Abstract
Cardiovascular–Kidney–Metabolic (CKM) syndrome, a concept recently introduced by the American Heart Association (AHA), emphasizes the intricate relationships among metabolic, renal, and cardiovascular diseases. The C-reactive protein–triglyceride–glucose index (CTI) has been proposed as an effective biomarker for insulin resistance (IR) and inflammation. Although there is substantial evidence demonstrating an association between the CTI and cardiovascular disease (CVD), its precise role in individuals with CKM syndrome stage 0–3 remains unclear. This prospective cohort study analysed data from China Health and Retirement Longitudinal Study (CHARLS), with a follow-up period of 10 years. The exposure variable was CTI at baseline, which was calculated as a combination of triglyceride (TG), fasting blood glucose (FBG), and C-reactive protein (CRP). The primary endpoint was the CVD. Cox proportional hazards regression and restricted cubic spline (RCS) analysis were conducted to examine the association between CTI and CVD risk. This study included 7,711 participants (52.38% female; mean age, 59.01 ± 9.37 years). An elevated CTI was significantly associated with a greater risk of developing CVD. Specifically, after fully adjusting for potential confounders, each one-unit increase in the CTI was associated with a 16% increase in CVD risk (hazard ratio [HR] = 1.16; 95% confidence interval [CI]: 1.06–1.27). Compared with participants in the lowest CTI group, those in the highest CTI group had a 42% greater CVD risk (HR = 1.42; 95% CI: 1.20–1.68). RCS analysis revealed a nonlinear association between CTI and the CVD risk among individuals with CKM syndrome stage 0–3 (overall P < 0.001; nonlinearity P = 0.01). This study revealed a positive association between CTI and CVD risk among individuals with CKM syndrome stages 0–3, suggesting that CTI may serve as a practical tool for CVD risk stratification in this population.
Similar content being viewed by others
Introduction
Cardiovascular–Kidney–Metabolic (CKM) syndrome is a complex, multisystem disease involving the interplay among cardiovascular disease (CVD), chronic kidney disease, and metabolic abnormalities. Recent studies have demonstrated that the synergistic interaction of these three components accelerates disease progression and significantly elevates mortality risk. Notably, CVD remains the leading cause of death worldwide, resulting in approximately 19.05 million deaths in 20201. In China, cardiovascular-related deaths account for 44.3% of total mortality, with a worrying trend toward younger individuals2. Large multicenter cohort studies—for example, the Framingham Heart Study and the CKD Prognosis Consortium—have confirmed that the concurrent presence of metabolic abnormalities and early-stage renal impairment markedly increases CVD risk3,4,5. The preclinical stages (0–3) of CKM syndrome are characterized by the presence of cardiovascular risk factors, kidney disorders, or metabolic disturbances in the absence of established cardiovascular events. Early identification and risk stratification in this population are critically important for the primary prevention of CVD6.
Insulin resistance (IR) refers to a diminished physiological response to insulin, resulting in impaired glucose uptake and utilization in target tissues. IR is not only the main cause of type 2 diabetes but also a key factor in CVD7. Epidemiological and experimental studies have demonstrated that IR may increase CVD risk by reducing nitric oxide (NO) synthesis, promoting subendothelial lipid accumulation, and enhancing oxidative stress8. The triglyceride‒glucose (TyG), which is calculated from fasting triglyceride and fasting glucose, is a simple and reliable surrogate marker of IR9. This method is highly correlated with traditional “gold standard” methods for IR assessment, such as the hyperinsulinaemic–euglycaemic clamp, across diverse populations and can effectively predict CVD risk10,11. Inflammation plays a vital role in the onset and progression of CVD. Chronic low-grade inflammation facilitates the formation and rupture of atherosclerotic plaques, resulting in thrombosis and potentially triggering major cardiovascular events such as myocardial infarction and stroke12. Moreover, inflammation is closely associated with adverse outcomes such as cardiac remodeling and heart failure13,14. C-reactive protein (CRP), a classic biomarker of inflammation, is an acute-phase protein. Elevated CRP not only reflects the presence of systemic inflammation but also serves as a predictor of CVD risk15. Many studies have shown a positive association between CRP and the CVD incidence. CRP has thus been widely used for CVD risk stratification and early disease warning16.
The C-reactive protein–triglyceride–glucose index (CTI), which is calculated as a combination of TyG and CRP, integrates both inflammation and insulin resistance, serving as a novel composite exposure variable. Preliminary studies have explored a positive association between the CTI and CVD risk17. However, prospective evidence on the relationship between the two among individuals with CKM syndrome stage 0–3 remains relatively limited.
This study utilized data from the China Health and Retirement Longitudinal Study (CHARLS) to systematically evaluate the association between CTI and the CVD risk among individuals with CKM syndrome stage 0–3 to achieve risk stratification and individualized management, ultimately reducing the incidence and mortality of CVD.
Methods
Data source and study population
The data for this study were obtained from the China Health and Retirement Longitudinal Study (CHARLS), a large-scale, nationally representative, long-term follow-up survey in China. CHARLS aims to collect information on health, economic status, and social and family conditions among individuals aged 45 years and older and their households. The project employs a multistage, stratified, probability sampling method to ensure the representativeness of the sample. Since its inception in 2011, CHARLS has conducted multiple follow-up waves, covering data on demographics, health status, blood tests, physical examinations, and medical service utilization, and has been widely used in academic research within China and internationally. The CHARLS project was initiated and organized by the National School of Development at Peking University, and all survey procedures were performed in strict accordance with ethical standards. The research team obtained ethical approval from the Biomedical Ethics Review Committee of Peking University (Approval No. IRB00001052-11015), and informed consent was obtained from all participants during data collection18. In this study, participants interviewed between 2011 and 2012 were used as the baseline, with follow-up conducted in 2013, 2015, 2018, and 2020.
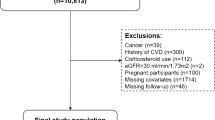
The flowchart (Fig. 1) outlines the inclusion and exclusion criteria applied in this study. At baseline, 17,708 participants were initially enrolled. We then excluded 2,408 individuals with preexisting CVD, 885 participants with missing CVD-related information, and 1,040 participants with less than two years of follow-up. In addition, 438 individuals who were younger than 45 years old were excluded. Further exclusions were made for 5,067 participants lacking triglyceride (TG) data, 3 participants missing CRP data, and 156 participants with abnormal or extreme CTI values. After these exclusions, a total of 7,711 participants were included in the final analysis.
Calculation of the C-reactive protein-triglyceride glucose index (CTI)
The CTI was calculated as follows19: CTI = 0.412 × ln (CRP [mg/L]) + ln ((TG [mg/dl] × FBG [mg/dl])/2).
CVD diagnosis
The primary outcome of this study was the incidence of new-onset CVD during the 10-year follow-up period from 2011 to 2020. Information regarding CVD history was collected via a standardized questionnaire. CVD was defined as a self-reported history of heart disease and/or stroke. To assess the presence of heart disease, participants were asked, “Has a doctor ever told you that you have had a heart attack, angina, coronary heart disease, heart failure, or any other heart-related problems?” For stroke diagnosis, participants were asked, “Has a doctor ever told you that you have had a stroke?”20. To ensure data reliability, the CHARLS research team implemented strict quality control procedures for data recording and verification21.
Definition of CKM syndrome stage 0 to 3
According to the American Heart Association, CKM syndrome is classified into stage 0 to 421. Stage 0 indicates the absence of metabolic risk factors, defined as a normal body mass index (BMI), blood glucose, blood pressure (BP), and blood lipids, with no evidence of chronic kidney disease (CKD) or subclinical/clinical CVD. Stage 1 is characterized by excess adiposity, including overweight (BMI ≥ 24 kg/m²), abdominal obesity (waist circumference ≥ 80 cm for female and ≥ 90 cm for male), and/or adipose tissue dysfunction (such as impaired glucose tolerance or prediabetes), but without other metabolic risk factors, CKD, or CVD. Stage 2 is defined by the presence of metabolic risk factors, such as dyslipidemia, hypertension, metabolic syndrome, diabetes, and/or moderate-to-high-risk CKD (estimated Glomerular Filtration Rate [eGFR] between 30 and 60 ml/min/1.73 m² or self-reported diagnosis of CKD). Stage 3 includes subclinical CVD (represented in this study by a 10-year CVD risk as predicted by the Framingham risk score, with a cut-off of ≥ 21.5 for female or ≥ 21.6 for male22 and/or very high-risk CKD (eGFR < 30 ml/min/1.73 m²). The estimation and classification of the eGFR were conducted via the CKD-EPI equation, according to the KDIGO guidelines. Stage 4 represents clinical CVD, including coronary heart disease, heart failure, or stroke. Therefore, individuals who were already at stage 4 at baseline were excluded from the study, while those at stages 0 to 3 were included.
Data collection
The collected data included the following :
-
(i)
Demographic information, such as gender, age and educational level.
-
(ii)
Physical measurements, including systolic blood pressure (SBP), diastolic blood pressure (DBP), height, weight, and waist circumference.
-
(iii)
Lifestyle factors, such as smoking status, drinking status, and sleep problems.
-
(iv)
Medical history and medication use, including a history of hypertension (defined as participants with a history of hypertension, those undergoing antihypertensive treatment, or baseline SBP ≥ 130 mmHg or DBP ≥ 80 mmHg)23, the use of antihypertensive medications, a history of diabetes (defined as those with a history of diabetes, those receiving antidiabetic treatment, or baseline FBG ≥ 7.0 mmol/L [126 mg/dL] or glycated Hemoglobin A1c (HbA1c) ≥ 6.5%)24,25, the use of antidiabetic medications, and a history of liver disease, lung disease, and cancer.
-
(v)
laboratory measurements, including FBG, HbA1c, total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), platelet count (PLT), serum creatinine (Scr), blood urea nitrogen (BUN), uric acid (UA) and CRP.
Handling of missing variables
We utilized multiple imputations to address the issue of missing values and mitigate potential bias.
Statistical analysis
The participants in this study were divided into four groups (Q1–Q4) according to their CTI quartiles. For quantitative variables following a normal distribution, the data were presented as the means ± standard deviations (SDs), and group differences were assessed via analysis of variance (ANOVA). For nonnormally distributed quantitative variables, the median and interquartile range (IQR) were reported, and the Kruskal‒Wallis test was used to assess differences among groups. Categorical variables were described as frequencies and percentages, and differences between groups were evaluated with the χ² test. The association between the CTI and the CVD risk was analysed via Cox proportional hazards regression models, and five different models were developed to facilitate a comprehensive analysis. Additionally, a fully adjusted restricted cubic spline (RCS) analysis was used to explore the dose‒response relationship between the CTI and the CVD risk because of their flexibility in modeling complex relationships without imposing a strict functional form. Four knots were placed at the 5th, 35th, 65th, and 95th percentiles of the CTI distribution. This approach balanced model flexibility and parsimony, helping to prevent overfitting and ensure interpretability. Kaplan–Meier curves were used to evaluate the CVD incidence across groups. Receiver operating characteristic (ROC) curves were also constructed to assess the predictive value of the CTI, CRP, and TyG for CVD risk, with the area under the ROC curve (AUC) being used to evaluate the incremental predictive effect of the CTI. Subgroup and interaction analyses were conducted to assess the associations between CTI and CVD risk across different demographic and clinical characteristics. Stratification factors included gender, age (45–60 years and ≥ 60 years), smoking status, drinking status, sleep problems, hypertension, diabetes, BMI (< 24, 24–28, and ≥ 28 kg/m²), uric acid (< 6.0 and ≥ 6.0 mg/dL), HbA1c (< 6.0 and ≥ 6.0%), low-density lipoprotein cholesterol (< 100, 100–130, and ≥ 130 mg/dL), total cholesterol (< 200, 200–240, and ≥ 240 mg/dL), and triglycerides (< 150, 150–200, and ≥ 200 mg/dL). These subgroups were predefined based on established clinical relevance and prior literature suggesting. Although some interaction terms were not statistically significant, we reported complete subgroup analyses to provide valuable information for clinical practice and hypothesis generation, and enhance the transparency and comparability of our findings within the existing literature.Statistical analyses were conducted using R software (version 4.4.0). Results with P < 0.05 were considered statistically significant.
Results
Baseline characteristics of the participants
A total of 7,711 participants were included in the analysis, 52.38% of whom were female, with a mean age of 59.01 ± 9.37 years. Baseline characteristics, as shown in Table 1, revealed a clear trend toward the accumulation of metabolic abnormalities and cardiovascular risk factors with increasing CTI values. Specifically, higher CTI quartiles were associated with elevated levels of FBG, TG, TC, LDL-C, HbA1c, UA, and CRP, whereas HDL-C levels were lower in these groups. In addition, higher CTI quartiles were correlated with increased BMI, waist circumference, blood pressure and a greater incidence of hypertension and diabetes. Furthermore, female participants tended to have higher CTI values than their male counterparts did.
The relationship between the CTI and the CVD risk in a population with CKM syndrome stage 0–3
In this study, a total of 1,936 participants developed CVD, yielding a cumulative incidence of 25.11%. Five Cox proportional hazards models were constructed to evaluate the association between the CTI and CVD risk (Table 2 is shown at the end of the manuscript). The results were robust and showed a statistically significant positive association across all models (p for trend < 0.001). In Model I, each one-unit increase in CTI was associated with a 25% higher risk of CVD (HR = 1.25, 95% CI: 1.18–1.32, P < 0.001). With sequential adjustment for multiple potential confounders in Models II, III, and IV, the risk estimates decreased slightly but remained significant, with HRs of 1.24 (95% CI: 1.17–1.31, P < 0.001), 1.15 (95% CI: 1.08–1.22, P < 0.001), and 1.10 (95% CI: 1.03–1.17, P = 0.00506), respectively. Even in the fully adjusted Model V, each one-unit increase in CTI was still associated with a 16% increased risk of CVD (HR = 1.16, 95% CI: 1.06–1.27; P < 0.001).
Furthermore, when the lowest quartile (Q1) was used as the reference, the CVD risk increased progressively across the second (Q2), third (Q3), and highest (Q4) CTI quartiles, demonstrating a clear dose‒response relationship. In Model I, the HRs for CVD in Q2, Q3, and Q4 were 1.25 (95% CI: 1.09–1.44), 1.46 (95% CI: 1.28–1.67), and 1.73 (95% CI: 1.52–1.97), respectively, all of which reached statistical significance. After further adjustment for confounding variables in Model V, the associations remained significant, with HRs of 1.16 (95% CI: 1.01–1.33, P = 0.037) for Q2, 1.24 (95% CI: 1.08–1.43, P = 0.003) for Q3, and 1.42 (95% CI: 1.20–1.68, P < 0.001) for Q4. The results indicated that, compared with those in Q1, participants in Q2, Q3, and Q4 had 16%, 24%, and 42% higher risks of developing CVD, respectively. These results collectively indicated a positive association between CTI and CVD risk among individuals in CKM syndrome stage 0–3. The Cox Model V was tested using Schoenfeld residuals. No significant violation was observed for the model (global P = 0.14).
To further explore the relationship between CTI and CVD incidence, we conducted a stratified analysis of incidence rates per 1,000 person-years across CTI quartiles (Table 3). The results demonstrated a clear increasing trend in CVD incidence with higher CTI quartiles (P for trend < 0.001).
The association between the CTI and CVD risk was further explored via a RCS Cox proportional hazards regression model (Fig. 2). The results revealed a significant nonlinear relationship (P-overall < 0.001; P-nonlinear = 0.01). At low to moderate CTI levels, CVD risk increased gradually, with a relatively gentle slope. However, once the CTI exceeded a certain threshold, the CVD risk increased sharply, indicating an accelerated dose–response relationship. Furthermore, we identified this threshold as the CTI value corresponding to the point of maximal slope on the risk curve (CTI = 8.72). This suggested that when the CTI exceeds 8.72, the CVD risk will rise markedly.
Additionally, Kaplan–Meier cumulative incidence curve analysis demonstrated a progressive increase in CVD events from the Q1 to Q4 groups (Fig. 3). A statistically significant difference was observed among these groups (P < 0.001), further supporting the observation that individuals with higher CTI have an increased CVD risk. Additionally, the number at risk, number of events, and cumulative risk at each follow-up time point were calculated and presented for each CTI quartile group (Tables 4, 5 and 6).
To further investigate the associations between CTI and CVD risk, subgroup and interaction analyses were performed according to age, gender, smoking status, drinking status, sleep problems, BMI, hypertension status, and diabetes status, among others (Fig. 4). Notably, significant interactions were observed in the BMI group, TC group, and diabetes status group (interaction P = 0.013, P = 0.032, and P = 0.041, respectively). These results suggested the potential universality of the CTI as an independent predictor of CVD risk.
To compare the predictive value of the CTI, CRP, and TyG for the CVD risk, we performed ROC curve analysis (Fig. 5). Compared with CRP and TyG alone, the CTI demonstrated the highest diagnostic performance for CVD incidence (AUC 0.559, 95% CI 0.545–0.575). These findings suggested that the CTI may serve as a more effective predictor for CVD risk than CRP and TyG, but its absolute performance was limited.
Sensitivity analysis
To assess the robustness of the findings, we performed several sensitivity analyses. Firstly, we re-categorized CTI using tertiles and sextiles and repeated the Cox regression analyses (Tables 7 and 8). The results remained consistent with the main analysis. Secondly, we applied logistic regression models using quartiles of CTI, and the association did not materially change (Table 9).
Discussion
This study is the first to investigate the relationship between the CTI and CVD risk among individuals with CKM syndrome stage 0–3. The results revealed a significant positive association, suggesting that the CTI may serve as an important predictor for future cardiovascular events in this population. Cox regression analyses indicated that, even after adjusting for multiple potential confounders, each one-unit increase in the CTI was associated with a 16% higher risk of CVD (HR = 1.16, 95% CI: 1.06–1.27), underscoring its value as an independent risk predictor in CVD risk assessment. Furthermore, quartile analyses of the CTI revealed a clear dose–response relationship. According to the fully adjusted model, individuals in the highest CTI quartile (Q4) had a 42% greater risk of CVD than those in the lowest quartile (Q1) did (HR = 1.42, 95% CI: 1.20–1.68). Kaplan–Meier curves further confirmed a significantly greater CVD incidence in the higher CTI group (log-rank test P < 0.001). RCS analysis revealed a nonlinear association between the CTI and CVD risk, with the hazard ratio rising sharply beyond a certain CTI threshold (CTI = 8.72). Identifying such a threshold may have important clinical implications, providing a basis for more precise risk stratification and highlighting the necessity for early initiation of primary prevention—such as adopting a healthy diet, increasing physical activity, quitting smoking, and controlling blood pressure, blood glucose and blood lipids—for patients with CTI values above this threshold. ROC curve analysis showed that CTI had greater predictive value for CVD risk than CRP or TyG. In addition, subgroup and interaction analyses revealed no significant interactions among the ten demographic subgroups examined (interaction p > 0.05), suggesting that the relationship between the CTI and CVD risk is consistent across different demographic groups. Although previous epidemiological studies have generally shown greater CVD risk in males26, our study revealed that the hazard ratio was slightly greater in females (HR = 1.25, 95% CI: 1.16, 1.35) than in males (HR = 1.23, 95% CI: 1.13, 1.33). This phenomenon may be closely related to the age structure of our study population, as all participants were aged 45 years or older, with the vast majority of female being perimenopausal or postmenopausal. Research has demonstrated that estrogen exerts multiple protective effects on the cardiovascular system, including promoting endothelial nitric oxide (NO) production to increase vasodilation, exerting anti-inflammatory and antioxidant effects, regulating lipid metabolism, and inhibiting thrombosis27. Following menopause, estrogen levels decrease markedly, resulting in the loss of these protective effects and a sharp increase in cardiovascular risk28. In a study involving 302,632 Chinese women, Ling Yang et al. found that postmenopausal women had a 49% higher CVD risk compared with premenopausal women (HR = 1.49, 95% CI:1.32–1.68)29. Therefore, clinicians should intensify their attention to cardiovascular health among perimenopausal and postmenopausal female. Furthermore, this study revealed that individuals with sleep problems had greater CVD risk than those without such problems. In recent years, the prevalence of sleep problems among middle-aged and elderly people has increased significantly. Substantial evidence suggested that inadequate sleep can induce chronic low-grade inflammation and excessive activation of the sympathetic nervous system, increase proinflammatory cytokine levels, and promote the development of IR30. Therefore, early clinical interventions to improve sleep quality should be considered important targets for reducing CVD risk and improving cardiovascular outcomes.
In recent years, the TyG, a surrogate marker for IR, has attracted increasing attention in the field of CVD. In a nested case‒control study including 3,745 patients with stable coronary artery disease (CAD), Jin et al. reported that each one-unit increase in the TyG was associated with a 36% increased risk of cardiovascular events (CVEs) (HR = 1.364, 95% CI: 1.100–1.691, P = 0.005)31. Similarly, a large prospective cohort study involving 96,541 Chinese adults conducted by Liu et al. demonstrated a significant positive association between the TyG and cardiovascular event risk, with participants in the highest TyG quartile showing a 34% higher risk of incident CVD than those in the lowest quartile (HR = 1.34, 95% CI: 1.23–1.45)32. These findings indicated that the TyG is an effective predictor of CVD risk. Nonetheless, as the TyG primarily reflects the state of IR, it does not provide a comprehensive evaluation of other major pathophysiological processes of CVD, such as chronic low-grade inflammation. In contrast, the CTI incorporates both the TyG and the CRP, thereby simultaneously capturing metabolic and inflammatory abnormalities to offer a more holistic assessment. The ROC curve analysis further demonstrated that the CTI had superior predictive value for CVD risk compared with the TyG alone. Furthermore, our results were consistent with two recent large-scale studies that reported a strong positive association between CTI and CVD risk. Xu et al. analysed data from 19,451 adult participants and reported a linear correlation between the CTI and the incidence of CHD, with those in the highest CTI quartile exhibiting an approximately 80.7% higher risk than those in the lowest quartile (HR = 1.807, 95% CI: 1.314–2.484, P < 0.001); importantly, the CTI demonstrated better predictive performance than CRP or TyG alone17. Similarly, Huo et al. analysed data from 10,443 participants and revealed a significant positive relationship between the CTI and stroke risk, particularly among individuals with normoglycemia and prediabetes, with hazard ratios of 1.33 and 1.20, respectively33. In comparison, our study specifically focused on individuals with CKM syndrome stage 0–3, a high-risk population commonly characterized by the coexistence of cardiovascular, renal, and metabolic risk factors, which may interact synergistically to exacerbate disease progression. Thus, early identification and accurate risk stratification in this high-risk group are crucial for implementing individualized interventions and effectively preventing adverse cardiovascular events.
As an integrated indicator reflecting both IR and inflammatory status, the CTI is associated with CVD risk through several mechanisms. Firstly, IR can inhibit the PI3K/Akt signaling pathway. This decreases endothelial Nitric Oxide Synthase (eNOS) activity and nitric oxide (NO) production, impairs vasodilation, and ultimately leads to endothelial dysfunction34. Secondly, IR is characterized by consistently elevated expression of proinflammatory cytokines, which aggravates chronic low-grade inflammation, promotes inflammatory cell infiltration, causes further structural damage to the vascular wall, and leads to the instability of atherosclerotic plaques35. Moreover, IR is often accompanied by lipid metabolism disorders, including increased release of free fatty acids (FFAs), elevated plasma triglyceride levels, reduced HDL-C, and accelerated formation of LDL particles, thereby facilitating lipid deposition in the arterial wall and related inflammatory responses36. On the other hand, CRP, a critical biomarker of inflammation, has been shown to play a significant role in the development and progression of CVD. Specifically, CRP can upregulate the expression of adhesion molecules (such as VCAM-1 and ICAM-1) on endothelial cells, increase leukocyte adhesion and transmigration, and accelerate atherosclerotic plaque formation37. Furthermore, CRP can bind to oxidized low-density lipoprotein (ox-LDL) and damaged cell membranes, thereby activating the classical complement pathway. This in turn induces a stronger local inflammatory response and promotes the progression and instability of atherosclerotic plaques38.
Furthermore, we explored the potential mechanisms underlying the nonlinear association between CTI and CVD risk. Firstly, when the CTI remains within a certain range, the body is able to maintain homeostasis. However, once CTI exceeds a critical threshold, homeostasis is disrupted and the CVD risk increases sharply. Secondly, inflammation induces IR, which in turn aggravates inflammatory processes. This bidirectional interplay establishes a vicious positive feedback loop, substantially increasing the CVD risk39,40.
This study has several notable strengths. Firstly, it is the first to explore the association between CTI and CVD risk, specifically in individuals with CKM syndrome stage 0–3. Secondly, this research is a prospective, nationwide longitudinal cohort study involving a relatively large sample of middle-aged and older adults with a balanced age distribution, which enhances the reliability of the findings. Additionally, we systematically adjusted for potential confounders and performed subgroup analyses to assess the consistency of associations across different demographic and clinical subpopulations, further strengthening the clinical applicability and external validity of the results.
Nevertheless, several limitations should be acknowledged. Firstly, the definition of subclinical CVD in this study was based on the Framingham 10-year cardiovascular risk score rather than the latest PREVENT equations. Secondly, although multiple known confounders were adjusted for, the possibility of residual unmeasured confounding influencing the results cannot be entirely excluded. Thirdly, since the CRP and TyG were assessed only once at baseline, dynamic changes in these biomarkers and their potential impact on CVD risk over time could not be evaluated. Fourthly, This study utilized data from the CHARLS, and thus the results are primarily applicable to middle-aged and older adults in China. Consequently, the generalizability of these findings to other populations may be limited by differences in ethnicity, culture, healthcare system, and socioeconomic context. Finally, CVD were identified based on self-reported information provided by participants. It is important to acknowledge that self-reported data are subject to misclassification bias, which may includes both under-reporting (failure to report existing CVD) and over-reporting (reporting CVD in the absence of a confirmed diagnosis). Such misclassification can arise from participants’ limited understanding of medical conditions or recall bias.
Conclusion
This study revealed a positive association between CTI and CVD risk among individuals with CKM syndrome stages 0–3, suggesting that CTI may serve as a practical tool for CVD risk stratification in this population.
Data availability
The data supporting the findings of this study are available in the CHARLS repository [http://charls.pku.edu.cn].
Abbreviations
- AHA:
-
American heart association
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under the ROC curve
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- BUN:
-
Blood urea nitrogen
- CHARLS:
-
China health and retirement longitudinal study
- CHD:
-
Coronary heart disease
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CKM:
-
Cardiovascular-kidney-metabolic
- CRP:
-
C-reactive protein
- CTI:
-
C-reactive protein–triglyceride–glucose index
- CVD:
-
Cardiovascular diseases
- CVEs:
-
Cardiovascular events
- DBP:
-
Diastolic blood pressure
- eGFR:
-
Estimated glomerular filtration rate
- eNOS:
-
Endothelial nitric oxide synthase
- FBG:
-
Fasting blood glucose
- FFAs:
-
Free fatty acids
- HbA1c:
-
Glycated Hemoglobin A1c
- HDL-C:
-
High-density lipoprotein cholesterol
- HR:
-
Hazard ratio
- ICAM-1:
-
Intercellular Cell Adhesion Molecule-1
- IQR:
-
Interquartile range
- IR:
-
Insulin resistance
- KDIGO:
-
Kidney disease improving global outcomes
- LDL:
-
Low-density lipoprotein
- LDL-C:
-
Low-density lipoprotein cholesterol
- NO:
-
Nitric oxide
- ox-LDL:
-
Oxidized low-density lipoprotein
- PLT:
-
Platelets
- ROC:
-
Receiver operating characteristic
- SBP:
-
Systolic blood pressure
- Scr:
-
Serum creatinine
- SDs:
-
standard deviations
- RCS:
-
Restricted cubic spline
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- TyG:
-
Triglyceride glucose index
- UA:
-
Uric acid
- VCAM-1:
-
Vascular Cell Adhesion Molecule-1
References
Tsao, C. W. et al. Heart disease and stroke Statistics—2023 update: A report from the American heart association. Circulation 147(8), e93–e621 (2023).
Hu, S. et al. Report on cardiovascular health and diseases in China 2022: an updated summary. Chinese Circulation Journal 38 (6), 583-612 (2023) (in chinese).
Foster, M. C. et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham heart study. Am. J. Kidney Dis. 52 (1), 39–48 (2008).
Sarnak, M. J. et al. Kidney disease as a risk factor for development of cardiovascular disease. Hypertension 42 (5), 1050–1065 (2003).
Wilson, P. W. F., D’Agostino, R. B., Sullivan, L., Parise, H. & Kannel, W. B. Overweight and obesity as determinants of cardiovascular risk. Archives Intern. Medicine 162(16), 1867–1872 (2002).
Khan, S. S. et al. Novel prediction equations for absolute risk assessment of total cardiovascular disease incorporating cardiovascular-Kidney-Metabolic health: A scientific statement from the American heart association. Circulation 148 (24), 1982–2004 (2023).
Lebovitz, H. Insulin resistance: definition and consequences. Exp. Clin. Endocrinol. Diabetes. 109 (Suppl 2), S135–S148 (2001).
Kosmas, C. E. et al. Insulin resistance and cardiovascular disease. Journal Int. Med. Research 51(3), 3000605231164548 (2023).
Vineetha, K. R. N., Praseeda, S., Mohan, T. S. & Sanjay, K. Triglyceride glucose (TyG) index: A surrogate biomarker of insulin resistance. J. Pak. Med. Assoc. 72 (5), 986–988 (2022).
da Silva, A. et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovascular Diabetology 18 (1), 89 (2019).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the Euglycemic-Hyperinsulinemic clamp. J. Clin. Endocrinol. Metabolism. 95 (7), 3347–3351 (2010).
Steven, S. et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxidative Med. Cell. Longev. 2019, 1–26 (2019).
Murphy, S. P., Kakkar, R., McCarthy, C. P. & Januzzi, J. L. Inflammation in heart failure. J. Am. Coll. Cardiol. 75 (11), 1324–1340 (2020).
Prabhu, S. D. & Frangogiannis, N. G. The biological basis for cardiac repair after myocardial infarction. Circul. Res. 119 (1), 91–112 (2016).
Zhou, H-H., Tang, Y-L., Xu, T-H. & Cheng, B. C-reactive protein: structure, function, regulation, and role in clinical diseases. Frontiers Immunology 15, 1425168 (2024).
Emerging Risk Factors Collaboration et al. John Danesh: C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. The Lancet 375(9709):132–140. (2010).
Xu, M., Zhang, L., Xu, D., Shi, W. & Zhang, W. Usefulness of C-reactive protein-triglyceride glucose index in detecting prevalent coronary heart disease: findings from the National health and nutrition examination survey 1999–2018. Frontiers Cardiovasc. Medicine 11, 1485538 (2024).
Zhao, Y., Hu, Y., Smith, J. P., Strauss, J. & Yang, G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int. J. Epidemiol. 43 (1), 61–68 (2012).
Ruan, G-T. et al. A novel inflammation and insulin resistance related indicator to predict the survival of patients with cancer. Frontiers Endocrinology 13, 905266 (2022).
Cui, C. et al. Triglyceride-glucose index, renal function and cardiovascular disease: a National cohort study. Cardiovascular Diabetology 22 (1), 325 (2023).
Ndumele, C. E. et al. Cardiovascular-Kidney-Metabolic health: A presidential advisory from the American heart association. Circulation 148 (20), 1606–1635 (2023).
D’Agostino, R. B. et al. General cardiovascular risk profile for use in primary care. Circulation 117 (6), 743–753 (2008).
Tan, L. et al. Association between insulin resistance and uncontrolled hypertension and arterial stiffness among US adults: a population-based study. Cardiovascular Diabetology 22 (1), 311 (2023).
Harreiter, J. & Roden, M. Diabetes mellitus – Definition, klassifikation, diagnose, screening und Prävention (Update 2023). Wien. Klin. Wochenschr. 135 (S1), 7–17 (2023).
Qiu, S. et al. Muscle strength and prediabetes progression and regression in middle-aged and older adults: a prospective cohort study. J. Cachexia Sarcopenia Muscle. 13 (2), 909–918 (2022).
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J. Am. Coll. Cardiol. 76 (25), 2982–3021 (2020).
Mendelsohn, M. E., Epstein, F. H. & Karas, R. H. The protective effects of Estrogen on the cardiovascular system. N. Engl. J. Med. 340 (23), 1801–1811 (1999).
Yang, J. L., Hodara, E., Sriprasert, I., Shoupe, D. & Stanczyk, F. Z. Estrogen deficiency in the menopause and the role of hormone therapy: integrating the findings of basic science research with clinical trials. Menopause 31 (10), 926–939 (2024).
Yang, L. et al. Menopause characteristics, total reproductive years, and risk of cardiovascular disease among Chinese women. Circulation: Cardiovasc. Qual. Outcomes 10 (11), e004235 (2017).
Hong, S., Lee, D-B., Yoon, D-W., Yoo, S-L. & Kim, J. The effect of sleep disruption on cardiometabolic health. Life 15 (1), 60 (2025).
Jin, J-L. et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J. Thorac. Disease. 10 (11), 6137–6146 (2018).
Liu, Q. et al. Triglyceride-glucose index associated with the risk of cardiovascular disease: the Kailuan study. Endocrine 75 (2), 392–399 (2021).
Huo, G. et al. Association between C-reactive protein-triglyceride glucose index and stroke risk in different glycemic status: insights from the China health and retirement longitudinal study (CHARLS). Cardiovascular Diabetology 24 (1), 142 (2025).
Cersosimo, E. & DeFronzo, R. A. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diab./Metab. Res. Rev. 22 (6), 423–436 (2006).
Fernández-Real, J. M. & Ricart, W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr. Rev. 24 (3), 278–301 (2003).
Semenkovich, C. F. Insulin resistance and atherosclerosis. J. Clin. Invest. 116 (7), 1813–1822 (2006).
Kawanami, D. et al. C-reactive protein induces VCAM-1 gene expression through NF-κB activation in vascular endothelial cells. Atherosclerosis 185 (1), 39–46 (2006).
Chang, M-K., Binder, C. J., Torzewski, M. & Witztum, J. L. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proceedings of the National Academy of Sciences 99(20):13043–13048. (2002).
Shimobayashi, M. et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Invest. 128 (4), 1538–1550 (2018).
Shoelson, S. E. Inflammation and insulin resistance. J. Clin. Invest. 116 (7), 1793–1801 (2006).
Acknowledgements
The authors would like to thank all members of CHALRS for their valuable contributions, and the participants for providing data.
Funding
This work was supported by the Research Project of Shanxi Provincial Health and
Family Planning Commission (Nos. 2020068), the Key Medical Research Projects of Shanxi Province (Nos. 2020XM29 and 2023XM029).
Author information
Authors and Affiliations
Contributions
BL and SR were responsible for the study design. LL, LL, and XW were responsible for data extraction and organization. ZL was responsible for data analysis, as well as writing and revising the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
CHARLS was approved by the Institutional Review Board of Peking University (IRB approval number for the household survey: IRB00001052-11015; IRB approval number for blood sample collection: IRB00001052-11014). Written informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, Z., Li, L., Wang, X. et al. Association between C-reactive protein-triglyceride glucose index and Future cardiovascular disease risk in a population with cardiovascular-Kidney-metabolic syndrome stage 0–3. Sci Rep 15, 31152 (2025). https://doi.org/10.1038/s41598-025-17173-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17173-0