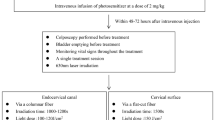Abstract
Currently, there is no relevant literature specifically discussing the human papillomavirus (HPV) negative conversion rate following Hiporfin photodynamic therapy (PDT) in HPV-associated high-grade squamous intraepithelial lesion (HSIL) of the female lower reproductive tract. Our study aims to fill this gap. Prospective study of 91 patients aged 37.4 ± 13.2 years old with HSIL in the female lower reproductive tract (cervical HSIL: 55, vaginal HSIL: 25, cervical HSIL combined with vaginal HSIL: 8, cervical HSIL combined with vulvar HSIL: 1, vaginal HSIL combined with vulvar HSIL: 2). Hiporfin® (2 mg/kg) was administered intravenously, and 48–72 h later, 630-nm laser irradiation was applied to the lesions. The median follow-up period was 36 months. Before treatment, all patients (91/91, 100%) tested positive for HPV, and only 6 patients (6/91, 6.6%) had been previously vaccinated against HPV. The HPV negative conversion rates were 74.5% (41/55), 89.1% (49/55), 89.8% (44/49) and 95.8% (23/24) at 3–6 months, 12 months, 24 months and 36 months respectively after PDT in treating cervical HSIL. For vaginal HSIL, the negative conversion rates were 28.0% (7/25) at 3–6 months, 52.0% (13/25) at 12 months, 60.9% (14/23) at 24 months, and 64.7% (11/17) at 36 months. In cases of multiple sites of female lower genital tract HSIL, the negative conversion rates were 18.2% (2/11), 45.5% (5/11), 60.0% (6/10), and 62.5% (5/8) at 3–6, 12, 24, and 36 months, respectively. Hiporfin-PDT was most effective in achieving HPV negative conversion for treating cervical HSIL, followed by vaginal HSIL, and finally for multisite female lower genital tract HSIL.
Similar content being viewed by others
Introduction
According to the 5th Edition of the World Health Organization (WHO) Classification, squamous intraepithelial lesions occurring in the lower reproductive tract (including the cervix, vagina and vulva) are classified into two types: HPV-related and non-HPV-related. Among them, HPV-related lesions include low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL)1. Cervical HSIL (cHSIL) is the most common, with prevalence rates of approximately 1.3–1.5% in China2. Vaginal HSIL (vaHSIL) rarely appears alone and often occurs alongside or following (cHSIL) or vulvar HSIL (vHSIL)3,4,5,6. Due to increased cervical cancer screening, improved colposcopy techniques, and greater awareness of vHSIL and vaHSIL, the incidence rates of vaHSIL and vHSIL have risen in recent years. HSIL has carcinogenic potential and is considered a precancerous lesion of female lower reproductive tract cancer7,8,9typically requiring clinical intervention.
HPV plays a crucial role in the development of HSIL in the female lower reproductive tract. While most HPV infections are temporary, some, particularly high-risk HPV (HR-HPV) infections with longer durations, can progress to HSIL10. HPV is a double-stranded deoxyribonucleic acid (DNA) virus. When the body fails to mount an effective immune response to HR-HPV, HSIL can develop. The viral DNA integrates into host cells, producing the oncoproteins E6 and E7, which interfere with normal cellular functions11. HPV is present in 93.06% of cHSIL12in 94.4% of vaHSIL cases13, and in 72–100% of vHSIL cases14.
Conventional treatments for HSIL include cold-knife surgery, loop electrosurgical excision procedure (LEEP), laser ablation, and drug treatments, et al.14,15,16,17,18,19,20. Surgery and ablation are generally non-selective in tissue removal. Common adverse effects of surgical excision include pain, cervical canal stenosis, scarring, cervical incompetence, premature birth, wound dehiscence, and altered appearance14,21,22,23,24. Laser ablation can also increase the risk of recurrence14,25. Drug treatments, such as 5-fluorouracil (5-FU) or imiquimod, are simple but often associated with high recurrence rates ranging from 13.6 to 38%, as well as adverse effects including local irritation, pain, erosions, and dyspareunia18,19,25,26. An increasing number of patients strongly desire organ preservation. Therefore, targeted and minimally invasive treatments are needed to meet clinical needs.
Hiporfin photodynamic therapy (PDT) is a clinically approved, targeted, and minimally invasive therapy. After administration, the photosensitizer accumulates selectively in pathologic tissue and is then activated by irradiation at a specific wavelength. In the presence of molecular oxygen, this energy transfer leads to a series of photochemical reactions and the generation of reactive oxygen species and various cytotoxic species, which induce cellular toxicity and kill pre-malignant or malignant cells through apoptosis and/or necrosis27. It also shuts down the tumor microvasculature and stimulates the host immune system28,29. Thus, PDT can not only eliminate the lesions but also aid in converting HPV to a negative status30.
Hiporfin is the only systemic photosensitizer approved for oncological indications by Chinese State Food and Drug Administration (SFDA)31. Some reports and our previous researches have confirmed its efficacy in treating precancerous lesions and cancers15,16,32,33,34,35,36,37,38. The effective rates of Hiporfin-PDT at 3–6 months were 96.8–100.0% in cHSIL, while the effective rate at 6 months was 83.3% in vaHSIL16,39,40. The Chinese expert consensus on Hiporfin-PDT for cHSIL has been published41. However, no systematic analysis has been conducted on HPV negative conversion following Hiporfin-PDT for HSIL in the female lower reproductive tract. As we know, persistent HR-HPV infection is associated with the recurrence of HSIL4,42,43.
The objective of the present study was to address this gap by exploring HPV negative conversion following Hiporfin-PDT for HSIL in the female lower reproductive tract.
Materials and methods
Study design
Ninety-one patients diagnosed with HSIL in the female lower reproductive tract were enrolled in the study from August 2021 to August 2023. In this prospective study, the size of the cohort was established according to clinical feasibility. All methods were performed in accordance with relevant guidelines and regulations. All participants provided written informed consent after being informed about the main objectives of the study, the treatment protocol, potential complications, and alternative traditional treatments. The study was approved by the Ethics Committee of our hospital (No. 2020(049)) and was registered with the Chinese Clinical Trial Registry (Date of registration: 29/05/2021, Registration number: ChiCTR2100046863).
Inclusion criteria included (1) a morphologically confirmed diagnosis of HSIL in the female lower reproductive tract, and (2) signed informed consent.
Exclusion criteria included (1) suspected invasive carcinoma of the female lower reproductive tract, (2) conditions such as hematoporphyria, diseases aggravated by light, or allergies to porphyrins or any excipients, (3) currently menstruating, pregnant or lactating, (4) patients with significantly abnormal liver function, coagulation dysfunction, or other serious uncontrolled medical complications, (5) acute inflammatory phase or active phase of a general infectious disease, (6) patients with immune system diseases taking high doses of immunosuppressive drugs, and (7) use of high doses of antithrombotic or antiplatelet aggregation drugs.
PCR diagnostics of HPV in cervical smears (Cobas® 4800) were performed before and after PDT.
Photodynamic therapy with Hiporfin
Perform a skin test before the intravenous injection of the photosensitizer. Dilute the HpD solution to 0.01 mg/mL and administer a 0.1 mL intradermal injection. Observe the injection site after 15–20 min; the absence of induration, redness, or swelling indicates a negative skin test. Hiporfin® (Chongqing Milelonge Biopharmaceutical Co., Ltd., China) was administered at a dose of 2 mg/kg via intravenous infusion in 250 ml of normal saline, 48–72 h prior to laser irradiation. Patients were positioned in the lithotomy position with an emptied bladder. The vulva, vagina, and cervix were cleaned with sterile 0.9% sodium chloride solution. Colposcopy (Electronic colposcopy imaging system CH1000 from Jiangsu Tongren Medical Electronic Technology Co., Ltd, China) was performed before PDT to reassess the location and size of lesions after the application of acetic acid and/or iodine solution. Vital signs were monitored throughout the treatment. PDT was administered as a single treatment session. For most patients with cHSIL and vaHSIL, oral or intravenous painkillers are not necessary before treatment. However, for patients who are sensitive to pain or those with vHSIL, diclofenac sodium suppositories may be inserted into the anterior anus prior to treatment, if there are no contraindications to the medication. Meanwhile, it is recommended to use a fan to blow on the irradiated area to help relieve pain during treatment for vHSIL.
For cervical lesions, light irradiation at a wavelength of 630 nm (PDT630 semiconductor laser treatment machine from Shenzhen Laser Medical Technology Co., Ltd, China) was first applied to the cervical canal using a 3 cm length columnar fiber, with an irradiation time of 1000–1200 s and a light dose of 100–120 J/cm². The fiber tip was positioned more than 1 cm from the internal cervical opening to prevent adhesion. Subsequently, light irradiation was applied to the cervical surface via a flat optical fiber (Shenzhen Laser Medical Technology Co., Ltd, China), with an irradiation time of 1500 s and a light dose of 150 J/cm². The size and number of irradiation fields, which overlapped the lesions by at least 5 mm at any edge, depended on the shape and dimensions of the lesions.
For vaginal lesions, light irradiation was applied with a light dose of 150 J/cm² via a flat optical fiber and/or a columnar fiber, depending on the requirements. The size and number of irradiation fields were tailored to completely cover the lesions, with at least a 0.5 cm margin around the edge. The lesions should be flattened as completely as possible using a speculum and cotton swabs.
For vulvar lesions, light irradiation was applied with a light dose of 150 J/cm² via a flat optical fiber. The size and number of irradiation fields were tailored to completely cover the lesions, with at least a 0.5 cm margin around the edge. The lesions should be flattened as fully as possible during treatment using cotton swabs (Fig. 1).
Because the photosensitizer can make the skin and eyes sensitive to light (primarily sunlight) for approximately 1–2 months after discharge, all patients were thoroughly instructed to avoid sunlight exposure for at least 1–2 months when outdoors. UV protection is not necessary when there is no sunlight, such as before sunrise, after sunset, at night, excluding cloudy days during the day. Patients can use mobile phones and computers without concern. When going outside, patients should use sun-protective items. Additionally, patients were advised to use contraception until 3 months after PDT.
Follow-up and statistical analysis
Follow-ups were conducted at 3–6 months, 12 months, and annually thereafter after PDT, in accordance with the Chinese expert consensus on follow-up for HSIL post-surgical treatment44. Adverse events related to PDT were documented at every follow-up visit. Adverse events occurring during and after PDT, including pelvic pain, vaginal discharge, photosensitivity, fever, hemorrhage, and others, were recorded in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. SPSS 27.0 software was used for all statistical analyses.
Results
Ninety-one patients, aged 37.4 ± 13.2 years (range: 31 to 69), with pathologically confirmed HSIL in the female lower reproductive tract were enrolled in the study. All patients were observed for at least a 12-month follow-up period. The median follow-up time was 36 months, with a maximum follow-up time of 42 months. No patients were lost to follow-up. Among the participants, 46 were nulliparous (46/91, 50.5%). Of the 91 patients enrolled, 55 (60.4%) had cHSIL, 25 (27.5%) had vaHSIL, and 11 (12.1%) had multisite female lower genital tract HSIL. Before treatment, the HPV infection rate was 100% (91/91), which may be attributed to the small sample size. HPV16/18 were the most common subtypes. The HPV vaccination rate prior to treatment was only 6.6%, with five patients having received the 9-valent HPV vaccine and one patient the 4-valent HPV vaccine (Table 1).
In the cHSIL group, 55 patients completed their follow-up at 12 months, 49 patients at 24 months, and 24 patients at 36 months. The incomplete follow-up for some patients was due to the follow-up period not yet being reached, rather than loss to follow-up. HPV DNA was no longer detectable in 74.5% (41/55), 89.1% (49/55), 89.8% (44/49), and 95.8% (23/24) of patients at 3–6, 12, 24, and 36 months after PDT, respectively. As of now, there are three patients with persistent infections in the cHSIL group. One patient, a 51-year-old with an HPV 16 infection, showed complete cytological and histological resolution following PDT. However, HSIL was detected in the vaginal vault 36 months post-PDT, indicating a new site of disease. The patient then underwent a second Hiporfin-PDT, after which both cytology and cervical biopsy results turned negative at 3–6 months post-treatment, although HPV-16 remained positive. In contrast, another patient, who had a lesion initially located in the cervical canal, demonstrated negative cytology and histology after PDT, with no recurrence observed during a 36-month follow-up period. The third patient, who had a history of LEEP for cHSIL and an HPV 18 infection, experienced downgrading of the lesions to LSIL, with no recurrence after 24 months of follow-up.
For vaHSIL, 25 patients completed their follow-up at 12 months, 23 patients at 24 months, and 17 patients at 36 months. The HPV negative conversion rates were 28.0% (7/25) at 3–6 months, 52.0% (13/25) at 12 months, 60.9% (14/23) at 24 months, and 64.7% (11/17) at 36 months. Up till now, there are 8 cases of persistent infection in the vaHSIL group. Of these, seven cases had a history of undergoing LEEP or hysterectomy for cHSIL, or had received surgery and chemoradiotherapy for cervical cancer. All lesions on the vaginal wall were multifocal, with combined lesions located either at the vaginal stump or the vaginal vault. Following PDT, two cases showed complete resolution of the lesions, while in another 2 cases, the lesions regressed to LSIL. The remaining 4 cases exhibited at least a 50% reduction in the HSIL area. One patient underwent hysterectomy, bilateral adnexectomy, and partial vaginal resection. However, three months post-operation, colposcopic biopsy revealed persistent vaHSIL at the vaginal remnant. Laser ablation treatment was performed, but HPV remained persistently positive. Another three patients underwent multiple laser ablation treatments and regular follow-ups, yet their HPV status also remained consistently positive.
In cases involving multiple sites of female lower genital tract HSIL, 11 patients completed their follow-up at 12 months, 10 patients at 24 months, and 8 patients at 36 months. The HPV negative conversion rates were 18.2% (2/11), 45.5% (5/11), 60.0% (6/10), and 62.5% (5/8) at 3–6 months, 12 months, 24 months, and 36 months, respectively (Table 2; Fig. 2). To date, five patients with multisite female lower genital tract HSIL have persistent infections. Notably, four of these patients had previously undergone LEEP or hysterectomy due to cHSIL. In one case, the lesions were downgraded to LSIL, with no recurrence observed during a 24-month follow-up period. A 62-year-old patient initially diagnosed with cervical and vaginal HSIL who did not adhere to regular follow-up developed vaginal cancer 32 months after PDT and was subsequently treated with radiotherapy; HPV remained positive. In three other cases, lesions showed significant reduction (over 50%) with residual lesions located in the vagina. One patient underwent hysterectomy, bilateral adnexectomy, and partial vaginal wall resection, while two patients declined surgery and are currently receiving laser ablation treatment. All of these patients had persistent HPV infection.
To further investigate whether Hiporfin-PDT affects the HPV negative conversion rate differently between primary and recurrent HSIL, we conducted a subgroup analysis stratifying the results by lesion type (Table 3). In the cHSIL group, three cases experienced recurrence after previous LEEP, and one case involved recurrence of stage IIB cervical cancer following radiotherapy and chemotherapy. Twelve months after PDT, the HPV negative conversion rate was 50% (2/4) in the recurrent cHSIL group, compared to 92.6% (47/51) in the primary cHSIL group. In the vaHSIL group, three cases experienced recurrence of stage IIA1 or higher cervical cancer after undergoing postoperative chemoradiotherapy or chemoradiotherapy. Twelve months after PDT, the HPV negative conversion rate was 33.3% (1/3) in the recurrent vaHSIL group, compared to 54.5% (12/22) in the primary vaHSIL group. In the group with multiple sites of lower female genital tract HSIL, seven cases had recurrence after previous LEEP. Twelve months after PDT for recurrent HSIL, the HPV negative conversion rate was 42.8% (3/7). In the primary HSIL group, the HPV negative conversion rate at 12 months post-PDT was 50.0% (2/4).
The procedure was generally well-tolerated, with no serious adverse effects reported among the 91 patients during and after PDT. 41.8% of patients experienced mild side effects. Fifteen patients (15/91, 16.5%) experienced pelvic pain (Grade 1) in the lower abdomen during irradiation, but no pain relief was required. Thirty-eight women (38/91, 41.8%) reported watery vaginal discharge (Grade 2), and twelve women (12/91, 13.2%) experienced pelvic pain (Grade 1) within 5–10 days post-treatment. Eighteen patients (18/91, 19.8%) suffered from photoallergic symptoms (Grade 3), such as slight edema and a burning sensation in exposed areas, due to insufficient sunblock education within 1–3 weeks after treatment. These symptoms resolved within 7–10 days following guidance and treatment with loratadine and ice. Two patients (2/91, 2.2%) experienced a fever below 38.5 °C (Grade 1) within 2 days after PDT and were treated with oral cefuroxime and metronidazole for infection control. No hemorrhage (Grade 2) occurred in any of the treated women (Table 4).
Among the 18 patients who planned to become pregnant, 13 achieved a total of 13 pregnancies, including 1 spontaneous abortion, 1 artificial abortion for personal reason, 9 full-term pregnancies, and 3 ongoing pregnancies. No fetal loss was attributed to cervical incompetence. Seven women delivered vaginally, and two underwent cesarean sections at term. Prenatal examinations showed no abnormalities, and the children exhibited normal physical growth.
Discussion
HSIL in the female lower reproductive tract is associated with HPV infection, and persistent HR-HPV infection is linked to recurrence45,46,47,48,49. Our study demonstrated that PDT with Hiporfin achieved high HPV negative conversion rates, particularly in cHSIL. Specifically, the HPV negative conversion rate was 89.1% at the 12-month follow-up after Hiporfin-PDT for cHSIL. These findings suggest that PDT has significant therapeutic potential in treating HPV-positive precancerous diseases and in preventing disease relapse50,51.
PDT is an organ-preserving therapeutic alternative for the treatment of precancerous diseases and cancer. It can initiate anti-tumor responses from the host’s inflammatory and immune systems as tumor cells are destroyed and cancer antigens are exposed52.
Choi et al.53 treated 59 patients with cHSIL using Photogem-PDT, reporting HPV negative conversion rates of 89.8% and 87.0% at 3 and 12 months, respectively. Gilyadova et al.54 treated 45 cases of cHSIL with chlorine E6-PDT, achieving HPV negative conversion rate of 100.0% at 12 months. Liu et al.15 used PDT with Hiporfin on 41 patients with cHSIL at childbearing age, achieving HPV negative conversion rates of 73.2% at 6 months and 92.7% at 12 months, with no recurrences observed during long-term follow-up. Qiao et al.40 applied PDT with Hiporfin for cHSIL, achieving an HPV negative conversion rate of 87.1% at 3–6 months post-treatment. In our study, the HPV negative conversion rates after PDT for cHSIL were 74.5%, 89.1%, 89.8%, and 95.8% at 3–6 months, 12 months, 24 months, and 36 months, respectively. These results are comparable to surgical outcomes, which showed conversion rates of 81.81%, 85.71%, and 90.91% at 6, 12, and 24 months, respectively49. Women with cHSIL are at high risk of anal HPV infection. It has been suggested that self-inoculation of the virus from the anal canal to the cervix may explain HPV recurrence in the cervix after treatment of cHSIL55,56,57,58. Pino et al.59found that women with anal HPV infection who were treated for cHSIL might have a higher risk of recurrent cervical HPV infection even after successful treatment. Therefore, monitoring HPV infection status in the anus will be an important focus for future research and clinical management of the female lower reproductive tract.
However, the HPV negative conversion rates for vaHSIL were not as high. Liu et al.16 treated 18 patients with vaHSIL using Hiporfin-PDT, achieving HPV negative conversion rates of 16.7%, 22.2%, and 44.4% at 3, 6, and 12 months, respectively. In this study, the HPV negative conversion rates for vaHSIL after Hiporfin-PDT were 28.0% at 3–6 months, 52.0% at 12 months, 60.9% at 24 months, and 64.7% at 36 months. The unique structure of the vagina, with its folds, can hinder complete light irradiation, leading to incomplete treatment. Traditional treatments for vaHSIL, such as CO2 laser or surgery, have shown similar HPV negative conversion rates, ranging from 38.2–77.2%17,60.
The HPV negative conversion rates were higher in the cHSIL group compared to the vaHSIL group and the multisite female lower genital tract HSIL group. This difference may be attributed to the adequate exposure of cervical lesions during PDT. Upon reviewing patients with vaHSIL and multisite female lower genital tract HSIL who have persistent HPV infection, it was found that the vast majority had undergone prior surgery and/or chemoradiotherapy for cHSIL or cervical cancer. These patients often have multisite lesions in the vaginal wall, with frequent involvement of the vaginal stump or vault, which may contribute to a relatively poor cure response and persistent HPV infection. Previous procedures such as LEEP or hysterectomy pose additional challenges for treatment16. Furthermore, in older patients with persistent HPV infection, increased vigilance is warranted for the potential risk of disease progression. Additionally, further analysis revealed that HPV negative conversion rates after Hiporfin-PDT were higher in primary HSIL compared to recurrent HSIL in the female lower reproductive tract.
Conclusion
In this study, the HPV negative conversion rate of Hiporfin-PDT was comparable to conventional treatments, with the added benefits of organ preservation and fertility conservation. However, the need to avoid light and the cost of photosensitizer limit the widespread use of this treatment. We hope that photosensitizer will be covered by medical insurance in the future to benefit more patients. The study had several limitations, including a small sample size, a single-arm trial design, the absence of a control group, and the lack of immune profiling. In the future, multi-center, larger prospective controlled trials are needed for validation, and exploring the potential immunological mechanisms of HPV clearance will also be a focus in subsequent stages.
Data availability
The data that support the findings of this study are available from the corresponding author, R.F.W. (E-mail addresses: wurf100@126.com), upon reasonable.
References
Cree, I. A., White, V. A., Indave, B. I. & Lokuhetty, D. Revising the WHO classification: female genital tract tumours. Histopathology 76, 151–156. https://doi.org/10.1111/his.13977 (2020).
Zhao, F. H. et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in china: a pooled analysis of 17 population-based studies. Int. J. Cancer. 131, 2929–2938. https://doi.org/10.1002/ijc.27571 (2012).
Aho, M., Vesterinen, E., Meyer, B., Purola, E. & Paavonen, J. Natural history of vaginal intraepithelial neoplasia. Cancer 68, 195–197. https://doi.org/10.1002/1097-0142(19910701)68:1<195::aid-cncr2820680135>3.0.co;2-l (1991).
Smith, J. S., Backes, D. M., Hoots, B. E., Kurman, R. J. & Pimenta, J. M. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obstet. Gynecol. 113, 917–924. https://doi.org/10.1097/AOG.0b013e31819bd6e0 (2009).
Watson, M., Saraiya, M. & Wu, X. Update of HPV-associated female genital cancers in the united states, 1999–2004. J. Womens Health (Larchmt). 18, 1731–1738. https://doi.org/10.1089/jwh.2009.1570 (2009).
Gunderson, C. C., Nugent, E. K., Elfrink, S. H., Gold, M. A. & Moore, K. N. A contemporary analysis of epidemiology and management of vaginal intraepithelial neoplasia. Am. J. Obstet. Gynecol. 208, 410e411–410e416. https://doi.org/10.1016/j.ajog.2013.01.047 (2013).
Tainio, K. et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta-analysis. Bmj 360, k499. https://doi.org/10.1136/bmj.k499 (2018).
Gustafsson, L. & Adami, H. O. Natural history of cervical neoplasia: consistent results obtained by an identification technique. Br. J. Cancer. 60, 132–141. https://doi.org/10.1038/bjc.1989.236 (1989).
Peto, J., Gilham, C., Fletcher, O. & Matthews, F. E. The cervical cancer epidemic that screening has prevented in the UK. Lancet 364, 249–256. https://doi.org/10.1016/s0140-6736(04)16674-9 (2004).
Burchell, A. N., Winer, R. L., de Sanjosé, S. & Franco, E. L. Chapter 6: epidemiology and transmission dynamics of genital HPV infection. Vaccine 24 (Suppl 3), 52–61. https://doi.org/10.1016/j.vaccine.2006.05.031 (2006).
Preti, M., Scurry, J., Marchitelli, C. E. & Micheletti, L. Vulvar intraepithelial neoplasia. Best Pract. Res. Clin. Obstet. Gynaecol. 28, 1051–1062. https://doi.org/10.1016/j.bpobgyn.2014.07.010 (2014).
Wu, E. Q. et al. Prevalence of type-specific human papillomavirus and pap results in Chinese women: a multi-center, population-based cross-sectional study. Cancer Causes Control. 24, 795–803. https://doi.org/10.1007/s10552-013-0162-8 (2013).
Preti, M. et al. Human papillomavirus genotyping in high-grade vaginal intraepithelial neoplasia: A multicentric Italian study. J. Med. Virol. 96, e29474. https://doi.org/10.1002/jmv.29474 (2024).
Ayala, M. & Fatehi, M. In StatPearls (StatPearls Publishing Copyright © 2025 (StatPearls Publishing LLC., 2025).
Liu, Y., Wu, R., Li, C., Wei, L. & Li, R. Photodynamic therapy with HiPorfin for cervical squamous intraepithelial lesion at childbearing age. Photodiagnosis Photodyn Ther. 46, 104018. https://doi.org/10.1016/j.pdpdt.2024.104018 (2024).
Liu, Y. et al. HiPorfin photodynamic therapy for vaginal high-grade squamous intraepithelial lesion. Arch. Gynecol. Obstet. 310, 1197–1205. https://doi.org/10.1007/s00404-024-07600-4 (2024).
Bogani, G. et al. LASER treatment for women with high-grade vaginal intraepithelial neoplasia: A propensity-matched analysis on the efficacy of ablative versus excisional procedures. Lasers Surg. Med. 50, 933–939. https://doi.org/10.1002/lsm.22941 (2018).
Fiascone, S., Vitonis, A. F. & Feldman, S. Topical 5-Fluorouracil for women with High-Grade vaginal intraepithelial neoplasia. Obstet. Gynecol. 130, 1237–1243. https://doi.org/10.1097/aog.0000000000002311 (2017).
Rountis, A., Pergialiotis, V., Tsetsa, P., Rodolakis, A. & Haidopoulos, D. Management options for vaginal intraepithelial neoplasia. Int. J. Clin. Pract. 74, e13598. https://doi.org/10.1111/ijcp.13598 (2020).
Schnürch, H. G. et al. Diagnosis, therapy and Follow-up of vaginal cancer and its precursors. Guideline of the DGGG and the DKG (S2k-Level, AWMF registry 032/042, October 2018). Geburtshilfe Frauenheilkd. 79, 1060–1078. https://doi.org/10.1055/a-0919-4959 (2019).
Noehr, B., Jensen, A., Frederiksen, K., Tabor, A. & Kjaer, S. K. Depth of cervical cone removed by loop electrosurgical excision procedure and subsequent risk of spontaneous preterm delivery. Obstet. Gynecol. 114, 1232–1238. https://doi.org/10.1097/AOG.0b013e3181bf1ef2 (2009).
Castanon, A. et al. Risk of preterm delivery with increasing depth of excision for cervical intraepithelial neoplasia in england: nested case-control study. Bmj 349, g6223. https://doi.org/10.1136/bmj.g6223 (2014).
Kyrgiou, M. et al. Morbidity after local excision of the transformation zone for cervical intra-epithelial neoplasia and early cervical cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 75, 10–22. https://doi.org/10.1016/j.bpobgyn.2021.05.007 (2021).
Loopik, D. L. et al. Cervical intraepithelial neoplasia and the risk of spontaneous preterm birth: A Dutch population-based cohort study with 45,259 pregnancy outcomes. PLoS Med. 18, e1003665. https://doi.org/10.1371/journal.pmed.1003665 (2021).
Wallbillich, J. J. et al. Vulvar intraepithelial neoplasia (VIN 2/3): comparing clinical outcomes and evaluating risk factors for recurrence. Gynecol. Oncol. 127, 312–315. https://doi.org/10.1016/j.ygyno.2012.07.118 (2012).
Mahto, M., Nathan, M. & O’Mahony, C. More than a decade on: review of the use of imiquimod in lower anogenital intraepithelial neoplasia. Int. J. STD AIDS. 21, 8–16. https://doi.org/10.1258/ijsa.2009.009309 (2010).
Rogers, L., Sergeeva, N. N., Paszko, E., Vaz, G. M. & Senge, M. O. Lead structures for applications in photodynamic therapy. 6. Temoporfin Anti-Inflammatory conjugates to target the tumor microenvironment for in vitro PDT. PLoS One. 10, e0125372. https://doi.org/10.1371/journal.pone.0125372 (2015).
Brown, S. B., Brown, E. A. & Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 5, 497–508. https://doi.org/10.1016/s1470-2045(04)01529-3 (2004).
Huang, Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 4, 283–293. https://doi.org/10.1177/153303460500400308 (2005).
Shanazarov, N. et al. Assessment of systemic reaction to inflammation induced by photodynamic therapy in cervical intraepithelial neoplasia. Photodiagnosis Photodyn Ther. 50, 104416. https://doi.org/10.1016/j.pdpdt.2024.104416 (2024).
Huang, Z. An update on the regulatory status of PDT photosensitizers in China. Photodiagnosis Photodyn Ther. 5, 285–287. https://doi.org/10.1016/j.pdpdt.2009.01.005 (2008).
Huang, Z. Photodynamic therapy in china: over 25 years of unique clinical experience part One-History and domestic photosensitizers. Photodiagnosis Photodyn Ther. 3, 3–10. https://doi.org/10.1016/s1572-1000(06)00009-3 (2006).
Huang, Z. Photodynamic therapy in china: over 25 years of unique clinical experience part two-Clinical experience. Photodiagnosis Photodyn Ther. 3, 71–84. https://doi.org/10.1016/j.pdpdt.2006.03.001 (2006).
Zeng, R. et al. Clinical efficacy of HiPorfin photodynamic therapy for advanced obstructive esophageal cancer. Technol. Cancer Res. Treat. 19, 1533033820930335. https://doi.org/10.1177/1533033820930335 (2020).
Liao, C., Shi, L., Wang, D. & Wang, X. Bimodal photodynamic therapy for treatment of a 91-year-old patient with locally advanced cutaneous basal cell carcinoma and postoperative Scar management. Photodiagnosis Photodyn Ther. 36, 102553. https://doi.org/10.1016/j.pdpdt.2021.102553 (2021).
Li, L. et al. Treatment of perianal paget’s disease using photodynamic therapy with assistance of fluorescence examination: case report. Lasers Med. Sci. 24, 981–984. https://doi.org/10.1007/s10103-009-0653-8 (2009).
Gao, S., Dong, C. & San, T. Expert consensus on the clinical application of photodynamic therapy for esophageal cancer. J. Esophageal Dis. 2, 1–7. https://doi.org/10.15926/j.cnki.issn2096-7381.2020.01.001 (2020).
Wang, H., Zou, H. & Jin, F. Chinese expert consensus on the clinical application of photodynamic therapy for respiratory tumors. Chin. J. Lung Dis. (Electronic Edition). 13, 6–12. https://doi.org/10.3877/cma.j.issn.1674-6902.2020.01.002 (2020).
Liu, Y. et al. Photodynamic therapy compared with loop electrosurgical excision procedure in patients with cervical high-grade squamous intraepithelial lesion. Sci. Rep. 14, 27090. https://doi.org/10.1038/s41598-024-78445-9 (2024).
Qiao, J. et al. Systemic photodynamic therapy vs. Loop electrosurgical excision: treatment of hight grade squamous intraepithelial lesion. Chin. J. Laser Med. Surg. 33, 181–188. https://doi.org/10.13480/j.issn1003-9430.2024.0181 (2024).
Liu, Y. et al. Consensus of Chinese experts on hematoporphyrin Injection-Based photodynamic therapy for High-Grade squamous intraepithelial lesions of cervices (2025). Chin. J. Laser Med. Surg. 34, 30–35 (2025).
Li, H., Guo, Y. L., Zhang, J. X., Qiao, J. & Geng, L. Risk factors for the development of vaginal intraepithelial neoplasia. Chin. Med. J. (Engl). 125, 1219–1223 (2012).
Zhang, S. et al. The prevalence of VAIN, CIN, and related HPV genotypes in Japanese women with abnormal cytology. J. Med. Virol. 92, 364–371. https://doi.org/10.1002/jmv.25611 (2020).
(CSCCP). E. C. O. T. C. S. F. C. A. C. P. Chinese expert C.nsensus O. C.rvical C.ncer screening A.d management O. A.normalities. Chin. J. Clin. Obstet. Gynecol. 18, 286–288 (2017).
Byun, J. M. et al. Persistent HPV-16 infection leads to recurrence of high-grade cervical intraepithelial neoplasia. Med. (Baltim). 97, e13606. https://doi.org/10.1097/md.0000000000013606 (2018).
Fernández-Montolí, M. E. et al. Vulvar High-Grade squamous intraepithelial lesions treated with imiquimod: can persistence of human papillomavirus predict recurrence?? Cancers (Basel). 14 https://doi.org/10.3390/cancers14194808 (2022).
Ao, M., Zheng, D., Wang, J., Gu, X. & Xi, M. Risk factors analysis of persistence, progression and recurrence in vaginal intraepithelial neoplasia. Gynecol. Oncol. 162, 584–589. https://doi.org/10.1016/j.ygyno.2021.06.027 (2021).
Zang, L., Huang, J., Zhu, J. & Hu, Y. Risk factors associated with the persistence of human papillomavirus after cervical excision in patients with high-grade squamous intra-epithelial neoplasia. Eur. J. Obstet. Gynecol. Reprod. Biol. 266, 175–181. https://doi.org/10.1016/j.ejogrb.2021.09.023 (2021).
Lu, J., Han, S., Li, Y., Na, J. & Wang, J. A study on the correlation between the prognosis of HPV infection and lesion recurrence after cervical conization. Front. Microbiol. 14, 1266254. https://doi.org/10.3389/fmicb.2023.1266254 (2023).
Baser, E. et al. Risk factors for human papillomavirus persistence among women undergoing cold-knife conization for treatment of high-grade cervical intraepithelial neoplasia. Int. J. Gynaecol. Obstet. 125, 275–278. https://doi.org/10.1016/j.ijgo.2013.12.012 (2014).
Chan Lee, J. K. & Jeong, C. H. Young Jeong Na, In Ho Kim, Sun Young Lee, Seung Jo Kim. Photodynamic therapy in the management of cervical intraepithelial neoplasia. Korean J Gynecol Oncol Colposc 15, 85–91, doi: (2004). https://doi.org/10.3802/kjgoc.2004.15.2.85
Korbelik, M. Induction of tumor immunity by photodynamic therapy. J. Clin. Laser Med. Surg. 14, 329–334. https://doi.org/10.1089/clm.1996.14.329 (1996).
Choi, M. C. et al. Photodynamic therapy for management of cervical intraepithelial neoplasia II and III in young patients and obstetric outcomes. Lasers Surg. Med. 45, 564–572. https://doi.org/10.1002/lsm.22187 (2013).
Gilyadova, A. V. et al. Comparative study of treatment efficacy in severe intraepithelial squamous cell lesions and preinvasive cervical cancer by conization and Chlorin e6-mediated fluorescence-assisted systemic photodynamic therapy. Photodiagn. Photodyn. Ther. 46 https://doi.org/10.1016/j.pdpdt.2024.104060 (2024).
Goodman, M. T. et al. Sequential acquisition of human papillomavirus (HPV) infection of the anus and cervix: the Hawaii HPV cohort study. J. Infect. Dis. 201, 1331–1339. https://doi.org/10.1086/651620 (2010).
Pino, M. D. et al. Natural history of anal HPV infection in women treated for cervical intraepithelial neoplasia. Cancers (Basel). 15 https://doi.org/10.3390/cancers15041147 (2023).
Nasioutziki, M. et al. Cervical, anal and oral HPV detection and HPV type concordance among women referred for colposcopy. Infect. Agent Cancer. 15 https://doi.org/10.1186/s13027-020-00287-7 (2020).
Moscicki, A. B. et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine 30 (Suppl 5), F24–33. https://doi.org/10.1016/j.vaccine.2012.05.089 (2012).
Pamnani, S. J. et al. Sequential acquisition of anal human papillomavirus (HPV) infection following genital infection among men who have sex with women: the HPV infection in men (HIM) study. J. Infect. Dis. 214, 1180–1187. https://doi.org/10.1093/infdis/jiw334 (2016).
Wang, Y., Kong, W. M., Wu, Y. M., Wang, J. D. & Zhang, W. Y. Therapeutic effect of laser vaporization for vaginal intraepithelial neoplasia following hysterectomy due to premalignant and malignant lesions. J. Obstet. Gynaecol. Res. 40, 1740–1747. https://doi.org/10.1111/jog.12383 (2014).
Acknowledgements
We sincerely thank all the patients who participated in the study.
Funding
This work was supported by Shenzhen Public Platform for Preservation of Fertility and Reproduction (XMHT20220104049); the National Key R&D Program of China (Program Nos. 2024YFC2707503); Shenzhen Key Medical Discipline Construction Fund(No. SZXK027).
Author information
Authors and Affiliations
Contributions
Y.L. contributed to project development, data analysis and manuscript writing. R.Z.L. supervised the project and was involved in manuscript editing and reviewing. H.D. supervised the project. R.F.W. and C.Z.L. contributed to support project development and manuscript reviewing. All of the authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study involving human participants was approved by the Ethics Committee from Peking University Shenzhen Hospital (No.2020(049)).
Consent to participate
Each patient provided their written informed consent to participate in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Li, R., Du, H. et al. HPV negative conversion following Hiporfin PDT for HPV associated HSIL in the female lower reproductive tract. Sci Rep 15, 31973 (2025). https://doi.org/10.1038/s41598-025-17292-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17292-8