Abstract
Within the EAGLE population-based case-control study, the present study aims to integrate previous analyses which suggested an increased lung cancer risk associated with particulate matter ≤ 10 μm (PM10) exposure estimated 2–5 years before diagnosis (year 2000), by considering pollutant levels estimated 12–15 years before diagnosis (year 1990), i.e., in a potentially more relevant time window. Odds ratios (OR) and 95% confidence intervals (CI) were calculated through multivariate unconditional logistic regression. Mean PM10 levels were higher in 1990 than in 2000 (68 vs. 46.6 µg/m3). Contrary to what we previously observed, among 1,665 cases and 1,808 controls we found no association between 1990 PM10 concentrations and lung cancer risk (OR: 0.96, 95%CI: 0.75–1.24). We further confirmed this difference also considering exposure from the two years combined, mutually adjusting for one another (0.89, 0.87–1.00 in 1990 and 1.49, 1.06–2.08 in 2000). The observed differences might be related to several factors: distinct methodologies used to estimate exposure, coarser granularity of 1990 exposure estimates, dissimilar levels in pollutant concentrations. However, our results might also suggest a greater relevance of more recent exposures in the carcinogenic process, thus contributing to the intriguing hypothesis that air pollution might act as a promoter of cancer development.
Similar content being viewed by others
Introduction
Lung cancer is the leading cause of cancer morbidity and mortality worldwide, with almost 2.5 million new cases in 2022 which represent about 12% of all cancer diagnoses1. It is also the leading cause of cancer deaths globally (18.7%)2,having a poor prognosis, with a 5-year survival rate that tends to be below 20% in most countries3. Incidence and mortality for this cancer are very different between countries, depending mainly on the distribution of smoking habits across populations4. Tobacco smoking is by far the main risk factor for lung cancer, but other determinants do play an important role, including environmental tobacco smoke, many occupational agents, and radon decay products5. Investigations on two very large study populations (i.e., the American Cancer Society [ACS] cohort and the Harvard Six Cities study) found that also long-term exposure to air pollution could increase the risk for lung cancer mortality6,7,8,9. In the ACS cohort, exposure to particulate pollution was considered as the median of a 5-year period occurring one to ten years preceding death, while in the Harvard Six Cities study, 1-year to 5-year lagged moving averages before death were used. In 2013, the International Agency for Research on Cancer (IARC) classified outdoor air pollution as a Group 1 carcinogen based primarily on evidence for lung cancer10. After the IARC evaluation, other studies reported positive associations between air pollution and lung cancer. In 2014, a meta-analysis summarizing 18 studies (17 cohort studies and one case-control study, mostly from Europe and the USA) estimated meta-relative risks for lung cancer of 1.09 (95% confidence interval [95%CI]: 1.04–1.14) and 1.08 (95%CI: 1.00–1.17) associated to a 10 µg/m3 increase in concentrations of particulate matter with diameter ≤ 10 (PM10) and ≤ 2.5 μm (PM2.5), respectively11. These represent two different fractions of particulate pollution (with PM2.5 being itself a fraction of PM10) and, at least in urban areas, share most of the same sources (i.e., nonindustrial combustion and road transport)12. Kim et al., in 2018, conducted a meta-analysis including 30 cohort studies from the USA, Europe, and China. The summary relative risks (RR) for lung cancer mortality for 10 µg/m3 increase in PM10 and PM2.5 concentrations were 1.07 (95%CI: 1.03–1.11) and 1.14 (95%CI: 1.07–1.21), respectively13. A more recent meta-analysis selected 15 high quality studies published between 2004 and 2019 to summarize the evidence on the association between particulate matter and lung cancer risk. The authors estimated summary relative risks of 1.23 (95%CI: 1.05–1.40) and 1.16 (95%CI: 1.09–1.23) for an increase of 10 µg/m3 in PM10 and PM2.5 exposure, respectively14. Also a study based on a large US cohort of adults (i.e., 1.5 million subjects from the National Health Interview Surveys conducted in 1986–2014, with mortality linkage through 2015) pointed out the role of air pollution in lung cancer mortality (HR: 1.13, 95%CI: 1.00–1.26, per 10 µg/m3 increase in PM2.5)15.
We previously conducted a study to evaluate the association between exposure to PM10 and lung cancer risk within the Environment And Genetics in Lung cancer Etiology (EAGLE) population-based case-control study, performed in Lombardy, North-West Italy in 2002–2005. We found suggestive evidence of a positive association, with an odds ratio per 10 µg/m3 of 1.28 (95%CI: 0.95–1.72). Average PM10 levels for year 2000 (i.e., 2–5 years before diagnosis) were estimated at each residential address from models integrating land-use regression (LUR) methodologies and aerosol optical depth (AOD) data from the Moderate Resolution Imaging Spectroradiometer (MODIS) satellite16. Several solid tumors occur many years or decades after exposure to carcinogenic agents. Therefore, starting from our earlier study, we integrated it by reconstructing pollutant levels estimated 12–15 years before diagnosis (year 1990), i.e., in a potentially more relevant time window from an etiological point of view, with the aim to deepen the knowledge on the relationship between lung cancer risk and long-term exposure to PM10. We could not move further away in time because of the unavailability of data from emission sources before 1990.
Materials and methods
The EAGLE study
EAGLE is a population-based case-control study conducted to investigate genetic and environmental risk factors for lung cancer, for which details can be found at the National Cancer Institute website17. Briefly, 216 municipalities belonging to five Lombardy areas were included: Brescia, Milan, Monza, Pavia, and Varese (Fig. S1). Two-thousand and ninety-nine incident lung cancer cases (participation rate: 86.6%) were recruited from April 2002 to June 2005 among subjects admitted to 13 hospitals, mostly located in the Milan metropolitan area. Two-thousand one-hundred and twenty healthy controls (participation rate: 72.4%) were randomly selected from the general population and frequency matched to lung cancer cases by gender, area, and age. Subjects of the study were of both genders, resident in one of the 216 municipalities, and with age between 35 and 79 years18. Informed consent was obtained from all individual participants included in the study. All participants signed informed consent regarding publishing their data as aggregate results of the study (single participants are not identifiable). All methods were performed in accordance with the relevant guidelines and regulations.
For each study subject, information on factors associated with lung cancer, including smoking, dietary habits, and occupational history were collected, and blood samples were taken.
Subjects’ addresses were translated into spatial coordinates using the web tool GPS Visualizer19. Using QGIS20all subjects were subsequently georeferenced into their cell of residence and assigned the corresponding estimated PM10 annual average value for year 1990.
Exposure assessment
PM10 concentrations for the year 1990 were reconstructed by an Air Quality Modelling System (AQMS) based on the Flexible Air quality Regional Model (FARM)21,22that considers the processes involving pollutants in the atmosphere, e.g., chemical reactions, transport, dispersion and deposition23,24. A detailed description of the adopted AQMS is reported in the Supplementary Material (in particular, Fig. S2 reports the AQMS scheme). FARM is the main component of the MINNI system, one of the models contributing to the Copernicus regional air quality production systems. It has been validated and compared with other similar models by numerous verification activities21. The air quality simulations have been performed considering two nested computational domains covering Northern Italy (with 9 km grid spacing) and Lombardy region (with 3 km grid spacing) respectively (Fig. S3).
The FARM model was coupled with the meteorological model WRF, which is a consolidated reference meteorological modelling system25. WRF was applied on 3 nested computational domains (Fig. S4) covering continental Europe and central Mediterranean, Northern Italy and Lombardy respectively (Fig. S3). Model results have been compared with meteorological observations available in Lombardy for the target period to verify the reliability of the meteorological reconstruction (Fig. S5-S6).
The Italian (Istituto Superiore per la Protezione e la Ricerca Ambientale, 2022) and the MACCity (ECCAD) emission inventories have been processed by the Emission treatment sub-system (Fig. S2) to provide the time-varying emission data needed by FARM. Fig. S7 shows hourly NO emission fluxes for a January 1990 weekday at 8:00 AM over Northern Italy and Lombardy produced by the Emission treatment sub-system. The variation recorded by air pollutant emissions from 1990 to 2010 and the contribution attributable to the human activity sectors are illustrated in Fig. S8, highlighting the decline of energy and industrial production emissions due to the occurred de-industrialization and to industrial emissions control measures. While SO2 and NOX emissions have been reduced by about 89% and 48% respectively from 1990 to 2010, PM10 showed a 16% only reduction due to the increased contribution of non-industrial combustion (mainly due to wood burning for residential heating), partially compensating the decrease of emissions from the industrial and transport sectors. The air quality boundary conditions were provided by the System for Integrated modelling of Atmospheric composition (SILAM) continental scale retrospective air quality reconstruction26.
PM10 concentrations over the Lombardy region have been then computed and stored with hourly time resolution. Fig. S9 and S10 show the yearly average concentration field and the comparison between observed and predicted levels at selected stations, confirming the capability of the modelling system to reconstruct the seasonal variability of PM10 levels.
Mean annual PM10 levels for the year 2000, which have been used for comparison and adjustment (see below), had been estimated at each subject’s residential address through a hybrid spatial-temporal model that integrates a LUR model and satellite measurements of AOD27. A complete description of the methods already applied to the EAGLE study population can be found in Consonni et al.16.
Statistical analysis
Analyses were performed only on subjects with a complete history of tobacco smoking and who had not changed their residence since 1980. Odds ratios (OR) and 95% confidence intervals (CI) were calculated through multivariable unconditional logistic regression28,29. Adjustment variables were selected a priori, either because they were matching variables or because they were plausibly related to both the exposure and the outcome at study, thus acting as potential confounders. For each analysis, we fitted two main models with additional levels of adjustment. Model 1, adjusted for the matching variables: gender, age (categorical, nine levels) and area (categorical, five levels) education level (none, elementary, middle, high, university), and smoking variables: ever smoked cigarettes, number of pack-years (in linear, quadratic and cubic transformations), years since quitting (categorical, eight levels), ever smoking other types of tobacco (i.e. cigars, cigarillos, pipe), and exposure to environmental tobacco smoking (in childhood, at work, or within the family). Model 2, further adjusted for occupational exposure to carcinogens (asbestos, respirable crystalline silica, polycyclic aromatic hydrocarbons, diesel motors exhausts and nickel/chromium compounds) and other factors possibly associated with lung cancer (assumption of red and processed meat30,fruit and vegetables31. In the present study we report results from Model 2: those deriving from model 1 can be found in the Supplementary Material. Several steps of analysis were performed. First, the association between PM10 exposure and lung cancer risk was assessed using exposure estimates for the year 1990, also evaluating its potential for nonlinearity by using cubic splines, with knots at the 25th, 50th, and 75th percentiles of the pollutant distribution. As a sensitivity analysis, we also performed this model in never and former smokers only (trying to rule out the effect of the most relevant carcinogen we have information on) and verified effect modification between smoking status and PM10. Second, results from the first analysis were compared to those obtained using exposure estimates for the year 2000 and already published16. Third, the two sets of exposure values (i.e., annual means for 1990 and 2000) were considered together in the same model. In the above analyses, where exposure levels are considered as a continuous variable, results are expressed per 10 µg/m3 increase in PM10 concentration and reported for the entire study sample, the Milan area alone and the other four areas combined. Finally, to try to improve the resemblance between the two models used to estimate air pollution levels in the two time points, we rescaled the spatial resolution of the exposure estimates for the year 2000 (available at each subjects’ residential address) to the same 3 × 3 km grid used for the year 1990 (i.e., by averaging exposure values estimated in 2000 for each subject who resided within each 3 × 3 km cell), assigned the annual average of exposure in each cell to each study subject (according to his/her residential address), and re-ran the models performed to assess the association between PM10 and lung cancer risk in the year 2000.
Results
Out of 4,219 subjects (2,099 cases, 2,120 controls), information was available for 4,060 (1,944 cases, 2,116 controls). Sixty-seven subjects were eliminated, as seven of them (3 cases, 4 controls) lacked data on active cigarette smoking, and 60 (35 cases, 25 controls) had no information on passive smoking. From the remaining 3,993 subjects (1,905 cases, 2,088 controls), 3,473 (1,665 cases and 1,808 controls) satisfied our inclusion criteria (i.e., complete history of tobacco smoking and fixed residence since 1980). The subjects included (both cases and controls) were a bit older and had slightly lower education than the excluded ones. In general, there were no substantial differences between cases and controls comparing included vs. excluded subjects (Tables S1-S3).
Most subjects were resident in Milan (2,411), followed by Brescia (400), Varese (258), Monza (204) and Pavia (200). Compared to controls, cases had a higher proportion of males, were slightly older and had lower educational levels. They also had a higher percentage of current smokers (49.8% vs. 24.4%) and a higher number of mean cigarette pack-years. More than 90% of both cases and controls were exposed to secondhand smoking. Adenocarcinoma was the most common morphology (41.1%), followed by squamous cell carcinoma (26%). Most frequent lung cancer stages were IIIA and IV (Table 1).
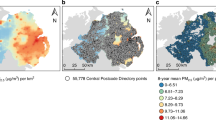
As regards air pollution exposure, annual 1990 average levels showed large differences when comparing Milan to the other areas (Fig. 1a), with a delta of about 30 µg/m3 between the first and second most polluted areas (80.7 vs. 53.3 µg/m3 as PM10 annual average). When looking at the pollutant mean levels for the year 2000, the different areas showed similar concentrations (Fig. 1b). Overall, median PM10 levels were higher in 1990 than in 2000 (79.1 vs. 47.8 µg/m3, p-value from Wilcoxon signed-rank test < 0.001), even if the distribution of the exposure between the two years was quite similar until the 25th percentile (47.7 in 1990 vs. 44.5 µg/m3 in 2000) (Table 2).
Distribution of PM10 concentrations by area, 1990 (a) and 2000 (b) (asterisk: adapted from Consonni et al.16).
No association was observed between PM10 concentrations in 1990 and lung cancer risk, neither in the overall population, nor separating Milan from the other areas (Table 3A). Analyses among never and former smokers (considered both as separate and conjunct categories) returned comparable findings (Tables S4-S6) and no formal interaction between smoking status and PM10 concentrations was detected. Even investigation of possible nonlinear associations did not show excess in risk (Fig. S11). These results differ from what had been observed considering exposure in 2000 (Table 3B), where increased risks were reported in both overall and stratified analyses. These differences were even more clear-cut in models considering the two years of exposure together (Table 3C): this was evident both in the entire population, where OR was 0.89 (95%CI: 0.87–1.00) in 1990 and 1.49 (1.06–2.08) in 2000, and when limiting the analysis to the Milan area [0.87 (0.76–1.00) vs. 1.87 (1.17–3.00), respectively].
Similar results were observed in models adjusting for fewer confounders (Table S7). Positive associations considering exposure in the year 2000 were observed also after rescaling PM10 estimates to a less fine spatial resolution (3 × 3 km, i.e., the same of the year 1990) (Table S8).
Discussion
In a large case-control study with thorough control of major confounders, we did not observe associations between lung cancer risk and air pollution values estimated as annual average for the year 1990, i.e., 12–15 years before diagnosis. Comparable findings were confirmed in the subgroup of never and former smokers. On the other hand, previously reported results in the same population had suggested an increased risk for exposures closer in time to the year of diagnosis (i.e., 2000)16. We further confirmed this difference not only by comparing the results from the two years but also by considering the two exposures combined, mutually adjusting for one another.
The observed differences might be related to several factors. First, distinct methodologies were used to estimate exposure: FARM chemical transport model was used to estimate PM10 in 199021 while PM10 estimates for the year 2000 were derived from a hybrid spatial-temporal model27. Second, the granularity of 1990 exposure estimates was coarser than in 2000. For this reason, we rescaled the spatial resolution of exposure estimates in 2000 to a coarser definition (3 × 3 km) and obtained results that were comparable to those from the old analysis. Third, dissimilar levels in pollutant concentrations in the two time periods might also justify the observed difference in air-pollution related lung cancer risk, although the direction of the association is counterintuitive: if we compare PM10 concentration levels, we observe that the average pollutant levels in 1990 were 46% higher than in 2000. This phenomenon is particularly evident in the Milan area, where PM10 levels markedly decrease in the timespan we are considering. Fourth, we might look at our results within the greater framework of tumorigenesis, which was proposed to occur as a two-step process: i.e., the first one inducing mutations in healthy cells, and the second one triggering cancer development32. Our results might thus support a greater relevance of more recent exposures, especially when considering the two exposures combined. To this regard, it is interesting to note that a recent paper by Hill and colleagues provided combined results from a plethora of observational, in vivo, and in vitro studies to support the hypothesis that “environmental particulate matter measuring ≤ 2.5 µm […] promotes lung cancer by acting on cells that harbour pre-existing oncogenic mutations in healthy lung tissue”33. In addition, in one of the studies they performed on about 250 female lung cancer cases, they observed an association with lung cancer risk when considering 3-year PM2.5 cumulative exposure, while this association was not evident after 20-year exposure to the same pollutant.
Several other studies investigated the association between long-term air pollution exposure and lung cancer. Starting from the studies evaluated by the IARC, Hamra et al. conducted a large meta-analysis including 18 studies from different countries in Europe and North America. Data on PM10 were available for nine studies (five in North America, three in Europe and one in New Zealand). Overall, a positive association was observed for PM10 (RR: 1.08; 95% CI: 1.00-1.17), with mean levels ranging from 8.3 to 51 µg/m11. Looking in more detail at the three studies conducted in Europe,
the Multicenter ESCAPE study investigated the effects of long-term PM10 exposure on lung cancer incidence, using data from 14 cohorts. The enrollment years ranged from 1985 to 2005. Air pollution concentrations at the baseline residential addresses of the study subjects were back-extrapolated using land-use regression models applied to pollution data collected between 2008 and 2011 (i.e., after the study period). Back-extrapolated PM10 levels ranged from about 5 to 90 µg/m3, with the highest levels estimated in Italy and Greece. The pooled analysis showed an association between the risk of lung cancer and increasing PM10 concentrations (HR: 1.22; 95% CI: 1.03–1.45 per 10 µg/m3)34.
The English study followed up a national cohort from 2003 to 2007 and observed positive associations for an interquartile range (IQR) increase in PM10 (HR: 1.03; 95% CI: 0.98–1.08), using exposure data in 2002 (1–5 years before death) and adjusting for smoking status, cigarettes/day and income35. PM10, estimated from emission-based models, ranged from 12.6 to 29.8 µg/m3.
The mortality of a small cohort of women enrolled between 1985 and 1994 was examined through 2008 in Germany. PM10 in the years 1985–1994 was derived from total suspended particles (TSP, applying a coefficient of 0.71) measured by fixed monitoring sites. An increase of 7 µg/m3 in PM10 was associated with an increased HR of 1.84 (95%CI: 1.23–2.74), in models adjusted for education and smoking status36.
Among the five US studies included in the meta-analysis by Hamra and colleagues, the Adventist Health Study on Smog (ASHMOG) followed up for cancer incidence (1977–1992) a large cohort of 6,338 non-smoking Californian adults. PM10 levels were derived from TSP from 1972 to 1987 and subsequently directly measured through fixed-site monitoring stations. PM10 ranged from 0 to 85 µg/m3 with mean values of 51.0 µg/m3. Incident lung cancer in males was positively associated with an IQR increase (24 µg/m3) in PM10 mean concentrations (RR: 5.21, 95%CI: 1.94–13.99, adjusted by cigarette pack-years, education, and alcohol)37.
A cohort study performed by the American Cancer Society within the Cancer Prevention Study (ACS-CPS-II) examined the association between air pollution and mortality among over 400,000 people enrolled in 1982–1998. Mean PM10 concentration derived from fixed monitoring stations in the enrollment period was 28.8 µg/m3. Unfortunately, numerical results for PM10 were not available, and a relative risk slightly greater than 1.00 in the period 1987–1996 (with 95%CI including the null value) could be derived only by looking at one of the paper figures38.
The association between PM10 exposure and cancer mortality (1985–2000) was investigated in about 40,000 workers employed in the U.S. trucking industry. PM10 annual average exposures were determined for 1985 through 2000 from a model using spatial smoothing and geographical information system-based covariates. The average exposure during the study period was 26.8 µg/m3. A HR for lung cancer of 1.08 (95%CI: 0.91–1.30) was estimated per 10 µg/m3. No data on smoking were available39.
The California teachers Study (TCS) involved a large cohort of women followed up for mortality (1997–2005)40. Monthly average PM10 concentrations were calculated from fixed-site monitors for the period 1996–2005. PM10 estimates ranged from 9.19 to 82.64 µg/m3 (mean: 29.21). The HR per 10 µg/m3, adjusted for smoking status, pack-years, body mass index, alcohol, passive smoking, dietary fat, fiber and calories, was 0.93 (95%CI: 0.81–1.07).
The relationship between lung cancer incidence and long-term residential exposure to PM10 was also evaluated within the Nurses’ Health Study, another large cohort of women followed up from 1994 to 201041. PM10 ranged from 3.17 to 74.79 µg/m3. Exposure was defined as the time-varying cumulative PM10 average experienced in the previous 72-months. The HR per 10 µg/m3, adjusted for income, (active and passive) smoking and dietary variables, was 1.04 (95%CI: 0.95–1.14).
Finally, in the New Zealand Census-Mortality study, records from the 1996 national census were probabilistically linked to mortality data (1996–1999)42. Average PM10 exposure at 1996 census were estimated using land use regression models and ranged from 0 to 30 µg/m3. The OR per 10 µg/m3 of PM10, adjusted for ethnicity, social deprivation, income, education, and smoking status was 1.14 (95%CI: 1.05–1.23) among subjects who had not changed residence since 1991.
In summary, the abovementioned studies return a heterogeneous picture, where different time windows of exposure are investigated, concentration levels cover a quite wide range of values, and results are often characterized by some uncertainty, notwithstanding the overall interpretation of the findings that point towards an increasing trend in risk.
Following the IARC evaluation other studies were published.
The Nation-wide Dutch Environmental Longitudinal Study (DUELS) examined the mortality (2004–2011) of a cohort of more than 7 million subjects43. Applying land-used regression models, annual mean PM10 concentrations in 2001 were derived. The median PM10 concentrations was 29 µg/m3. The HR, adjusted for age, sex, marital status, region of origin, household income, per 10 µg/m3 increase in PM10 was 1.26 (95%CI: 1.21–1.30). No information on smoking and diet was available.
In a multi-cohort Swedish study, high-resolution dispersion models were used to estimate annual mean concentrations of PM10 at individual address during each year of follow-up (1990–2011), and moving averages were calculated for the time-windows 1–5 years and 6–10 years preceding the outcomes of interest (i.e., natural and cause-specific deaths). No associations were observed between PM10 exposure (range: 6.3–41.9 µg/m3) and lung cancer mortality, in models adjusted for sex, calendar year, sub-cohort, smoking status, alcohol consumption, physical activity, marital status, socioeconomic status, educational level, and occupation44.
Moving to lung cancer incidence, several studies were conducted in South Korea. A large cohort study included more than 6.5 million subjects from the Korean National Health Insurance Service (NHIS) database for 2006–2007, who were linked to Korea Central Cancer Registry data to confirm lung cancer incidence for 2006–2013. Individual exposure to PM10 was assessed as five-year average concentrations predicted at subjects’ district-specific home addresses for 2002–2007 via a geo-statistical model (mean: 55.8 µg/m3). An increased risk in lung cancer incidence (comparing the upper vs. the lower quartile of exposure) was estimated only for adenocarcinoma in current male smokers (HR: 1.14, 95%CI: 1.03–1.25, in models adjusted for age, gender, income, BMI, pack-year, history of COPD, interstitial lung disease, lung disease due to external factor, physical activity and alcohol consumption)45. Another NHIS-based study was conducted in the Seoul metropolitan area, where about 83,000 people were followed up for 2007–2015. PM10 exposure was estimated predicting district-specific annual-average concentrations (mean: 56.2 µg/m3). HRs for lung cancer incidence ranged from 1.05 (95%CI: 0.81–1.36) to 1.14 (95%CI: 0.84–1.54) considering either a 1-year or 5-year exposure average, respectively46. A further update of this study was provided, taking also advantage of improved exposure assessment: about 2 million people without history of lung cancer in 2002–2006 were selected from the NHIS database. Lung cancer incident cases were identified up to 2016, and PM10 concentration levels were estimated as 5-year averages at subjects’ residential addresses geocoded on a 100-m grid through a prediction model. PM10 levels ranged from 28.6 to 100.7 µg/m3 and were not associated with increased lung cancer risk, regardless of the various adjustments applied, which included both individual and areal covariates47.
The potential of the UK Biobank was exploited to investigate whether air pollution and genetic factors could jointly contribute to incident lung cancer. Almost 460,000 participants aged 40–69 years, recruited between 2006 and 2010, and without previous cancer at baseline, were included. PM10 concentrations at residential address were estimated applying land-use regression models: estimates were available for years 2007 and 2010, and the average of these sets of values was used as mean exposure of the study participants (19.3 µg/m3). In the fully adjusted model (i.e., including age, sex, BMI, household income, education level, smoking status, and pack-years of smoking), a 10 µg/m3 increase in PM10 concentration was associated with an HR of 1.53 (95%CI: 1.20–1.96). Interestingly, subjects with a high “genetic risk” (based on gene polymorphisms) had a higher risk of incident lung cancer (HR: 1.77, 95%CI: 1.50–2.10)48. Another analysis on a smaller population selected from the UK biobank (N ≈ 367,000) tried to consider also the effect of lifestyle factors, and the association between PM10 exposure and lung cancer risk was smaller and slightly more uncertain (HR: 1.05, 95%CI:0.99–1.10 per IQR increase in PM10, in models adjusted for age, sex, race, educational level, household income, region, BMI, alcohol status, smoking status, physical activity, and diet)49.
The role of PM10 exposure on both mortality and hospitalization for lung cancer was investigated in a recent cohort study conducted in Guangzhou, China, where about 650,000 participants were recruited between 2009 and 2015, and followed up for an average of 8.2 years. Annual mean concentration of PM10 (58.8 µg/m3) was estimated using a satellite-based random forest algorithm. Applying a causal inference model, for every 1 µg/m3 increase in PM10 concentration the HR for mortality due to lung cancer was 1.032 (95%CI: 1.024–1.041), and that for hospitalization was 1.067 (95%CI: 1.020–1.115)50.
The only case-control study that we know of specifically investigating PM10 exposure and lung cancer was performed in South Korea51. Nine hundred and eight incident lung cancer cases were matched to population controls by sex, smoking status and age. PM10 exposure assessment was done using land-use regression models in a 20-year period preceding subjects’ enrollment and complete residential histories since 1995. PM10 levels ranged from 18.25 to 95.58 µg/m3. The OR adjusted for education, smoking status, education, exposure to occupational carcinogens, and fruit intake was 1.09 (95%CI: 0.96–1.23) per 10 µg/m3 of PM10. Compared to the EAGLE study, this investigation is limited by the poor characterization of information regarding smoking habits (i.e., only smoking status was available).
In summary, the evidence trying to link PM10 and lung cancer risk has been steadily growing and points towards the carcinogenic effects of this air pollutant. Nonetheless, uncertainties characterizing the various investigations must be acknowledged, probably related to the variability of the confounders considered in the analyses, the different methods applied for the exposure assessment, the wide range of the pollutant concentration values, and the different time windows investigated (very often close to the health event of interest).
To this regard, the role of exposure to air pollution 10 years before lung cancer diagnosis has been recently examined in a large Southern Californian cohort study including more than 45,000 participants aged 50 + years at enrollment. Lung cancer risk was estimated considering 5-year moving averages of ultrafine particles (UFP) exposure, lagged 10 years52. Interestingly, the authors found only a modest association between UFP exposure and lung cancer risk when considering the entire cohort (HR: 1.03, 95%CI: 0.99–1.08), while an increased risk was observed for adenocarcinoma when comparing most exposed vs. least exposed males (HR: 1.39, 95%CI: 1.05–1.85), and also in male participants who were oldest at the time of enrollment (HR: 1.13, 95%CI: 1.02–1.26). Notwithstanding the far smaller sample size of our study population and the difference in the considered pollutants (although combustion-derived ultrafine particles are numerous in urban PM1053), we tried to replicate the analyses by Jones and colleagues: however, no positive associations were observed neither when looking at adenocarcinoma in males, nor when further restricting our analysis to the same histological subtype among males aged 60 years or more at enrollment (results not shown). Possible explanations of these partially contrasting findings (apart from the obvious difference in sample size and pollutants considered among the two studies) might be the finer spatial resolution of exposure levels in the California study (LUR models to predict average levels at participant residential addresses) but also, on the other hand, the lack of control for important confounders such as occupational exposures, for which indeed we were able to control for.
Our study has several strengths. First, EAGLE is a population-based case-control study with a large number of enrolled subjects18. Many variables regarding residential history, smoking habits, and several others factors relevant for lung cancer were collected. This makes us confident of the quality of our data and allowed us to control for many potential confounders or risk factors that could bias the effect of air pollution in the analysis. Second, we assessed a past exposure to air pollution temporally located 12–15 years before the enrollment which, as far as we know, has rarely been evaluated. Third, we are dealing with exceptionally high values of air pollution concentrations: the mean PM10 estimate of exposure of our study population in 1990 was 68 µg/m3, i.e., more than four times the air quality guideline level recommended by the World Health Organization (15 µg/m3)54. Notably, we also had estimates of PM2.5 values in 1990. We did not focus our analyses on PM2.5 because our main aim was to make a comparison with our previous results regarding the year 2000, where only PM10 estimates were available. However, PM2.5 levels represented about 96% of PM10 values in our dataset. As such, the annual average PM2.5 level in our study population would roughly be 65 µg/m3, i.e., more than three times higher than the mean of the highest quartile of exposure in the study by Jones and colleagues (21.8 µg/m3). In any case, we replicated our main analysis considering PM2.5 exposure and found comparable results (Table S9). Fourth, by comparing exposures in two time points, we tried to address the insidious topic of temporality in cancer development.
This study has also limitations. First, the spatial distribution of exposure estimates in 1990 is very different from that in 2000. This is particularly evident in Milan, which has much higher PM10 values as compared with other areas. A second limitation regards the low spatial resolution of our exposure data. The model applied to estimate 1990 PM10 concentration levels used a 3 × 3 km grid, thus implying that all subjects localized within the same cell were assigned the exact same exposure value, possibly introducing a nondifferential misclassification of exposure. To this regard, we must also consider that about 70% of our study population was resident within the metropolitan city of Milan. These subjects all fall within four cells only: as such, in our analysis for the year 1990 most subjects share the same exposure. However, the sensitivity analysis where we rescaled exposure estimates for the year 2000 to the 3 × 3 km grid basically reconfirmed previous results obtained with exposure estimates at a much finer spatial resolution, suggesting that exposure granularity is not the main driver of the different findings. Finally, as already discussed, we are comparing different methodologies to estimate exposures in the two time points, which could affect results with their own uncertainties55.
Conclusions
In conclusion, in a large case-control study characterized by a vast set of information on individual confounders and conducted in a very polluted area of northern Italy, we did not observe an increased lung cancer risk when considering air pollution exposures 12–15 years before diagnosis. On the other hand, the positive association suggested for exposures to air pollution in a time point closer to diagnosis were confirmed (and strengthened) by analyses that were able to consider both past and more recent exposures at the same time. Our results thus might contribute to the intriguing hypothesis that air pollution could act as a promoter of cancer development, and stimulate further research aimed to deepen our knowledge on the biological role that air pollution could play in the carcinogenic process. Further research is required to verify this hypothesis: nonetheless, should it be confirmed, it would have groundbreaking public health implications, as interventions to reduce air pollution levels could have beneficial and relevant effects in a relatively short period of time (e.g., a few years).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Berg, C. D. et al. Air pollution and lung cancer: A review by international association for the study of lung cancer early detection and screening committee. J. Thorac. Oncol. https://doi.org/10.1016/J.JTHO.2023.05.024 (2023).
American Cancer Society. Cancer Facts and Figures 2018. (2018).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Gharibvand, L. et al. The association between ambient fine particulate air pollution and lung cancer incidence: results from the AHSMOG-2 study. Environ. Health Perspect. 125, 378–384 (2017).
Dockery, D. W. et al. An association between air pollution and mortality in six U.S. Cities. N. Engl. J. Med. 329, 1753–1759 (1993).
Krewski, D. et al. Mortality and Long-Term exposure to ambient air pollution: ongoing analyses based on the. J. Toxicol. Environ. Health A. 68, 1093–1109 (2005).
Lepeule, J., Laden, F., Dockery, D. & Schwartz, J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard six cities study from 1974 to 2009. Environ. Health Perspect. 120, 965–970 (2012).
Pope, C. A. et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. Adults. Am. J. Respir Crit. Care Med. 151, 669–674 (1995).
International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 109 - Outdoor Air Pollution. (2015).
Hamra, G. B. et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 122, 906–911 (2014). https://doi.org/10.1289/ehp.1408092
Eurostat. Glossary: Particulate matter - environment. (2025). https://tinyurl.com/349h3bfd.
Kim, H. B., Shim, J. Y., Park, B. & Lee, Y. J. Long-term exposure to air pollutants and cancer mortality: A meta-analysis of cohort studies. Int. J. Environ. Res. Public Health 15, 2608 (2018).
Ciabattini, M., Rizzello, E., Lucaroni, F., Palombi, L. & Boffetta, P. Systematic review and meta-analysis of recent high-quality studies on exposure to particulate matter and risk of lung cancer. Environ. Res. 196 (2021).
Pope, C. A. et al. Mortality risk and fine particulate air pollution in a large, representative cohort of U.S. adults. Environ. Health Perspect. 127 (2019).
Consonni, D. et al. Outdoor particulate matter (PM10) exposure and lung cancer risk in the EAGLE study. PLoS One 13 (2018).
National Cancer Institute - Division of Cancer Epidemiology and Genetics. EAGLE - Environment And Genetics in Lung cancer Etiology. https://dceg.cancer.gov/research/who-we-study/cancer-cases-controls/eagle-study. Last accessed June 2025 (2025).
Landi, M. T. et al. Environment and genetics in lung cancer etiology (EAGLE) study: An integrative population-based case-control study of lung cancer. BMC Public Health 8 (2008).
GPS Visualizer. https://www.gpsvisualizer.com. Last accessed June 2025.
QGIS Association. QGIS Geographic Information System. http://www.qgis.org. Last accessed June 2025.
Mircea, M. et al. Assessment of the AMS-MINNI system capabilities to simulate air quality over Italy for the calendar year 2005. Atmos. Environ. 84, 178–188 (2014).
Silibello, C. et al. Modelling of PM10 concentrations over Milano urban area using two aerosol modules. Environ. Model. Softw. 23, 333–343 (2008).
Bessagnet, B. et al. Presentation of the EURODELTA III intercomparison exercise-evaluation of the chemistry transport models’ performance on criteria pollutants and joint analysis with meteorology. Atmos. Chem. Phys. 16, 12667–12701 (2016).
Kukkonen, J. et al. A review of operational, regional-scale, chemical weather forecasting models in Europe. Atmos. Chem. Phys. 12, 1–87 (2012).
Skamarock, W. C. et al. A Description of the Advanced Research WRF Model Version 4.
Sofiev, M. et al. Springer,. A long-term re-analysis of atmospheric composition and air quality. In Springer Proceedings in Complexity. 55–59 (2018). https://doi.org/10.1007/978-3-319-57645-9_9
Nordio, F. et al. Estimating spatio-temporal resolved PM10 aerosol mass concentrations using MODIS satellite data and land use regression over lombardy, Italy. Atmos. Environ. 74, 227–236 (2013).
He, Z., Wang, Y., Kuo, C. L., Duan, Y. & Grady, J. Unconditional or conditional logistic regression model for age-matched case-control data? Front. Public Health6, 2 (2018).
Pearce, N. Analysis of matched case-control studies. BMJ (Online) 352 (2016).
Lam, K. et al. Intakes of red meat, processed meat, and meat mutagens increase lung cancer risk. (2009). https://doi.org/10.1158/0008-5472.CAN-08-3162
Lam, T. K. et al. Dietary quercetin, quercetin-gene interaction, metabolic gene expression in lung tissue and lung cancer risk. Carcinogenesis 31, 634–642 (2010).
Berenblum, I. & Shubik, P. A new, quantitative, approach to the study of the stages of chemical cartinogenesis in the mouse’s skin. Br. J. Cancer. 1, 381–393 (1947).
Hill, W. et al. Lung adenocarcinoma promotion by air pollutants. Nature 616, 159–167 (2023).
Raaschou-Nielsen, O. et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European study of cohorts for air pollution effects (ESCAPE). Lancet Oncol. 14, 813–822 (2013).
Carey, I. M. et al. Mortality associations with long-term exposure to outdoor air pollution in a National English Cohort. Am. J. Respir Crit. Care Med. 187, 1226–1233 (2013).
Heinrich, J. et al. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup. Environ. Med. 70, 179–186 (2013).
Beeson, W. L., Abbey, D. E. & Knutsen, S. F. Long-term concentrations of ambient air pollutants and incident lung cancer in California adults: results from the AHSMOG study.adventist health study on smog. Environ. Health Perspect. 106, 813–823 (1998).
Pope, C. A. 3rd et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287, 1132–1141 (2002).
Hart, J. E. et al. Long-term ambient multipollutant exposures and mortality. Am. J. Respir Crit. Care Med. 183, 73–78 (2011).
Lipsett, M. J. et al. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am. J. Respir Crit. Care Med. 184, 828–835 (2011).
Hales, S., Blakely, T. & Woodward, A. Air pollution and mortality in New Zealand: cohort study. J. Epidemiol. Community Health. 66, 468–473 (2012).
Fischer, P. H. et al. Air pollution and mortality in seven million adults: the Dutch Environmental Longitudinal Study (DUELS). Environ. Health Perspect. 123, 697–704 (2015).
Nilsson Sommar, J. et al. Long-term exposure to particulate air pollution and black carbon in relation to natural and cause-specific mortality: a multicohort study in Sweden. BMJ Open. 11, e046040 (2021).
Moon, D. H., Kwon, S. O., Kim, S. Y. & Kim, W. J. Air pollution and incidence of lung cancer by histological type in Korean adults: A Korean National health insurance service health examinee cohort study. Int J. Environ. Res. Public. Health 17 (2020).
Yang, S., Kim, O. J., Shin, M., Kim, W. J. & Kim, S. Y. Association between long-term exposure to high levels of ambient air pollution and incidence of lung cancer in a population-based cohort. Environ. Res. 198, 111214 (2021).
Park, J. et al. Long-term exposure to air pollution and lung cancer incidence: findings from improved exposure assessment and extended population. Cancer Causes Control 1–12. https://doi.org/10.1007/S10552-025-02010-6/FIGURES/2 (2025).
Huang, Y. et al. Air pollution, genetic factors, and the risk of lung cancer. A prospective study in the UK biobank. Am. J. Respir Crit. Care Med. 204, 817–825 (2021).
Liang, H. et al. Association of outdoor air pollution, lifestyle, genetic factors with the risk of lung cancer: A prospective cohort study. Environ. Res. 218, 114996 (2023).
Guo, T. et al. Potential causal links of long-term air pollution with lung cancer incidence: from the perspectives of mortality and hospital admission in a large cohort study in Southern China. Int. J. Cancer. 154, 251–260 (2024).
Lamichhane, D. K. et al. Lung cancer risk and residential exposure to air pollution: A Korean Population-Based Case-Control study. Yonsei Med. J. 58, 1111 (2017).
Jones, R. R. et al. Outdoor ultrafine particulate matter and risk of lung cancer in Southern California. Am. J. Respir Crit. Care Med. https://doi.org/10.1164/RCCM.202305-0902OC (2023).
MacNee, W. & Donaldson, K. Mechanism of lung injury caused by PM10 and ultrafine particles with special reference to COPD. Eur. Respir. J. 21, 47s–51s (2003).
Organización Mundial de la Salud (OMS). WHO Global Air Quality Guidelines. Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. 1–360. (2021).
Holnicki, P. & Nahorski, Z. Emission data uncertainty in urban air quality Modeling—Case study. Environ. Model. Assess. 20, 583–597 (2015).
Acknowledgements
This work is part of L. Fedrizzi’s dissertation project for the Epidemiology Master Program, University of Turin, Italy. We thank Mikhail Sofiev and the Finnish Meteorological Institute for providing results of their long-term re-analysis of atmospheric composition and air quality at continental scale.
Funding
This work was partially supported by: the Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Division of Cancer Epidemiology and Genetics, Bethesda, Maryland, USA; the Lombardy Regional Government (research grant “Regione Lombardia Ricerca Indipendente 2012: ESSIA - Effetti Sulla Salute degli Inquinanti Aerodispersi in Regione Lombardia”), Milan, Italy.
Author information
Authors and Affiliations
Contributions
According to the CRediT author statement- L.F.: conceptualization, data curation, formal analysis, methodology, writing – original draft, writing – review & editing. - M.C.: conceptualization, methodology, supervision, writing – original draft, writing – review & editing. - D.C.: conceptualization, data curation, methodology, supervision, writing – review & editing. - C.S.: data curation, formal analysis, methodology, writing – review & editing. - S.F.: data curation, formal analysis, methodology, writing – review & editing. - P.R.: data curation, formal analysis, methodology, writing – review & editing. - A.C.P.: conceptualization, funding acquisition, methodology, supervision, writing – review & editing. - M.T.L.: conceptualization, funding acquisition, supervision, writing – review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the institutional review boards (IRBs) of the following institutions: USA: National Cancer Institute, Bethesda, MD; Italy: Università degli Studi di Milano, Milan; Istituti Clinici di Perfezionamento, Milan and Fondazione IRCCS Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena, Milan (now Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan); Ospedale Niguarda, Milan; Istituto Clinico Humanitas, Rozzano; Istituto Scientifico Universitario Ospedale San Raffaele, Milan; Ospedale Luigi Sacco, Milan; AO San Paolo, Milan; AO Ospedale San Carlo Borromeo, Milan; Ospedale Fatebenefratelli e Oftalmico, Milan; Ospedale San Giuseppe, Milan; Ospedale San Gerardo, Monza; Spedali Civili, Brescia; Fondazione IRCCS Policlinico San Matteo, Pavia; Ospedale di Circolo e Fondazione Macchi, Varese. The original IRB number was 02-C-N211. However, since the National Cancer Institute only received de-identified samples and data from collaborating centers, had no direct contact or interaction with the study participants, and did not use or generate identifiable private information, EAGLE has been later determined to constitute “Not Human Subject Research (NHSR)” based on the federal Common Rule (45 CFR 46; https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fedrizzi, L., Carugno, M., Consonni, D. et al. Past exposure to PM10 and lung cancer risk in the EAGLE case–control study. Sci Rep 15, 31530 (2025). https://doi.org/10.1038/s41598-025-17159-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17159-y