oenanthe). Primate Conserv. 26, 17–23 (2011).
Vermeer, J. & Shanee, S. Plecturocebus oenanthe (San Martin titi monkey), (2020). https://www.iucnredlist.org/species/3553/17975319
Shanee, S., Aldrich, B. C., Pacheco, V. & Serrano-Villavicencio, J. E. Callicebus oenanthe (Primates: Pitheciidae). Mamm. Species 56, seae002, (2024). https://doi.org/10.1093/mspecies/seae002
van Kuijk, S. M., García-Suikkanen, C., Tello-Alvarado, J. C., Vermeer, J. & Hill, C. M. Estimating population density of the San Martin Titi monkey (Callicebus oenanthe) in Peru using vocalisations. Folia Primatol. 86, 525–533 (2015).
Aldrich, B. C. A song-based survey of the Andean titi monkey (Callicebus oenanthe) at Taranque, with notes on its vocalizations. MSc thesis, Oxford Brookes University, (2006).
Schaffer-Smith, D., Swenson, J. J. & Bóveda-Penalba, A. J. Rapid conservation assessment for endangered species using habitat connectivity models. Environ. Conserv. 43, 221–230. https://doi.org/10.1017/S0376892915000405 (2016).
Gómez-Puerta, L. A., López-Urbina, M. T. & González, A. E. Occurrence of tapeworm Bertiella mucronata (Cestoda: Anoplocephalidae) in the Titi monkey callicebus oenanthe from peru: new definitive host and geographical record. Vet. Parasitol. 163, 161–163. https://doi.org/10.1016/j.vetpar.2009.04.008 (2009).
Shanee, N., Mendoza, A. P. & Shanee, S. Diagnostic overview of the illegal trade in primates and law enforcement in Peru. Am. J. Primatol. 79, e22516. https://doi.org/10.1002/ajp.22516 (2017).
Shanee, N. Trends in local wildlife hunting, trade and control in the tropical Andes hotspot, Northeastern Peru. Endanger. Species Res. 19, 177–186 (2012).
Allgas, N. et al. Natural re-establishment of a population of a critically endangered primate in a secondary forest: the San Martin titi monkey (Plecturocebus oenanthe) at the Pucunucho Private Conservation Area, Peru. Primates, 1–8, (2016). https://doi.org/10.1007/s10329-016-0581-8
Barr, S. Conservation Efforts for Pied Tamarins (Saguinus bicolor) - Evaluating Ecological Corridors for Restoring the Forest Fragments of Urban Manaus, Brazil Msc thesis, Lund University, (2015).
DeLuycker, A. M. Insect prey foraging strategies in Callicebus oenanthe in Northern Peru. Am. J. Primatol. 74, 450–461. https://doi.org/10.1002/ajp.22002 (2012).
DeLuycker, A. M. The ecology and behavior of the Rio Mayo titi monkey (Callicabus oenanthe) in the Alto Mayo, Northern Peru Ph.D thesis, Washington University in St Louis, (2007).
Heiduck, S. The use of disturbed and undisturbed forest by masked Titi monkeys Callicebus personatus melanochir is proportional to food availability. Oryx 36, 133–139. https://doi.org/10.1017/S0030605302000200 (2002).
Kulp, J. & Heymann, E. W. Ranging, activity budget, and diet composition of red Titi monkeys (Callicebus cupreus) in primary forest and forest edge. Primates 56, 273–278. https://doi.org/10.1007/s10329-015-0471-5 (2015).
DeLuycker, A. M. Diet and feeding ecology of the critically endangered San Martín Titi monkey (Plecturocebus oenanthe) in Peru. Int. J. Primatol. https://doi.org/10.1007/s10764-021-00256-w (2021).
Wearn, O. R., Reuman, D. C. & Ewers, R. M. Extinction debt and windows of conservation opportunity in the Brazilian Amazon. Science 337, 228–232. https://doi.org/10.1126/science.1219013 (2012).
Chiarello, A. G. & de Melo, F. R. Primate population densities and sizes in Atlantic forest remnants of Northern Espírito santo, Brazil. Int. J. Primatol. 22, 379–396. https://doi.org/10.1023/A:1010751527749 (2001).
Caser, C. Anti-predator behaviour of black-fronted titi monkeys (Callicebus nigrifrons) PhD thesis, University of St Andrews, (2012).
Eppley, T. M., Santini, L., Tinsman, J. C. & Donati, G. Do functional traits offset the effects of fragmentation? The case of large-bodied diurnal Lemur species. Am. J. Primatol. 82, e23104. https://doi.org/10.1002/ajp.23104 (2020).
Lootvoet, A. C., Philippon, J. & Bessa-Gomes, C. Behavioral correlates of primates conservation status: intrinsic vulnerability to anthropogenic threats. PLOS ONE. 10, e0135585. https://doi.org/10.1371/journal.pone.0135585 (2015).
Shannon, G. et al. A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 91, 982–1005. https://doi.org/10.1111/brv.12207 (2016).
Duquette, C. A., Loss, S. R. & Hovick, T. J. A meta-analysis of the influence of anthropogenic noise on terrestrial wildlife communication strategies. J. Appl. Ecol. 58, 1112–1121. https://doi.org/10.1111/1365-2664.13880 (2021).
Gómez-Espinosa, E., Dias, P. A. D. & Rangel-Negrín, A. The influence of anthropogenic noise on the behavior of male mantled howler monkeys. Am. J. Primatol. 84, e23377. https://doi.org/10.1002/ajp.23377 (2022).
Dias, P. A. D., Gómez Espinosa, E. E. & Chavira Ramírez, D. R. Rangel negrín, A. Noise intensity modulates the responses of mantled howler monkeys to anthropophony. Am. J. Primatol. 86, e23568. https://doi.org/10.1002/ajp.23568 (2024).
de la Torre, S., Snowdon, C. T. & Bejarano, M. Preliminary study of the effects of ecotourism and human traffic on the howling behavior of red howler monkeys, Alouatta seniculus, in Ecuadorian Amazonia. Neotropical Primates. 7, 84–86 (1999).
Duarte, M. H. L., Kaizer, M. C., Young, R. J., Rodrigues, M. & Sousa-Lima, R. S. Mining noise affects loud call structures and emission patterns of wild black-fronted Titi monkeys. Primates 59, 89–97. https://doi.org/10.1007/s10329-017-0629-4 (2018).
Bittencourt, E., Vasconcellos, A. D., Sousa-Lima, R. S., Young, R. J. & Duarte, M. H. Acoustic monitoring of black-tufted marmosets in a tropical forest disturbed by mining noise. Animals 13, 352 (2023).
Williams, J. Wind suppresses calling in Northern buff-cheeked crested Gibbons (Nomascus annamensis). The Hum. Voyage 1, 1–15 (2017).
Piel, A. K. Temporal patterns of chimpanzee loud calls in the Issa valley, tanzania: evidence of nocturnal acoustic behavior in wild chimpanzees. Am. J. Phys. Anthropol. 166, 530–540. https://doi.org/10.1002/ajpa.23609 (2018).
Ferrario, V. et al. Singing in the rain! Climate constraints on the occurrence of indri’s song. Am. J. Primatol. 86, e23673. https://doi.org/10.1002/ajp.23673 (2024).
Ewers, R. M. & Banks-Leite, C. Fragmentation impairs the microclimate buffering effect of tropical forests. PLOS ONE. 8, e58093. https://doi.org/10.1371/journal.pone.0058093 (2013).
Sales, L., Ribeiro, B. R., Chapman, C. A. & Loyola, R. Multiple dimensions of climate change on the distribution of Amazon primates. Perspect. Ecol. Conserv. 18, 83–90. https://doi.org/10.1016/j.pecon.2020.03.001 (2020).
Mitman, S. et al. Challenges to IUCN guideline implementation in the rehabilitation and release of trafficked primates in Peru. Primate Conserv. 35, 87–102 (2021).
Mormile, J. E., Hill, C. M. & and Living with urban baboons: exploring attitudes and their implications for local baboon conservation and management in knysna, South Africa. Hum. Dimensions Wildl. 22, 99–109. https://doi.org/10.1080/10871209.2016.1255919 (2017).
Back, J. P., Suzin, A. & Aguiar, L. M. Activity budget and social behavior of urban capuchin monkeys, Sapajus sp. (Primates: Cebidae). Zoologia 36, 1–10 (2019).
Saraswat, R., Sinha, A. & Radhakrishna, S. A god becomes a pest? Human-rhesus macaque interactions in Himachal pradesh, Northern India. Eur. J. Wildl. Res. 61, 435–443. https://doi.org/10.1007/s10344-015-0913-9 (2015).
Alcocer-Rodríguez, M. et al. Evaluating extinction debt in fragmented forests: the rapid recovery of a critically endangered primate. Anim. Conserv. 24, 432–444. https://doi.org/10.1111/acv.12648 (2021).
Robinson, J. G. Vocal regulation of inter- and intragroup spacing during boundary encounters in the Titi monkey, Callicebus moloch. Primates 22, 161–172. https://doi.org/10.1007/bf02382607 (1981).
Kinsey, W. G. in In Ecology and Behavior of Neotropical Primates Volume 1. 241–276 (eds Coimbra-Filho, A. F. & Mittermeier, R. A.) (Academiz Brasileira de Ciencias, 1981).
PRESENCE -. Software To Estimate Patch Occupancy and Related Parameters (USGS-PWRC, 2006).
Krebs, C. J. Ecological Methodology 2nd edn (Benjamin Cummings, 1999).
ArcGIS Desktop v. 10.0 (Environmental Systems Research Institute, 2018).
R Core Team. R: a language and environment for statistical computing., (2019). http://www.R-project.org/
Berg, S. S. The package sigloc for the r software: A tool for triangulating transmitter locations in ground-based telemetry studies of wildlife populations. Bull. Ecol. Soc. Am. 96, 500–507. https://doi.org/10.1890/0012-9623-96.3.500 (2015).
Brockleman, W. Y. & Ali, R. in Primate Conservation in the Tropical Rainforest (eds C.W. Marsh & R.A. Mittermeier) 23–62Alan R. Liss, inc, (1987).
McGarigal, K. & Marks, B. J. FRAGSTATS: spatial pattern analysis program for quantifying landscape structure. Vol. 351 122US Department of Agriculture, Forest Service, Pacific Northwest Research Station, (1995).
Acknowledgements
We thank the editor and two anonymous reviewers for their helpful comments which greatly improved the manuscript. We are grateful to our students and interns who helped in field data collection, namely Brenda, Danitza, David, Edgar, James, Jer, Keylin, Liz, Mayra, Meritxell, Nicole, Raquel and Vanessa. This work was carried out under permits: RDG N° 173-2016-SERFOR-DGGSPFFS, RDG N° 213-2016-SERFOR, RDG N° 350-2017, and RDG N° 389-2019-MINAGRI-SERFOR-DGGSPFFS.
Funding
This work was funded by Neotropical Primate Conservation.
Author information
Authors and Affiliations
Contributions
SS, VV, JW, SK and BA wrote the main manuscript text and SS prepared figures. SS, ERRP, LF-H and NA led conception and design of the study. ERRP led field data acquisition and SS, ERRP and VV analyzed the data. All authors reviewed and revised the manuscript, and have approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shanee, S., Roque Perez, E.R., Vero, V. et al. A survey of critically endangered plecturocebus oenanthe in moyobamba’s urban forests, Peru. Sci Rep 15, 31172 (2025). https://doi.org/10.1038/s41598-025-17148-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17148-1