Abstract
Acute Myeloid Leukemia (AML) is a highly heterogeneous malignant hematologic cancer with poor clinical outcome. The presence of leukemia stem cells (LSC) is a significant factor contributing to the failure of AML treatments and frequent relapses. The quiescent and plastic nature of LSC decreases cell death under conventional chemotherapy. Programmed cell death (PCD) plays a critical role in the development and progression of various cancers including AML. We hypothesized that the expression of PCD gene in LSCs may predict the therapeutic outcome of AML patients in the clinic. In this study, we comprehensively analyzed the expression of PCD gene and identified the unique expression patterns of cell death genes of LSC. By integrating PCD- and LSC-related genes, we identified eight LSC death genes with prognostic values: OAZ1, S100A4, MPG, IL2RA, MMRN1, CDK6, HOXA9, and XIRP2. Based on these genes, we developed a leukemia stem cell prognostic death score (LSCD) and a prognostic nomogram. Our findings revealed that LSC, particularly Quiescent LSPC, exhibits a high LSCD score. AML patients with high LSCD score group showed characteristics of significant immune dysfunction and worse prognosis. Additionally, predictions regarding FDA-approved drugs indicated that the high LSCD score group is less sensitive to Venetoclax but more sensitive to Crenolanib, Tandutinib, or Midostaurin. In summary, we developed an LSCD model that shows the predictive potential of clinical prognosis and drug sensitivity. This model provides meaningful insights for personalized treatment of AML patients.
Similar content being viewed by others
Introduction
AML, characterized by clonal expansion of undifferentiated myeloid precursors, is the most common type of acute leukemia in adults, with a five-year survival rate of only 29%1,2. Clinical treatment options for AML rely on intensive chemotherapy regimens, such as 7 + 3 therapy (cytarabine combined with anthracycline), and hypomethylating agents (azacitidine, etc.)3. The allogeneic hematopoietic stem cell transplantation (allo-HSCT) therapy has significantly improved the survival of AML patients4. Recently, the emergence of Venetoclax, a BCL2 inhibitor, has dramatically increased the initial response and prolonged survival of AML patients5. Despite these treatments leading to complete remission in many patients, initial drug resistance and frequent relapse after treatment remain major challenges. Therefore, further study on the pathogenesis and resistance mechanisms, along with the development of prognostic and therapeutic biomarkers, is critical for personalized treatment and drug development for AML patients.
LSCs, also known as leukemia-initiating cells, are characterized by their ability to self-renew, engraft in recipient mice and generate leukemia6. Clinical evidence from the past 30 years has demonstrated that AML exhibits a hierarchical structure similar to normal hematopoiesis, with LSCs at its apex7,8. LSCs are also recognized as key cells initiating relapse and are critical in minimal residual disease post-chemotherapy. LSCs reside in a highly specialized microenvironment within the bone marrow, which protects them from drug-induced genotoxicity9. Moreover, LSCs are often in a quiescent or dormant state, which contributes to their resistance to chemotherapy6. Although Venetoclax targets BCL2 and induces cell death of LSCs, the monocytic subclone of LSCs confers drug resistance and promotes disease relapse10. A comprehensive analysis of the characteristics of LSCs is urgently needed for guidance on therapeutic choice.
Programmed cell death (PCD) is a highly ordered and regulated process that precisely controls cell death through complex molecular mechanisms, enabling the elimination of disordered or destined-for-death cells11. An increasing number of cell death modalities have been discovered, including alkaliptosis, anoikis, apoptosis, autophagy, autosis, cuproptosis, disulfidptosis, entotic cell death, ferroptosis, immunogenic cell death, lysosome-dependent cell death, mitotic catastrophe, mitochondrial permeability transition-driven necrosis (MPT), necroptosis, netotic cell death, oxeiptosis, parthanatos, pyroptosis and others12,13,14. PCD acts as a natural barrier against cancer progression. Frequent mutation and dysregulated expression of genes involved in PCD lead to the initiation and progression of multiple cancers15. A handful of drugs targeting the PCD pathway to induce cell death have been invented and translated into clinics. Venetoclax targets and degrades BCL2 to promote cell apoptosis of AML cells including LSCs16. Due to the initial and post-treatment resistance frequently occurring, additional studies focusing on PCD are required to develop new therapeutic strategies to predict drug response and avoid and overcome drug resistance in AML.
Here we developed a novel prognostic score based on LSC death characteristics (LSCD) that accurately predicts the survival outcomes of AML patients. Integrating this score with clinical features enhances the predictive ability for patient survival time. We observed that patients with high LSCD scores exhibit immune dysregulation. Additionally, these patients demonstrate less sensitivity to Venetoclax, and we identified potential alternative drugs that may be effective, including Crenolanib, Tandutinib, and Midostaurin.
Results
The gene expression patterns of PCD in AML
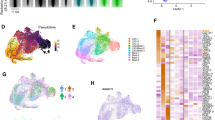
We analyzed single-cell sequencing data (GSE116256)17 from 16 patients diagnosed with AML and 5 normal bone marrow donors, which included six types of malignant AML cells(-like), along with their corresponding normal cells and other cell types (Fig. 1A). We calculated the ssGSEA scores for each malignant cell using the 18 collected PCD genes (Supplementary Table S1). Our results revealed that the overall score for disulfidptosis was highest in AML, indicating it is a core PCD type in AML malignant cells (Fig. 1B). In all AML malignant cells, there is a positive correlation between most PCD patterns; however, in certain malignant cell types, such as cDC-like cells, specific PCD patterns, including oxeiptosis and netotic cell death, exhibit a negative correlation (Fig. 1C).
Single-cell analysis reveals levels of PCD across various cell types in AML and normal bone marrow. (A) UMAP plots of single-cell RNA sequencing data from AML and normal bone marrow samples. (B) Heatmap displaying the ssGSEA scores for 18 PCD patterns across different cell types within malignant AML cells. The bar plot in the right panel represents the sum of the PCD score. (C) Heatmap showing the correlation between any two PCD scores across six types of AML malignant cells. (D) Dot plot showing the correlation between PCD scores across six types of AML malignant cells and cancer-related signaling pathways from CancerSEA. (E) Heatmap highlighting the differences in PCD pattern among the six types of malignant AML cells. (F) Violin plot comparing PCD score between AML and normal bone marrow cells. (G) The violin plot shows the 18 PCD scores in malignant and normal hematopoietic stem cells in leukemia. Statistical significance is indicated as follows: *, P < 0.05, **, P < 0.01, ***, P < 0.001, ****, P < 0.0001.
Furthermore, we investigated the association between PCD levels and functional pathway signaling in AML. The results indicate that PCD scores are positively correlated with DNA repair, angiogenesis, and quiescence, while they are negatively correlated with invasion, EMT, stemness, and the mTOR pathway (Fig. 1D, Supplementary Fig. 1 A). Different AML cell types exhibit distinct modes of programmed cell death (PCD). Several death scores gradually increased from the original AML cell type. cDC-like cells are sensitive to multiple forms of cell death, whereas HSC-like cells exhibit consistently low scores across almost all cell death pathways (Fig. 1E). Compared to normal bone marrow cells, we found that the overall PCD scores in malignant cells were significantly higher, except for the Promono cells (Fig. 1F). Additionally, we compared the differences in 18 forms of PCD between normal and malignant cells. Consistent with the overall PCD scores, most forms of PCD are significantly elevated in malignant cells (Supplementary Fig. 1B). In HSC-like cells, 17 PCD patterns show significant differences compared to HSCs, with 16 types being elevated (Fig. 1G).
Characterization of leukemia stem cell specific programmed cell death genes
Compared to other cell types, HSC-like cells exhibit markedly lower levels of PCD (Fig. 1E). To investigate the death characteristics of LSCs, we integrated several gene sets, including differentially expressed genes (DEGs) between HSC-like and normal HSC cells derived from scRNA data17DEGs between LSC+ and LSC- AML cells (with LSC activity determined by xenotransplantation)18and 18 PCD-related genes (Supplementary Table S1). We ultimately identified 45 LSC-related PCD genes (Fig. 2A). These genes comprise 12 PCD modules, as shown in Fig. 2B. Figure 2C presents the volcano plot of the differential expression of LSC-related PCD genes between HSC-like and normal HSC cells. GO enrichment analysis revealed that these genes are involved in various hematopoietic, immune regulatory, and cancer pathways, including cytokine production, negative regulation of lymphocyte activation and leukocyte proliferation, negative regulation of mononuclear cell proliferation and B cell activation, and tumor necrosis factor production (Fig. 2D). The protein-protein interaction network of these genes is depicted in Fig. 2E.
Landscape of leukemia stem cell-specific modes of cell death. (A) Venn diagram illustrating differential LSC genes from GSE116256 and GSE199452, as well as the 18 PCD-related genes (blue represents differential genes between HSC-like and HSC cells in GSE116256, green represents differential genes between LSC and non-LSC cells in GSE199452, and orange represents the 18 PCD genes). (B) Sankey diagram showing the relationships between 45 LSC-related PCD genes and the 18 modes of cell death. (C) Volcano plot of the 45 LSC-related PCD genes in HSC-like and HSC cells. (D) GO enrichment analysis of LSC-related PCD genes. (E) Protein-protein interaction (PPI) network of the LSC-related PCD genes.
Identifying an 8-gene prognostic signature (LSCD) from leukemia stem cell PCD genes
Using the TCGA dataset, we conducted univariate Cox regression analysis on 45 LSC-related PCD genes and uncovered 18 survival risk genes (Fig. 3A). We then applied LASSO regression to these 18 genes, performing 10-fold cross-validation with bootstrap resampling. Genes selected in over 70% of bootstrap iterations were retained as 8 critical candidate genes (Fig. 3B, Supplementary Fig. 2A). Using multivariate Cox regression, we constructed a prognostic model in the TCGA training set based on these 8 genes. The model was used to export a leukemia stem cell prognostic death score (LSCD) for patients using the following formula:
Construction of a prognostic LSCD signature for AML patients. (A) The hazard ratios (HR) for the 18 risk genes selected through Cox regression in the TCGA dataset. (B) Model gene selection from 18 LSC-related PCD genes using LASSO regression. (C-F) Kaplan-Meier curves of overall survival for patients in the low- and high-LSCD score group in the TCGA-LAML, GSE71014, GSE146173, and BeatAML. (G) Differential expression levels of the eight LSCD-related genes between paired AML patients and healthy controls.
LSCD score = (0.111962 × OAZ1 exp.) + (0.315936 × S100A4 exp.) + (0.208408 × MPG exp.) + (0.159514 × IL2RA exp.) + (0.144387 × MMRN1 exp.) + (− 0.208729 × CDK6 exp.) + (0.051381 × HOXA9 exp.) + (0.378294 × XIRP2 exp.)
We then stratified patients into high LSCD score and low LSCD score groups based on the median LSCD score. In the TCGA validation cohort, patients in the high LSCD score group had significantly worse prognoses compared to those in the low LSCD score group (Fig. 3C). This finding was consistently replicated across three independent AML datasets, demonstrating the robustness of our LSCD model (Fig. 3D-F). All model genes significantly affected overall survival (OS) and were classified as high-risk genes (Supplementary Fig. 2B-I). To further explore the potential of key LSCD-related genes as prognostic markers for AML, we utilized the GSE138702 dataset, which includes data from 9 AML patients and healthy donors matched for age and sex. The results showed that the expression levels of MPG, IL2RA, MMRN1, CDK6, HOXA9, and XIRP2 were significantly higher in AML patients compared to their healthy counterparts (Fig. 3G).
Comprehensive evaluation of the LSCD prognostic model
We performed a comparison between the LSCD score and the established LSC17 score. First, we assessed the concordance between the two scores, finding that 66.2% of samples shared the same classification (high or low) by both scores, indicating substantial agreement (Supplementary Fig. 3 A). We then evaluated the clinical utility using Decision Curve Analysis (DCA). The results demonstrate that the LSCD model provides a higher net benefit across a range of threshold probabilities compared to the LSC17 model. Furthermore, the net benefit of the LSCD model alone was similar to that of a combined LSCD + LSC17 model, suggesting that the LSCD score captures certain predictive features present in the LSC17 score (Supplementary Fig. 3B). We analyzed the five most prevalent genetic subtypes within the TCGA cohort. Our results show distinct patterns of LSC death characteristics: Subtypes characterized by FLT3 + NPMc, FLT3 mutation, and IDH mutation exhibited significantly higher LSC death scores. In contrast, subtypes characterized by NPMc mutation and PML-RARA displayed lower LSC death scores. This pattern of LSC death score variation aligns with the recognized clinical heterogeneity among these genetic subtypes (Supplementary Fig. 3 C).To address the impact of molecular subtypes on the model’s predictive sensitivity, we performed survival analyses stratified by the five major genetic subtypes. The results confirmed that the LSCD score effectively stratified prognosis (high vs. low LSCD) within the FLT3 + NPMc, FLT3 mutation, and IDH mutation subtypes. While the p-values did not reach statistical significance in the NPMc and PML-RARA subtypes, a consistent trend towards poorer survival in the high LSCD group was observed (Supplementary Fig. 3D-H). Furthermore, we conducted a multivariable Cox proportional hazards regression analysis adjusting for these five key molecular subtypes. The LSCD score remained a significant independent predictor of survival (Supplementary Fig. 3I), indicating that its prognostic value is not solely driven by these common genetic alterations.
Construction and evaluation of the nomogram survival model
To determine whether the LSCD score serves as an independent prognostic factor, both univariate and multivariate Cox regression analyses were conducted. The univariate Cox regression analysis revealed that the LSCD score is the most significant prognostic factor compared to other features (P < 0.001) (Fig. 4A). Multivariate Cox regression further confirmed that the LSCD score remains an independent prognostic factor for AML patients (P < 0.001) (Fig. 4B). Based on the results of the multivariate Cox regression analysis, we developed a prognostic nomogram model using the TCGA cohort to predict 1-, 3-, and 5-year survival probabilities for AML patients (Fig. 4C). Calibration curves demonstrated the nomogram’s accuracy in predicting survival rates at 1-, 3-, and 5-year (Fig. 4D). Decision curve analysis (DCA) further confirmed that the nomogram model outperforms other predictive factors used in the study (Fig. 4E). Moreover, the nomogram score indicated significant survival differences between high-score and low-score groups (Fig. 4F). To evaluate the performance of the nomogram, its predictive ability was tested in the TCGA cohort and two independent cohorts. Our results showed that the model achieved a high area under the curve (AUC) score for predicting 1-, 3-, and 5-year survival rates in TCGA AML patients, with similar results observed in the validation cohorts (Fig. 4G-I).
Establishment and evaluation of a nomogram survival model. (A) Univariate Cox regression analysis of clinical characteristics and LSCD score in the TCGA cohort. (B) Multivariate Cox regression analysis of clinical characteristics and LSCD score in the TCGA cohort. (C) Prognostic nomogram prediction model for TCGA patients. (D) Calibration plots for predicting 1-, 3-, and 5-year overall survival rates in the TCGA cohort. (E) Decision curve analysis for clinical characteristics and LSCD in the TCGA cohort. (F) Kaplan-Meier analysis of overall survival for high- and low-risk patients in the TCGA-LAML cohort based on the nomogram score. (G-I) ROC analysis of the 1-, 3-, and 5-year survival rates in AML patients in the (G) TCGA-LAML, (H) GSE146173, and (I) BeatAML cohorts.
LSCD score correlates with stemness of AML cells
To investigate the relationship between the LSCD score and stemness, we calculated the hematopoietic HSC stemness score using TCGA data19. The results revealed that the high LSCD score group had elevated stem scores, which were positively correlated with the HSC stemness score (Fig. 5A-B). Furthermore, we explored the relationship between LSC and LSCD scores using the LSC17 gene signature developed by Stanley W. K. Ng et al.20. Our analysis revealed that samples within the high-LSCD score group exhibited elevated LSC17 gene scores, with a strong correlation between the LSCD and LSC17 scores (Fig. 5C-D). According to Andy G. et al., leukemia stem cells are categorized into three subsets: Quiescent LSPC, Cycling LSPC, and Primed LSPC18. Our results indicated that the high LSCD score group showed a stronger correlation with Quiescent LSPC (Fig. 5F). In the LSCD groups, there is a significant difference in quiescent LSPC scores rather than in other LSPC populations (Fig. 5E). Additionally, we analyzed TCGA patients based on the FAB classification. The Sankey diagram showed that the majority of M0 stage patients were classified within the high LSCD score group (Fig. 5G).
Association between LSCD score and hematopoietic stem cell characteristics. (A) Boxplot showing the differences in HSC score between high and low LSCD score groups in the TCGA cohort. (B) Scatter plot illustrating the correlation between HSC score and LSCD score. (C) Boxplot displaying the differences in LSC17 gene score between high and low LSCD score groups. (D) Scatter plot showing the correlation between LSC17 gene score and LSCD score. (E) Boxplot showing differences in the three LSPC cell types between high- and low-LSCD score groups in the TCGA cohort. (F) Scatter plot illustrating the correlation between LSPC_Quiescent score and LSCD score. (G) Sankey diagram showing the relationship between FAB classification and LSCD score group in TCGA-LAML.
High LSCD score is associated with immune dysfunction in AML cells
Using the ESTIMATE algorithm to assess the immune scores of AML patients, we found that patients in the high LSCD score group had higher immune score (Fig. 6A). Furthermore, we assessed the status of various immune-related pathways and observed upregulation of several pathways in the high LSCD score group, including antigen processing and presentation, BCR signaling pathway, TCR signaling pathway, and chemokine receptor pathway (Fig. 6B). This suggests that patients with high LSCD scores may experience immune dysregulation. Additionally, we analyzed the correlation between the LSCD score and 78 immune modulators. Most immune modulators were expressed at higher levels in patients with high LSCD scores. This includes key genes associated with antigen presentation (HLA-A, HLA-B, HLA-C), co-inhibitors (CD274, CD276), co-stimulators (CD28, CD80) and others (Fig. 6C). To further explore immune modulation, we conducted a TIDE (Tumor Immune Dysfunction and Exclusion) analysis for patients with high and low LSCD score groups. High LSCD score patients exhibited higher levels of immune dysfunction and lower levels of immune exclusion, correlating with a reduced response to immunotherapy (Fig. 6D-F). This suggests that immune dysfunction may partly explain the lack of response to immunotherapy in patients with high LSCD scores. Notably, high LSCD score patients had lower immune exclusion in their microenvironment, and the expression of immune checkpoint proteins such as PDL1 and PDL2 was upregulated (Fig. 6G-H). This suggests the potential for targeted immune checkpoint therapies.Furthermore, we have deepened our analysis of the immune microenvironment associated with high LSCD scores. Using CIBERSORT to infer immune cell infiltration, we found that high LSCD score samples exhibited a significantly increased abundance of immunosuppressive cell populations, particularly monocytes and Tregs, alongside a decrease in plasma cells. This feature suggests an immunosuppressive tumor microenvironment. Although we did not observe significant differences in T-cell proportions, we noted significant upregulation of key T-cell exhaustion genes (PDCD1/PD-1 and LAG3) in the high LSCD group, indicating a state of T-cell dysfunction/exhaustion (Supplementary Fig. 4A, B). These findings provide a more comprehensive link between the LSCD score, specific immune phenotypes, and the underlying microenvironmental structure in AML.
Immune Characteristics of High and Low LSCD Score Groups. (A) ESTIMATE immune scores for high and low LSCD score groups in the TCGA LAML cohort. (B) Heatmap of immune signaling pathway ssGSEA scores for high and low LSCD score groups. (C) Correlation between LSCD scores and the expression levels of 78 immune modulators. (D-F) Boxplots of TIDE-calculated immune response metrics: (D) Dysfunction, (E) Exclusion, and (F) Predicted Immune Response, comparing high and low LSCD score groups. (G-H) Expression levels of PDL1 and PDL2 between high and low LSCD score groups.
LSCD predicts drug sensitivity
To explore the relationship between LSCD and drug efficacy in AML patients, we utilized in vitro drug sensitivity data from the BeatAML dataset. We examined the sensitivity of each drug using the AUC values to identify drugs with significant differences. The correlations between drug sensitivity and LSCD model genes are illustrated in Fig. 7A and Supplementary Fig. 5A. Correlation analyses and differential analysis between LSCD score and drug sensitivity are shown in Fig. 7B and Supplementary Fig. 5B. The results indicate that, among AML-related drugs, the high LSCD score group exhibited higher AUC values for Venetoclax, whereas AUC values for Crenolanib, Tandutinib, and Midostaurin were lower (Fig. 7C-E, Supplementary Fig. 5C). These findings suggest that patients with high LSCD scores may have low sensitivity to Venetoclax, but high sensitivity to three other FDA-approved AML drugs: Crenolanib, Tandutinib, and Midostaurin. Additionally, predictions based on BeatAML’s in vitro drug models applied to TCGA data confirmed these results, demonstrating that the high LSCD score group is similarly less sensitive to Venetoclax while remaining sensitive to Crenolanib, Tandutinib, and Midostaurin (Fig. 7F-G, Supplementary Fig. 5A, D-E). Given that LSCD is derived from LSC death signatures, we specifically investigated its relationship with venetoclax resistance. The LSCD score showed a significant positive correlation with venetoclax resistance, whereas the LSC17 score showed no significant correlation. Importantly, the LSCD score maintained superior prognostic power for overall survival compared to the LSC17 score within both venetoclax-resistant and venetoclax-sensitive patient subgroups (Supplementary Fig. 6A-D). These results indicate that while the LSCD score shows additional advantages in predicting venetoclax resistance and providing prognostic stratification regardless of venetoclax resistance status.
Effectiveness of predicting drug sensitivity based on LSCD grouping. (A) Dot plot showing the correlation between LSCD model genes and BeatAML in vitro drug AUC values. (B) Volcano plot illustrating the correlation between AML drug AUC values and LSCD score. (C-E) Boxplots comparing AUC score of significantly different drugs between high and low LSCD score group in BeatAML: Venetoclax, Crenolanib, and Tandutinib. (F-G) Predicted differences in drug sensitivity between high and low LSCD score group for Venetoclax and Crenolanib in the TCGA cohort. Statistical significance is indicated as follows: *, P < 0.05, **, P < 0.01, ***, P < 0.001, ****, P < 0.0001.
Discussion
Various PCD modalities are closely associated with tumor initiation, progression, and metastasis21. The therapy with BCL2 inhibitor venetoclax, which activates the intrinsic cell death pathway, has significantly improved the clinical outcome of AML patients. However, relapse occurs in many patients post venetoclax treatment, highlighting the importance of understanding the survival mechanisms of leukemia stem cells (LSCs). By summarizing different modes of cell death and understanding the responses of leukemia and LSCs to these modalities, we can further understand the biology of LSCs and mechanisms of drug resistance, which help enhance the design of new drugs or revise existing therapeutic strategies for more precise treatment of leukemia.
In this study, we conducted a comprehensive analysis of 18 different PCD modalities in the context of LSCs for the first time. By integrating RNA sequencing data from single-cell and patient sample derived xenograft, we identified genes differentially expressed in LSCs which were subsequently applied for death modalities screening. As a result, we identified 45 LSC-related PCD genes which were further narrowed down to 8 genes (OAZ1, S100A4, MPG, IL2RA, MMRN1, CDK6, HOXA9, and XIRP2) by combing the factor of patient prognosis. Our data indicates that the 8-gene signature (LSCD) accurately predicts patient survival in multiple datasets (Fig. 3). To further assess the clinical relevance of our model, we developed a nomogram for AML that incorporates clinical parameters. This model confirmed the efficacy of LSCD in AML and demonstrated its clinical applicability. Consequently, the prognostic nomogram model effectively predicts the 1-, 3-, and 5-year overall survival of AML patients based on their clinical characteristics and LSCD score.
Among the 8 genes in LSCD models, OAZ1 encodes antizyme, an inhibitor of ornithine decarboxylase, which suppresses polyamine synthesis and thereby inhibits cell proliferation. In transgenic mice, OAZ1 may promote apoptosis and inhibit cell proliferation by upregulating the expression of the pro-apoptotic protein Bax, thus contributing to the suppression of forestomach tumor development22. In addition, rearrangements involving OAZ1 and MAFK have also been identified in cases of AML23. S100A4 exhibits a complex and critical role in regulating AML cell death: it promotes cell survival not only by suppressing basal apoptosis and conferring resistance to ferroptosis, but also by facilitating necroptosis under specific conditions, highlighting its “dual nature” as a potential therapeutic target24,25. MPG, a DNA N-glycosylase, activates alkyl base DNA N-glycosylase activity involved in base excision repair. It is associated with temozolomide (TMZ) resistance in glioblastoma, and silencing MPG weakens the repair of TMZ-induced DNA damage and enhances apoptosis26. IL2RA has been reported to be specifically upregulated in LSCs, promoting AML cell proliferation while inhibiting apoptosis and differentiation, and is linked to chemotherapy resistance and poor prognosis in AML, consistent with our findings27. MMRN1, a member of the EMILIN/multimerin family mediating cell adhesion, has been identified as a novel biomarker for improved AML risk stratification in a retrospective study of childhood leukemia28. CDK6, a core kinase driving the G1/S phase transition, is particularly crucial for LSC self-renewal (functioning downstream of HCK). CDK6 inhibitors induce apoptosis in specific AML subtypes (e.g., FLT3-ITD), establishing CDK6 as a highly promising therapeutic target for the selective elimination of LSCs29,30. HOXA9, a homeobox transcription factor frequently overexpressed in AML, is a central driver maintaining the leukemic transformation state and LSC properties. It precisely regulates the self-renewal/differentiation balance switch and directly promotes cell cycle progression and survival by transcriptionally activating downstream targets such as CDK631,32,33. XIRP2, an actin-binding protein, demonstrates anti-apoptotic potential: its knockout in cardiomyocytes significantly increases apoptosis, while overexpression in a hepatocellular carcinoma model does not alter basal apoptosis but markedly attenuates oxaliplatin-induced apoptotic responses34,35,36. This indicates a potential anti-apoptotic role for XIRP2 in supporting cell survival.
To evaluate the specificity of our LSCD score in LSCs, we conducted a Sankey diagram analysis on patients classified according to French-American-British (FAB) staging. As expected, a majority of M0 patients exhibited a high LSCD score. Additionally, patients in the high LSCD score group demonstrated higher scores for hematopoietic stem cells (HSC) and the LSC17 gene signature. Notably, the high LSCD score group showed a positive correlation with quiescent LSC and the proportion of quiescent LSC exhibited significant differences among the LSCD groups compared to cycling LSPC and primed LSPC. These results confirm the specificity of our LSCD model in identifying cell death modalities in LSCs.
Furthermore, our study indicates that high LSCD score are associated with a dysregulated immune environment, impairing the ability of immune regulation to effectively target cancer cells. However, our data also reveal that samples in the high LSCD score group exhibit higher expression of PD-L1, and PD-L2, indicating a potential choice with immune checkpoint blockade. To identify drugs effective against high LSCD score, we analyzed drug sensitivity data from BeatAML datasets. Our analysis revealed patients in the LSCD score higher group may not be responded to BCL2 inhibitor venetoclax but are likely sensitive to Crenolanib, Tandutinib, and Midostaurin.
This study also has several limitations. First, our analysis relies on public datasets. Consequently, further experiments are essential to elucidate the specific roles and molecular mechanisms of leukemia stem cell death characteristics and associated genes in AML. Second, although we found that the LSCD score correlates with predicted sensitivity to Venetoclax, Crenolanib, Tandutinib, and Midostaurin in AML, these findings are inferential and lack experimental validation. Future work is needed to directly verify the role of LSCD features in drug sensitivity. Finally, our model lacks validation in phase 3 clinical randomized controlled trials. Therefore, verification through high-quality, multicenter randomized controlled trials is required.
In summary, Our study developed a leukemia stem cell death characteristic model. This model provides new insights into the relationship between leukemia stem cell death and AML, offering a valuable reference for the personalized treatment and prognosis assessment of AML patients.
Materials and methods
Data collection
We compiled a list of 18 PCD-related genes from previously published articles13,37 (Supplementary Table S1). RNA-seq data and clinicopathological information for LAML samples were obtained from The Cancer Genome Atlas (TCGA). RNA-seq data for additional AML validation cohorts were sourced from the Gene Expression Omnibus (GEO) database (GSE71014, GSE146173) and the BeatAML database38,39,40. Drug sensitivity predictions for AML were derived from the BeatAML dataset. Single-cell RNA-seq data for AML patients and normal samples were retrieved from the GSE116256 dataset17. RNA-seq data for xenograft experiments were obtained from the GSE199452 dataset18. Additionally, Healthy and AML-paired RNA-seq data are derived from GSE13870241.
Reanalysis of single-cell data
scRNA data from normal and AML samples were processed using the Seurat (version 4.4.0) package, following standard procedures for data filtering and normalization42. To map normal cell labels to AML data, we employed Seurat’s Mapping and Annotating Query Datasets workflow to transfer non-tumor cell labels to the AML dataset. Furthermore, we referred to the original annotations provided by Peter van Galen et al. along with the new classification labels for leukemia stem and progenitor cells introduced by Andy G. X. Zeng et al.17,18.
Identification of leukemia stem cell-specific PCD-related genes
Differentially expressed genes between HSC-like and HSC cells in the single-cell RNA-seq data were identified using the FindMarkers function. Differential expression analysis between LSC+ and LSC- cells in the GSE199452 dataset was conducted using DESeq243. A significance threshold of |log2FoldChange| ≥ 0.5 and padj < 0.05 was applied to identify significantly differentially expressed LSC genes.
Functional enrichment analysis
For scRNA data, we utilized the scGSVA package to calculate scores for each of the 18 PCD gene sets using the ssGSEA method. Enrichment analysis for LSC-specific PCD-related genes, as well as other enrichment analyses, was conducted using the clusterProfiler R package44. Gene sets were sourced from various signatures available in the Molecular Signatures Database (MSigDB)45 cancerSEA46 and import database47.
Construction of the PCD-related gene signature
We filtered expression profiles based on clinical data from TCGA and validation cohorts, retaining samples with available clinical information and survival outcomes. Univariate Cox regression analysis was employed to identify LSC-related cell death genes associated with survival in AML patients, using a significance threshold of p < 0.05. Subsequently, LASSO regression was utilized to select the eight most critical candidate genes. These eight candidate genes were incorporated into a multivariate Cox regression model to construct the LSCD score for AML patients. The TCGA dataset was partitioned into training and validation sets using the createDataPartition function, with a 7:3 ratio for model training and validation48.
Construction and evaluation of the nomogram
To validate the LSCD score as an independent prognostic marker for AML patients, we performed univariate and multivariate Cox regression analyses, incorporating clinical features (age, sex, FAB classification, and cytogenetic risk category) along with the LSCD score. Subsequently, we utilized the rms and regplot R packages for nomogram construction and prediction. The performance of the nomogram was evaluated using decision curve analysis, calibration curves, and ROC curves49.
Immunological analysis
We assessed the immune status of AML patients using the ESTIMATE R package. The Tumor Immune Dysfunction and Exclusion (TIDE) framework was employed to calculate immune dysfunction, immune exclusion, and the potential efficacy of immune therapies based on expression profiles50. The correlation of 78 immune modulators was analyzed using the gene expression levels of these modulators51.
Drug sensitivity analysis
Drug sensitivity was analyzed using the area under the curve (AUC) values for 122 small-molecule inhibitors from the BeatAML dataset, which were matched with RNA-seq data. Correlation analyses were performed between high and low LSCD score groups and LSCD-related genes, with thresholds set at p < 0.05 and |r| > 0.1. The oncoPredict R package was utilized to predict drug sensitivity for 122 small-molecule inhibitors in the TCGA cohort, based on BeatAML drug sensitivity data and RNA expression profiles52.
Statistical analysis
All analyses adhered to standard statistical procedures and were conducted using R (v4.3.2). Differences between the two groups were assessed using t-tests. Survival curves were generated using the survival and survminer R packages, with comparisons made using the log-rank test. Statistical significance for all analyses was set at p < 0.05.
Data availability
The datasets generated and/or analysed during the current study are available in the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) repository, with accession numbers GSE116256, GSE71014, GSE146173, GSE199452, and GSE138702. Additionally, data from The Cancer Genome Atlas (TCGA, https://cancergenome.nih.gov/) and BeatAML (http://vizome.org/).
References
Dohner, H., Weisdorf, D. J. & Bloomfield, C. D. Acute myeloid leukemia. N. Engl. J. Med. 373, 1136–1152. https://doi.org/10.1056/NEJMra1406184 (2015).
Shimony, S., Stahl, M. & Stone, R. M. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 98, 502–526. https://doi.org/10.1002/ajh.26822 (2023).
Wang, H. et al. Venetoclax plus 3 + 7 Daunorubicin and cytarabine chemotherapy as first-line treatment for adults with acute myeloid leukaemia: a multicentre, single-arm, phase 2 trial. Lancet Haematol. 9, e415–e424. https://doi.org/10.1016/S2352-3026(22)00106-5 (2022).
Liu, H. Emerging agents and regimens for AML. J. Hematol. Oncol. 14 https://doi.org/10.1186/s13045-021-01062-w (2021).
Souers, A. J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208. https://doi.org/10.1038/nm.3048 (2013).
Marchand, T. & Pinho, S. Leukemic stem cells: from leukemic niche biology to treatment opportunities. Front. Immunol. 12, 775128. https://doi.org/10.3389/fimmu.2021.775128 (2021).
Kellaway, S. G. et al. Leukemic stem cells activate lineage inappropriate signalling pathways to promote their growth. Nat. Commun. 15, 1359. https://doi.org/10.1038/s41467-024-45691-4 (2024).
Khaldoyanidi, S. K., Hindoyan, A., Stein, A. & Subklewe, M. Leukemic stem cells as a target for eliminating acute myeloid leukemia: gaps in translational research. Crit. Rev. Oncol. Hematol. 175, 103710. https://doi.org/10.1016/j.critrevonc.2022.103710 (2022).
Barreto, I. V. et al. Leukemic stem cell: A Mini-Review on clinical perspectives. Front. Oncol. 12, 931050. https://doi.org/10.3389/fonc.2022.931050 (2022).
Pei, S. et al. Monocytic subclones confer resistance to Venetoclax-Based therapy in patients with acute myeloid leukemia. Cancer Discov. 10, 536–551. https://doi.org/10.1158/2159-8290.CD-19-0710 (2020).
Dai, X., Wang, D. & Zhang, J. Programmed cell death, redox imbalance, and cancer therapeutics. Apoptosis 26, 385–414. https://doi.org/10.1007/s10495-021-01682-0 (2021).
Chen, Y., Li, X., Yang, M. & Liu, S. B. Research progress on morphology and mechanism of programmed cell death. Cell. Death Dis. 15, 327. https://doi.org/10.1038/s41419-024-06712-8 (2024).
Zhang, W. et al. Pan-cancer evaluation of regulated cell death to predict overall survival and immune checkpoint inhibitor response. NPJ Precis Oncol. 8, 77. https://doi.org/10.1038/s41698-024-00570-5 (2024).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell. Death Differ. 25, 486–541. https://doi.org/10.1038/s41418-017-0012-4 (2018).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674. https://doi.org/10.1016/j.cell.2011.02.013 (2011).
Buettner, R. et al. Targeting the metabolic vulnerability of acute myeloid leukemia blasts with a combination of venetoclax and 8-chloro-adenosine. J. Hematol. Oncol. 14, 70. https://doi.org/10.1186/s13045-021-01076-4 (2021).
van Galen, P. et al. Single-Cell RNA-Seq Reveals AML Hierarchies Relevant to Disease Progression and Immunity. Cell 176, 1265–1281 e1224, (2019). https://doi.org/10.1016/j.cell.2019.01.031
Zeng, A. G. X. et al. A cellular hierarchy framework for Understanding heterogeneity and predicting drug response in acute myeloid leukemia. Nat. Med. 28, 1212–1223. https://doi.org/10.1038/s41591-022-01819-x (2022).
Shi, K. et al. Bone marrow hematopoiesis drives multiple sclerosis progression. Cell 185, 2234–2247 e2217, (2022). https://doi.org/10.1016/j.cell.2022.05.020
Kim, D. D. H. et al. The 17-gene stemness score associates with relapse risk and long-term outcomes following allogeneic haematopoietic cell transplantation in acute myeloid leukaemia. EJHaem 3, 873–884, (2022). https://doi.org/10.1002/jha2.466
Su, Z., Yang, Z., Xu, Y., Chen, Y. & Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer. 14, 48. https://doi.org/10.1186/s12943-015-0321-5 (2015).
Fong, L. Y., Feith, D. J. & Pegg, A. E. Antizyme overexpression in Transgenic mice reduces cell proliferation, increases apoptosis, and reduces N-nitrosomethylbenzylamine-induced forestomach carcinogenesis. Cancer Res. 63, 3945–3954 (2003).
Padella, A. et al. Novel and rare fusion transcripts involving transcription factors and tumor suppressor genes in acute myeloid leukemia. Cancers (Basel). 11 https://doi.org/10.3390/cancers11121951 (2019).
Alanazi, B. et al. Integrated nuclear proteomics and transcriptomics identifies S100A4 as a therapeutic target in acute myeloid leukemia. Leukemia 34, 427–440. https://doi.org/10.1038/s41375-019-0596-4 (2020).
Tan, X. et al. The dual role of FSP1 in programmed cell death: resisting ferroptosis in the cell membrane and promoting necroptosis in the nucleus of THP-1 cells. Mol. Med. 30, 102. https://doi.org/10.1186/s10020-024-00861-4 (2024).
Agnihotri, S. et al. Alkylpurine-DNA-N-glycosylase confers resistance to Temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J. Clin. Invest. 122, 253–266. https://doi.org/10.1172/JCI59334 (2012).
Nguyen, C. H. et al. IL2RA promotes aggressiveness and stem Cell-Related properties of acute myeloid leukemia. Cancer Res. 80, 4527–4539. https://doi.org/10.1158/0008-5472.CAN-20-0531 (2020).
Laszlo, G. S. et al. Multimerin-1 (MMRN1) as novel adverse marker in pediatric acute myeloid leukemia: A report from the children’s oncology group. Clin. Cancer Res. 21, 3187–3195. https://doi.org/10.1158/1078-0432.CCR-14-2684 (2015).
Uras, I. Z., Sexl, V. & Kollmann, K. CDK6 inhibition: A novel approach in AML management. Int. J. Mol. Sci. 21 https://doi.org/10.3390/ijms21072528 (2020).
Uras, I. Z. et al. Palbociclib treatment of FLT3-ITD + AML cells uncovers a kinase-dependent transcriptional regulation of FLT3 and PIM1 by CDK6. Blood 127, 2890–2902, (2016). https://doi.org/10.1182/blood-2015-11-683581
Wright, S. et al. Systematic characterization of the HOXA9 downstream targets in MLL-r leukemia by noncoding CRISPR screens. Nat. Commun. 14, 7464. https://doi.org/10.1038/s41467-023-43264-5 (2023).
Zhong, X. et al. HoxA9 transforms murine myeloid cells by a feedback loop driving expression of key oncogenes and cell cycle control genes. Blood Adv. 2, 3137–3148. https://doi.org/10.1182/bloodadvances.2018025866 (2018).
Pineault, N., Abramovich, C. & Humphries, R. K. Transplantable cell lines generated with NUP98-Hox fusion genes undergo leukemic progression by Meis1 independent of its binding to DNA. Leukemia 19, 636–643. https://doi.org/10.1038/sj.leu.2403696 (2005).
Paul, M. R. et al. Genomic landscape of metastatic breast cancer identifies preferentially dysregulated pathways and targets. J. Clin. Invest. 130, 4252–4265. https://doi.org/10.1172/JCI129941 (2020).
Guo, H. et al. Intercalated disc protein Xinbeta is required for Hippo-YAP signaling in the heart. Nat. Commun. 11, 4666. https://doi.org/10.1038/s41467-020-18379-8 (2020).
Li, D. et al. Identification of clinical value and biological effects of XIRP2 mutation in hepatocellular carcinoma. Biology (Basel). 13. https://doi.org/10.3390/biology13080633 (2024).
Luo, Y., Liu, L. & Zhang, C. Identification and analysis of diverse cell death patterns in diabetic kidney disease using microarray-based transcriptome profiling and single-nucleus RNA sequencing. Comput. Biol. Med. 169, 107780. https://doi.org/10.1016/j.compbiomed.2023.107780 (2024).
Chuang, M. K. et al. An mRNA expression signature for prognostication in de Novo acute myeloid leukemia patients with normal karyotype. Oncotarget 6, 39098–39110. https://doi.org/10.18632/oncotarget.5390 (2015).
Bamopoulos, S. A. et al. Clinical presentation and differential splicing of SRSF2, U2AF1 and SF3B1 mutations in patients with acute myeloid leukemia. Leukemia 34, 2621–2634. https://doi.org/10.1038/s41375-020-0839-4 (2020).
Tyner, J. W. et al. Functional genomic landscape of acute myeloid leukaemia. Nature 562, 526–531. https://doi.org/10.1038/s41586-018-0623-z (2018).
Celik, H. et al. Highly multiplexed proteomic assessment of human bone marrow in acute myeloid leukemia. Blood Adv. 4, 367–379. https://doi.org/10.1182/bloodadvances.2019001124 (2020).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 e3529, (2021). https://doi.org/10.1016/j.cell.2021.04.048
Love, M. I., Huber, W. & Anders, S. Moderated Estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. https://doi.org/10.1186/s13059-014-0550-8 (2014).
Wu, T. et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innov. (Camb). 2, 100141. https://doi.org/10.1016/j.xinn.2021.100141 (2021).
Liberzon, A. et al. The molecular signatures database (MSigDB) hallmark gene set collection. Cell. Syst. 1, 417–425. https://doi.org/10.1016/j.cels.2015.12.004 (2015).
Yuan, H. et al. CancerSEA: a cancer single-cell state atlas. Nucleic Acids Res. 47, D900–D908. https://doi.org/10.1093/nar/gky939 (2019).
Bhattacharya, S. et al. ImmPort: disseminating data to the public for the future of immunology. Immunol. Res. 58, 234–239. https://doi.org/10.1007/s12026-014-8516-1 (2014).
Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 28, 1–26. https://doi.org/10.18637/jss.v028.i05 (2008).
Blanche, P., Dartigues, J. F. & Jacqmin-Gadda, H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat. Med. 32, 5381–5397. https://doi.org/10.1002/sim.5958 (2013).
Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 24, 1550–1558. https://doi.org/10.1038/s41591-018-0136-1 (2018).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 12, 453–457. https://doi.org/10.1038/nmeth.3337 (2015).
Maeser, D., Gruener, R. F. & Huang, R. S. OncoPredict: an R package for predicting in vivo or cancer patient drug response and biomarkers from cell line screening data. Brief. Bioinform. 22 https://doi.org/10.1093/bib/bbab260 (2021).
Acknowledgements
This work was supported by Noncommunicable Chronic Diseases-National Science and Technology Major Project (2024ZD0519600); National Natural Science Foundation of China (82370184); Natural Science Foundation of Jiangsu Province (BK20231220); The Suzhou Municipal Key Laboratory (SZS2022005); CAMS Innovation Fund for Medical Sciences (2022-I2M-1-024, 2022-I2M-2-004); The Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2022-RC180-02); The Special Research Fund for Central Universities, Peking Union Medical College (3332023082); The NCTIB Fund for R&D Platform for Cell and Gene Therapy.
Author information
Authors and Affiliations
Contributions
G.Z. and W.F. conceived and designed the study. W.F. and L.M. completed the data analysis. C.N. and F.Q. wrote the manuscript. G.Z. performed the language editing. G.Z. and L.F. carefully and critically revised this manuscript, and all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, F., Li, M., Cui, N. et al. Signature of leukemia stem cell death pattern predicts prognosis and therapeutic response of acute myeloid leukemia patients. Sci Rep 15, 31612 (2025). https://doi.org/10.1038/s41598-025-17288-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17288-4