Abstract
Signaling lymphocytic activation molecule (SLAM) family receptors are widely expressed on immune cells, often acting as self-ligands and playing crucial roles in cellular communication and adhesion, thereby modulating immune responses. Several studies have demonstrated that SLAM family receptors are associated with potential immune checkpoints on T cells and play a role in tumor immunity in various cancers. However, the effect of SLAMF1 expression in tumors has been rarely investigated. Here, we confirmed SLAMF1 expression using tissue microarray analysis in breast cancer tissues with diverse pathological characteristics and subtypes. Additionally, SLAMF1 expression in triple-negative breast cancer (TNBC) cells was analyzed using flow cytometry and real-time PCR. Public clinical data analysis suggests that a positive correlation exists between SLAMF1 expression and overall survival and that SLAMF1 levels are slightly increased in patients with breast cancer who received radiation therapy. Similarly, when TNBC cells were irradiated, SLAMF1 expression specifically increased compared to that in non-irradiated cells. To study the biological function of SLAMF1 in mice, we established 4T1-SLAMF1 overexpressing a stable cell line. In the 4T1 syngenetic tumor model, SLAMF1 overexpression triggered strong infiltrating-CD8+ T cell responses and significantly reduced the tumor growth. Our results provide clear evidence for SLAMF1 expression in breast cancer and provide insights into the recent advances in SLAM-based targeted immunotherapies.
Similar content being viewed by others
Introduction
Breast cancer is the most common cancer diagnosed in females worldwide and is a leading cause of mortality and morbidity1. This heterogeneous disease is characterized by extensive molecular alterations that drive invasion and metastasis2,3. Various studies have identified numerous gene mutations in breast cancer cells and categorized them into four subtypes in clinical practice based on immunohistochemical results: luminal A, luminal B, epidermal growth factor receptor 2 (HER2)-enriched, and triple-negative breast cancer (TNBC). Luminal A is characterized by high estrogen receptor (ER), progesterone receptor (PR)-positive, and HER2-negative, which indicates slower cell growth, better prognosis, and better responses to hormon therapy. While luminal B are also hormone receptor-positive but can be either HER2 positive or negative, and often more aggressive than luminal A. It indicating faster cell growth and may be treated with hormon therapy and chemotherapy. HER2-enriched cancer have high levels of HER2, which are often more aggressive but can benefit from HER2-targeted therapy. TNBC dose not express ER, PR, and HER2 and accounts for 15–20% of breast cancer cases4. Despite the lack of drug-targetable receptors and an overall poorer prognosis5there is no specific treatment strategies for TNBC. Currently, TNBC is managed with conventional treatment, including systemic therapy and locoregional therapy, such as surgery and radiation therapy (RT). However, the cure rate of TNBC is low due to significant toxicity, relapse, or treatment refractoriness, highlighting the urgent need for developing innovative therapeutic strategies using predictive biomarkers for TNBC.
Cancer immunotherapy has recently emerged as a ground-breaking advancement in oncology. Clinical trials conducted between 2010 and the present have demonstrated the remarkable antitumor activity of diverse immunotherapeutic approaches, including immune checkpoint inhibitors (ICIs) and adoptive cellular therapies, across a range of cancer types. Although breast cancer has traditionally shown limited immunogenicity6,7recent challenges in immunotherapy and increasing evidence of the important role of the immune system in cancer epidemiology have led to extensive investigation of immunotherapeutic strategies for patients with breast cancer8,9. The KEYNOTE-012 trial evaluated the efficacy of pembrolizumab in pretreated programmed cell death ligand 1 (PD-L1)-positive metastatic TNBC and reported an overall response rate (ORR) of 18.5% was observed10. KEYNOTE-086 and JAVELIN trials demonstrated an ORR of 21.4% (pembrolizumab) and 5.2% (avelumab), respectively6,7. Moreover, the combination of chemotherapy with ICIs has demonstrated significantly improved ORR results compared to ICI monotherapy or chemotherapy alone for treating metastatic TNBC11,12. A recent area of clinical interest is the use of radiation to enhance response to immunotherapy. Radiation increases mutational load of tumors, optimizes antigen presentation, and may act to promote the recruitment and infiltration of immune cells in the tumor microenvironment (TME), priming the tumor for immunotherapy13,14,15. Radiotherapy is used as a tool to elicit or amplify clinically actionable signalling pathways in cancer patients, and as a means to convert immunologically cold and therefore ICI-resistant tumors into ICI-responsive hot lesions16. Overall, it reflects a well-defined and predictable safety profile, and a non-negligible potential for immunostimulation (at least in some settings, depending on dose, fractionation schedule and target volume)17. RT and anti-PD-L1 treatment synergistically promoted antitumor immunity and reversed immune resistance18. Pilones et al. showed that anti-CTLA-4 combined with RT effectively suppressed the lung metastasis of breast cancer in a mouse model19. Other molecules that target ICIs to prevent the inhibition of T cells (such as CTLA-4, LAG3, and TIGIT) or to stimulate T cells (such as OX-40 and 4-1BB) have been developed9. Given these previous challenges, further research is essential to develop effective combination strategies of personalized RT and immunotherapy.
The SLAMF1/CD150 receptor is a member of the signaling lymphocyte activation molecule (SLAM) family of cell surface receptors. Following the name of SLAM, SLAMF1 is widely expressed in immune cells, including T- lymphocytes, B-lymphocytes, natural killer cells (NKs), macrophages, and dendritic cells (DCs)20,21,22,23. It plays a pivotal role in regulating both innate and adaptive immunity and in maintaining the tissue microenvironment. SLAMF1 engages with its ligands to recruit intercellular signaling molecules such as SH2-containing tyrosine phosphatase 2 (SHP-2), phosphatidylinositol 3-kinase (PI3K), and mitogen-activated protein kinase (MAPK) p38, thereby leading to lymphocyte activation22,24. Recent studies have shown that SLAMF is variably expressed in several non-hematologic cancers. SLAMF1 may increase methotrexan resistance by triggering protective autophagy in choriocarcinomas25. In central nervous system (CNS) tumors, SLAMF1 can serve as a new diagnostic marker and a potential target for innovative therapeutic approaches in the future26. Assessment of The Cancer Genome Atlas (TCGA) databases demonstrated that the SLAMF1/CD27/SELL multigene signature can be used as a biomarker to predict the prognosis and personalized immunotherapy for cervical cancer27. Particularly, most of the SLAM family receptors are expressed within breast cancer, affecting patient survival and prognosis. According to a study by Gordiienko et al., SLAMF1 expression in breast tumor was demonstrated in an in silico study based on public data and patient DNA microarray and the authors reported the subellular localization and several isoform expression of SLAMF1 in breast cancer cell lines28.
To sum up, although SLAMF1 has been reported to be expressed in several cancer types, no studies have validated its protein expression in breast cancer on evidence, and the precise role of its incrased expression in tumors remains largely unknown. Therefore, this study aims to investigate SLAMF1 expression in breast cancer tissues and TNBC cell lines. Additionally, we examined whether SLAMF1 exerts its anti-tumor effect by suppressing tumor progression through immune cell activation in an in vivo mouse model. Our results demonstrated that SLAMF1 is expressed in breast cancer and influences immune cell activity within the tumor microenvironment, which requires further clarification and interpretation.
Results
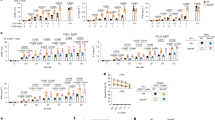
SLAMF1 gene expression in various cancer types
We first investigated SLAMF1 expression in various normal and tumor tissues using the publicly accessible TCGA data-enabled UCSC Xena server. SLAMF1 expression was significantly upregulated in multiple cancer types, including brain (p < 0.001), breast (p < 0.001), cervical (p = 0.002), kidney (p = 0.005), liver (p = 0.01), pancreas (p < 0.001), stomach (p < 0.001), and ovarian (p < 0.001) cancers, compared to normal tissue samples. Conversely, bladder and lung cancers exhibited a notable decrease in SLAMF1 expression (Fig. 1A). We specifically analyzed carcinomas in patients undergoing RT and validated the SLAMF1 expression in these patients receiving RT treatment. In particular, SLAMF1 expression was significantly elevated in patients with breast cancer who received RT (p = 0.0016) (Fig. 1B). Furthermore, high SLAMF1 levels exhibited significantly better survival rates than its low expression in patients with breast carcinoma (BRCA, p = 0.041, HR = 0.71), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC, p = 0.00039, HR = 0.49), head and neck squamous cell carcinoma (HNSC, p = 0.00059, HR = 0.62), and lung adenocarcinoma (LUAD, p = 0.00034, HR = 0.57) patients, which obtained through analysis using the GEPIA2 sever (Fig. 1C), suggesting that SLAMF1 is expressed in various solid tumors and is associated with the long-term survival of patients with cancer.
SLAMF1 expression and survival analysis in various cancers. (A) SLAMF1 gene levels were compared in various normal and tumor tissues using the UCSC Xena platform (https://xenabrowser.net) with data from TCGA. (B) The analysis included comparisons of SLAMF1 expression in patient tissues with and without radiotherapy. (C) The association of SLAMF1 expression with overall survival was evaluated using data from the GEPIA2 server (http://gepia2.cancer-pku.cn/#index).
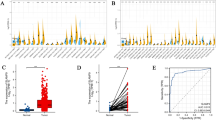
SLAMF1 upregulation in malignant breast cancer
According to the above TCGA data analysis results, higher SLAMF1 expression was associated with improved survival rates and in patients with four types of cancer, but SLAMF1 expression levels was elevated only in breast cancer patients receiving radiotherapy. Because we are investigating a novel biomarker to increase the efficacy of RT or a predictor of response to combined treatment of immunotherapy and radiotherapy, elucidating the role of SLAMF1 in breast cancer would be relevant for these purposes. We conducted an immunofluorescence staining analysis of a tissue microarray (TMA) comprising 192 human cancer spots to investigate SLAMF1 protein expression levels in breast cancer. Analysis of 10 pathological areas revealed varying expression levels: weak expression in benign, adjacent normal breast cancer, and cancer-adjacent breast cancer; moderate expression in ductal carcinoma, inflammation, and hyperplasia; and very strong expression in metastatic carcinoma, invasive carcinoma, invasive lobular carcinoma, and squamous cell carcinoma (Fig. 2A, B). Additionally, upon the classification of breast cancers into four subtypes, TNBC (ER−PR−HER2−) exhibited significant SLAMF1 expression upregulation compared to luminal A (ER+PR+HER2−) and HER2 positive (ER−PR−HER2+) tissues (Fig. 2C). Moreover, SLAMF1 expression was higher in grade 2 and 3 tumors compared to negative stage (Fig. 2D). No significant correlation was observed with patient age (p = 0.0865) (Fig. 2E). These results demonstrate that SLAMF1 is highly expressed in malignant breast cancer stages. Before this study, evidence of SLAMF1 protein expression in breast cancer was lacking. The results obtained through TMA and bioinformatics analysis indicated the presence of SLAMF1 mRNA and protein expression in breast cancer; however, further discussion and validation experiments are needed to elucidate the importance and potential clinical significance of SLAMF1.
SLAMF1 expression in breast cancer subtypes. (A) Individual tissue spots on a slide containing both normal breast tissue and six types of breast cancer were stained with an anti-SLAMF1 antibody, followed by an Alexa488 polyclonal antibody. Nuclei were counterstained with DAPI. SLAMF1 is shown in green, and nuclei are shown in blue. (B) Positive expression of SLAMF1 was counted and quantified in the six types of human breast cancers. (C) SLAMF1 expression in four different breast cancer subtypes—luminal A, luminal B, HER2 positive, and TNBC—was quantified by immunofluorescence positive score. The relationship between SLAMF1 expression and breast cancer grade (D), as well as patient age (E), was visualized. Statistical significance is indicated as * p < 0.05, ** p < 0.01, *** p < 0.001.
SLAMF1 expression in RT-treated breast cancer cell lines
As shown in Fig. 1, SLAMF1 expression was increased in patients with BRCA who received RT. Therefore, we further investigated the effects of RT on SLAMF1 expression in human TNBC cell lines. MDA-MB-231 and BT549 cells were selected as representative TNBC cell lines, and SLAMF1 gene levels were analyzed after irradiation with 3 and 12 Gy. High-dose irradiation increased SLAMF1 mRNA expression in TNBC cell lines (Fig. 3A). Next, we confirmed the cell surface expression of SLAMF1 after irradiation using flow cytometry. As shown in Fig. 3B, SLAMF1 expression was significantly increased by high-dose irradiation of MDA-MB-231 and BT549 cells. Therefore, our results provide new evidence that irradiation can activate sufficient cellular signaling mechanisms to enable SLAMF1 detection on breast cancer cell surfaces.
SLAMF1 expression in human breast cancer cell lines. (A) Human TNBC cell lines, MDA-MB-231 and BT549, were irradiated with 3 Gy and 12 Gy for 24 h, and then total RNA was isolated. SLAMF1 gene expression was determined by RT-qPCR. (B) SLAMF1 protein expression in TNBC cell lines was detected by flow cytometry. The data shown are representative of those obtained from three independent experiments. Statistical significance is indicated as * p < 0.05, ** p < 0.01, *** p < 0.001.
Antitumor activity of SLAMF1 in a murine tumor transplant model
To investigate SLAMF1-mediated immune responses in an in vivo model, we employed a tumor model transplanted with 4T1 murine breast cancer cells overexpressing SLAMF1. First, 4T1 cells were stably transfected with either an SLAMF1 overexpression plasmid (4T1-SLAMF1over) or an empty vector (4T1-Mock), and SLAMF1 expression levels were determined via RT-qPCR and flow cytometry. SLAMF1 expression in 4T1-SLAMF1over cells was significantly increased at both mRNA and protein levels compared to 4T1-Mock cells, confirming successful transfection (Fig. 4A). Following the transplantation of 4T1-Mock and SLAMF1over cells into mice, tumor volume was measured at 2–3 times per week. The result demonstrated that SLAMF1 overexpression delayed tumor growth compared to the control group, as shown in the xenograft growth curve (Fig. 4B). On day 15 post-transplantation, tumor volume was significantly reduced in SLAMF1 overexpression group (1085.0 ± 80.6 mm3 compared to the control group (1352.2 ± 91.2 mm3, p < 0.05) (Fig. 4C). Additionally, SLAMF1-overexpressing mice exhibited reduced spleen mass and splenocyte counts compared to those of controls (Fig. 4D), while total leukocyte counts in peripheral blood remained unchanged between groups. Notably, SLAMF1-overexpressing 4T1 mice demonstrated an increase in lymphocyte counts and a reduction in neutrophils, whereas monocyte, erythrocyte, and platelet levels were unaffected (Fig. 4E). To further examine SLAMF1-mediated systemic immune responses, we analyzed immune cell subpopulations in the spleen and tumor-infiltrating immune cells by flow cytometry. SLAMF1-overexpressing 4T1 mice demonstrated increased proportions of T cell subpopulations (TCRβ+, CD3+, CD4+, and CD8+) and a decrease in macrophages (CD11b+) within spleens (Fig. 4F). Similarly, SLAMF1 overexpression was notably increased infiltrating-CD8+ T cell and decreased infiltrating-macrophages (CD11b+) in tumor tissues (Fig. 4G). Overall, these results suggested that high SLAMF1 expression in breast cancer induced antitumor effects via an increase in CD8+ cytotoxic T cells and a decrease in tumor-infiltrating macrophages.
Antitumor activity of SLAMF1 overexpression in murine breast cancer cells. (A) The establishment of SLAMF1 overexpression in 4T1 cells was analyzed using RT-qPCR and flow cytometry. (B) BALB/c mice were injected subcutaneously in the right flank with either 4T1-Mock or 4T1-SLAMF1over cells on day 0. Tumor growth was monitored in each experimental group throughout the study period. (C) Tumor volume was presented at the end of the experiment. (D) Spleen weight and total splenocyte counts were measured post-sacrifice. (E) Complete blood cell counts were analyzed from EDTA-treated whole blood obtained from each mouse at the end of the study. (F) Splenocyte subpopulations were labeled with fluorescence-conjugated antibodies and analyzed by flow cytometry. (G) Tumor-infiltrating lymphocytes were examined by flow cytometry. Data represent means ± SEM of 6–8 mice per experiment. Statistical significance is represented as * p < 0.05, ** p < 0.01, *** p < 0.001 compared to 4T1-Mock.
Discussion
SLAMF1 acts as an immune costimulatory factor on T-and B-lymphocytes, NK cells, and antigen-presenting cells29,30. SLAMF1 functions as a self-ligand to initiate a signal transduction pathway and increase lymphocyte activation during B-T lymphocyte interactions. SLAMF1 is highly expressed in thymocytes, T- and B-lymphocytes (overexpressed upon activation), DCs, platelets, hematopoietic stem cells, and macrophages31,32. Additionally, the SLAM family has been implicated in tumor immunity and signaling pathways across various carcinoma types, such as breast cancer28, CNS tumors26, prostate cancer28, gastric cancer33, colorectal cancer34, and gynecological cancer25,35. As previously described, SLAMF1 expression is associated with immune cell responses and the tumor microenvironment. However, these findings can not fully explain the characteristics and major functions of SLAMF1 expression beyond the hematopoietic system, especially within solid tumors.
This study focused on SLAMF1 expression in malignant breast cancer and its role in promoting antitumor immune response. TCGA data analysis revealed upregulated SLAMF1 gene expression in breast, brain, cervix, kidney, liver, pancreas, stomach, and ovarian cancers compared to normal tissues, with particularly high expression in breast cancer patients receiving RT treatment. Furthermore, elevated SLAMF1 levels correlated with improved survival rates in breast carcinoma (Fig. 1). This is consistent with findings in gastric cancer and colorectal cancer where SLAMF1 expression is associated with favorable survival outcomes33,34supporting a potential role for SLAMF1 in enhancing antitumor immunity.
When we analyzed SLAMF1 expression in breast cancer patient samples using TMA staining, SLAMF1 expression was detected in various breast cancer pathology subtypes, and higher expression levels were associated with more advanced breast cancer stages (Fig. 2), suggesting that SLAMF1 expression in malignant tumors may promote immune cell infiltration and shift an immunosuppressive TME to an immune-friendly one. Collectively, these results underscore the relevance of SLAMF1 as a favorable immune target for the treatment of malignant tumors, offering novel insights into its role in tumor immunology and patient prognosis.
RT is one of the traditional oncology treatments and is the most common therapeutic modality for cancers. Many studies have accepted the concept that RT can not only induce cellular damage but also trigger a systemic immune response leading to immunogenic cell death (ICD)36,37. After triggering ICD, multiple damage-associated molecular patterns were released and recognized by DCs. DCs can further present these antigens to cytotoxic T cells and also activate NK cells thus inducing immune modulations38,39,40. The immunomodulatory effects of RT lead to systemic antitumor responses. However, tumor elimination by RT-induced immune microenvironment regulation also triggers immunosuppression responses such as the polarization of macrophages with the M2 phenotype41, neutrophils with N2 phenotype42,43, and accumulation of myeloid-derived suppressor cells44,45. These cells can generally induce immunosuppressive effects via the expression of PD-L1 and other mechanisms46. Some studies have shown that PD-L1 expression is upregulated in the irradiated tumor sites, mediating radioresistance18,47. Our recent data reveal that radiation exposure increases the expression of inhibitory immune checkpoint PVR (CD155) expression in breast cancer. Notably, blocking PVR enhances tumor cell killing by promoting cytotoxic T lymphocyte activation48. RT simultaneously modulates various ICs of cancer cells, including immunosuppressive and costimulatory factors, and the outcome may vary depending on the intrinsic immunogenicity of tumors as well as the surrounding environment. Therefore, it is very difficult to predict the immunological efficacy of RT against tumors, and the underlying mechanisms are largely unknown, requiring comprehensive investigation.
Herein, we noted that SLAMF1 expression was specifically increased in breast cancer after RT, and confirmed RT-induced SLAMF1 expression in malignant TNBC cell lines through additional in vitro experiments. Radiation significantly and dose-dependently increased SLAMF1 expression in both TNBC cell lines (Fig. 3). The increase in SLAMF1 expression induced by RT could be expected to be a positive event, leading to the activation of the immune cells and antitumor activity. This is supported by previous studies that the survival rate of patients with high SLAMF1 expression in colorectal cancer was significantly higher than that of patients with low SLAMF1 expression, strongly suggesting that SLAMF1 may act as an antitumor biomarker in colorectal cancer34. The precise mechanism underlying radiation-induced SLAMF1 expression has not yet been fully elucidated, but it is likely to function in the same manner as the case presented below. As mentioned above, RT has been shown to significantly induce PD-L1 expression, with the potential mechanisms including the DNA damage signaling pathway49, the interferon gamma signaling pathway46,50, the cGAS-STING pathway51,52, and the epidermal growth factor receptor pathway53,54. Notably, all these mechanisms are associated with the JAK-STAT pathway55,56. Therefore, research should be conducted to explore these signaling pathways.
We further examine whether SLAMF1 overexpression in tumor cells could induce antitumor immunity and suppress tumor growth in an in vivo transplant model (Fig. 4). SLAMF1 overexpression in breast cancer cells led to a significant reduction in tumor progression, attenuation of tumor-induced splenomegaly, and decrease of neutrophils counts. Moreover, increased SLAMF1 expression in tumors modulated immune activation by enhancing the infiltration of CD8+ T lymphocytes while decreasing macrophage populations within both spleen and tumor tissues. These results suggest that increased SLAMF1 expression in breast cancer may enhance anticancer effect via immune activation, but the exact role of SLAMF1 in immune response within the TME remains unclear. Therefore, further investigations are required to elucidate the target immune cells, secreted cytokines, and mechanisms of action of SLAMF1 in modulating tumor immunity.
Most immunotherapy targeting immune inhibitory mechanisms mediated by CTLA-4 and PD-1 or its ligand PD-L1 have shown durable responses and survival benefits in melanoma, lung, and renal cell carcinoma57,58,59,60. Conversely, activating immune stimulatory pathways can enhance tumor immunogenicity and improve tumor antigen presentation, thereby promoting tumor suppression. Andarini et al. reported that costimulatory factor OX40 ligand-transduced tumor cells could elicit tumor-specific Th1 immune responses, generate anti-tumor immunity, and inhibit tumor growth in vivo61. Gough MJ et al. showed that OX40 agonistic therapy contributed to the priming of anti-tumor CD8+ effector T cells and augmented CD8+ T cell responses to tumor-derived antigens62. Therefore, the activation of immune stimulatory factors helps to modulate the quality, duration, and amplitude of the immune response and promote T cell expansion and activation signal.
This study has the following limitations. First, despite using a large number of breast cancer patient tissues, it could not provide results that accurately distinguished tumor cells from immune cells. In addition to our TMA analysis results, it would have been better to provide more definitive evidence using tumor cell-specific or immune cell-specific multiple staining. Second, the role of SLAMF1 induced by radiation was not confirmed through animal experiments. Although most researchers expect that in vitro results will translate well to in vivo results, differences in responses are inevitable when using human breast cancer cell lines and animal-derived tumor cell lines. Therefore, in follow-up studies, we are confirming the anticancer effect of SLAMF1 overexpression itself and the anticancer enhancement effect when combined with radiotherapy through a humanized mouse model.
Overall, our findings demonstrate that SLAMF1 expression is significantly elevated in various cancers compared to normal tissues, and high SLAMF1 levels are associated with improved survival in certain cancer patient populations. In breast cancer tissues, particularly in high-grade malignancies, SLAMF1 expression was notably upregulated, and SLAMF1 expression was further increased in TNBC cell lines following radiation exposure. Breast cancer cells with high SLAMF1 expression demonstrated suppressed tumor growth and heightened infiltration of CD8+ T cells, suggesting that SLAMF1 plays an important role as a potential mediator of RT-induced immune stimulation in breast cancer, providing new evidence for overcoming the limitations of immunotherapy and as a good therapeutic target candidate.
Materials and methods
Cell culture and irradiation
Human TNBC cell lines BT549, MDA-MB-231, and mouse breast cancer 4T1 cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). These cells were cultured in RPMI1640 medium (Welgene, Gyengsangbuk-do, Republic of Korea) supplemented with 10% fetal bovine serum (FBS, Welgene) and 100 U/mL penicillin/streptomycin (Welgene) at 37 ℃ under a 5% CO2 atmosphere. The human TNBC cells were exposed to various radiation doses (3–12 Gy) using 137Cs γ-source Biobeam 8000 irradiator (Gamma-Service Medical GmbH, Leipzig, Germany) at a dose rate of 3.5 Gy/min.
Bioinformatics analysis
Data retrieved from TCGA via the UCSC Xena platform (https://xenabrowser.net) were used to analyze the comparative SLAMF1 levels in normal and tumor tissues with and without radiotherapy. SLAMF1 mRNA expression-based overall survival of various patients with cancer was analyzed using GEPIA2 (gepia2.cancer-pku.cn/#index).
Tissue microarray with Immunofluorescence (IF) staining
The breast cancer tissue microarray (TMA, #BR2082b) was purchased from TissueArray.Com LLC (Derwood, MD, USA) and comprised 192 individual tissue spots. Within this set, 192 tumor samples contained 10 distinct pathological regions, and four subtypes were identified. Patient and tumor characteristics were sourced from the pathological reports provided by the manufacturer for subsequent analysis. The TMA slides were deparaffinized and rehydrated. The slides were boiled in citrate for antigen retrieval and blocked with 3% bovine serum albumin (GenDEPOT, Katy, TX, USA) for 1 h. Primary antibodies were incubated at 4 °C overnight: CD150 polyclonal antibody (#PA5-21123, Thermo Fisher Scientific, Waltham, MA, USA) at 20 µg/ml. On the next day, the slide was incubated with an Alexa Fluor® 488 polyclonal antibody (#A-11094, Invitrogen, Waltham, MA, USA) and normalized to those of DAPI (4′,6-diamidino-2-phenylindole). Quantitative immunofluorescence analysis of antibody-stained breast cancer TMA slides was conducted using the Woodang Network. Inc. (Seoul, Republic of Korea), a specialized analytical institution for precise data extraction. Images were acquired using a Carl Zeiss Microscope GmbH, Axioscan 7 (Zeiss, Jena, Germany) using the Zen 2.3 software (Zeiss) for image processing and quantitative analysis. The IF score was determined based on the extent and positive counts of staining.
Quantitative real-time PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen). The mRNA expression was assessed by real-time quantitative RT-PCR using the PowerUp™ SYBR™ Green Master Mix for qPCR (Applied Biosystems™, Waltham, MA, USA) with a LightCycler® 96 Real-Time PCR System (Roche, Basel, Switzerland). The sequences of primers were as follows and were purchased from Bioneer (Daejeon, Korea): human SLAMF1 forward 5’-TGTGGCTTACAGCTGGAGTG-3’, reverse 5’-TGCTGATAGGGTTGCTCACG-3’; human 18S ribosomal RNA forward 5’- CACGCCAGTACAAGATCCCA-3,’ reverse 5’-TTCACGGAGCTTGTTGTCCA-3’; mouse SLAMF1 forward 5’-CATGCAAGCAGGAATCCTCCTC-3’, reverse 5’- TTGCCGTGAAAACCAGGATGAAG-3’; mouse 18S ribosomal RNA forward 5’- CGAAAGCATTTGCCAAGAAT-3’, reverse 5’-AGTCGGCATCGTTTATGGTC-3’. The quantity of the test genes and internal control 18S ribosomal RNA was determined from a standard curve using the LightCycler® 96 software and was compared with that of controls.
Flow cytometry
Surface protein SLAMF1 expression in human breast cancer cell lines MDA-MB-231 and BT549, mouse breast cancer cell line 4T1 was detected by direct immunofluorescence using a FITC anti-human CD150 antibody (A12(7D4), 306306, BioLegend, San Diego, CA, USA) and APC anti-mouse CD150 antibody (TC15-12F12.2, 115910, BioLegend). The harvested cells were incubated with the antibody (5 µl per 100 µl staining volume, as recommended by the manufacturer) for 1 h at 4 ℃, followed by washing with 2% FBS in phosphate-buffered saline (PBS) to remove unbound antibody. Stained cells were obtained using the CyFlow® Cube6 flow cytometer (Sysmex-Partec GmbH, Görlizt, Germany) and analyzed using the FlowJo software (v.10, Tree Star, Ashland, OR, USA).
Establishment of a stable cell line
To establish SLAMF1-overexpressing breast cancer cells, the full length of SLAMF1 (MG226063, Slamf1 Mouse Tagged ORF Clone, NM_013730) or negative control scramble (PS100010, pCMV6-AC-GFP Mammalian expression vector) was transfected into murine breast cancer cell 4T1 using a lentivirus vector (Origene, Rockville, MD, USA). Briefly, 10 × 104 cells were seeded into a 60 mm dish and medium containing Lipofectamin 3000 (Thermo Fisher Scientific) and a lentiviral vector was added the following day. The next day, a total of 1 µg/ml neomycin (Invivogen, San Diego, CA, USA) was used following culture for 3 days to select the stable cell lines. Following the selection, the cells were cultured in a standard neomycin-containing medium, confirming mRNA and protein expression levels.
Mice and tumor model
Female BALB/c mice (6–8 weeks old) were purchased from Orient Bio (Seongnam-si, Gyeonggi-do, Republic of Korea) and maintained under specific pathogen-free conditions. 4T1 cells and SLAMF1 overexpression stable cells (5 × 105 cells/100 µL PBS) were subcutaneously injected into the right thigh. Tumor volume was calculated 2–3 times per week after tumor administration using three orthogonal planes (V = (L × W × W)/2, where V (mm3 is tumor volume, L (mm) is tumor length, and W (mm) is tumor width). Body weights from each group were measured 2–3 times per week. Mice were administered intraperitoneally appropriate concentrations of alfaxalone (80 mg/kg, Alfaxan®, Jurox Inc., MO, USA) and xylazine (10 mg/kg, Rompun®, Elanco Animal Health, IN, USA) for sacrifice. After the anesthesia, blood samples were collected from individual mice at the sacrificed time points via retro-orbital plexus bleeding using heparinized capillary tubes. Complete blood cell counts were determined using a VETSCAN HM5 hematology analyzer (Abasix, CA, USA). Spleen and tumor tissues were harvested from experimental mice and subjected to immunophenotypic analysis. The animal experiments were conducted in accordance with the ARRIVE guidelines and approved by the Institutional Animal Care and Use Committee of Korea Institute of Radiological & Medical Sciences (KIRAMS 2022-0141).
Isolation and phenotypic analysis of splenocytes and tumor-infiltrating lymphocytes (TILs)
Isolated splenocytes were subjected to immunostaining as previously described63. For TIL isolation, tumor tissues were mechanically cut into small pieces and enzymatically dissociated with RPMI 1640 medium (Welgene) with 500 µL collagenase/hyaluronidase solution (#07912, STEMCELL Technologies, Vancouver, BC, Canada) and 150 µg/mL DNase I solution (#07900, STEMCELL Technologies) according to the manufacturer’s instructions, and incubated for 25 min at 37 °C with shaking. The digested samples were passed through a 70 μm nylon cell strainer to obtain a single-cell suspension. The filtered cells were incubated with ammonium-chloride-potassium lysis buffer (BP10-548E, Lonza, Basel, Switzerland) for 5 min at room temperature to lyse red blood cells. After lysis, cells were immediately washed with RPMI1640 by centrifugation at 300 × g for 5 min to remove residual lysis buffer. The collected cells were stained with the following monoclonal antibodies (5 µl per 100 µl staining volume, according to the manufacturer’s instructions) for 1 h at 4 ℃; TCRβ-APC (H57-597) and CD11c-PE (HL3) purchased from BD Biosciences (San Diego, CA, USA); and CD3-FITC (17A2), CD4-APC (RM4-5), CD8a-APC (53 − 6.7), B220-APC (RA36B2), NK1.1-FITC (PK136), and CD11b-FITC (M1/70) purchased from BioLegend. Stained cells were obtained using the CyFlow® Cube6 (Sysmex-Partec) and analyzed using the FlowJo software (v.10, Tree Star).
Statistical analysis
GraphPad Prism software version 8 (GraphPad, La Jolla, CA, USA) was used to perform statistical analyses. All data are expressed as mean ± standard error of the mean. Analysis of variance and Tukey’s post-hoc test were performed to determine significant between-group differences.
Data availability
The original data generated and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
References
Loibl, S., Poortmans, P., Morrow, M., Denkert, C. & Curigliano, G. Breast cancer. Lancet 397, 1750–1769. https://doi.org/10.1016/S0140-6736(20)32381-3 (2021).
Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70. https://doi.org/10.1038/nature11412 (2012).
Januskeviciene, I. & Petrikaite, V. Heterogeneity of breast cancer: the importance of interaction between different tumor cell populations. Life Sci. 239, 117009. https://doi.org/10.1016/j.lfs.2019.117009 (2019).
Yin, L., Duan, J. J., Bian, X. W. & Yu, S. C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 22, 61. https://doi.org/10.1186/s13058-020-01296-5 (2020).
Derakhshan, F. & Reis-Filho, J. S. Pathogenesis of Triple-Negative breast cancer. Annu. Rev. Pathol. 17, 181–204. https://doi.org/10.1146/annurev-pathol-042420-093238 (2022).
Adams, S. et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 30, 397–404. https://doi.org/10.1093/annonc/mdy517 (2019).
Dirix, L. Y. et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res. Treat. 167, 671–686. https://doi.org/10.1007/s10549-017-4537-5 (2018).
Esteva, F. J., Hubbard-Lucey, V. M., Tang, J. & Pusztai, L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 20, e175–e186. https://doi.org/10.1016/S1470-2045(19)30026-9 (2019).
Debien, V. et al. Immunotherapy in breast cancer: an overview of current strategies and perspectives. NPJ Breast Cancer. 9 https://doi.org/10.1038/s41523-023-00508-3 (2023).
Nanda, R. et al. Pembrolizumab in patients with advanced Triple-Negative breast cancer: phase Ib KEYNOTE-012 study. J. Clin. Oncol. 34, 2460–2467. https://doi.org/10.1200/JCO.2015.64.8931 (2016).
Adams, S. et al. Atezolizumab plus nab-Paclitaxel in the treatment of metastatic Triple-Negative breast cancer with 2-Year survival Follow-up: A phase 1b clinical trial. JAMA Oncol. 5, 334–342. https://doi.org/10.1001/jamaoncol.2018.5152 (2019).
Schmid, P. et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 21, 44–59. https://doi.org/10.1016/S1470-2045(19)30689-8 (2020).
Weichselbaum, R. R., Liang, H., Deng, L. & Fu, Y. X. Radiotherapy and immunotherapy: a beneficial liaison? Nat. Rev. Clin. Oncol. 14, 365–379. https://doi.org/10.1038/nrclinonc.2016.211 (2017).
Demaria, S., Coleman, C. N., Formenti, S. C. & Radiotherapy Changing the game in immunotherapy. Trends Cancer. 2, 286–294. https://doi.org/10.1016/j.trecan.2016.05.002 (2016).
Demaria, S., Bhardwaj, N., McBride, W. H. & Formenti, S. C. Combining radiotherapy and immunotherapy: a revived partnership. Int. J. Radiat. Oncol. Biol. Phys. 63, 655–666. https://doi.org/10.1016/j.ijrobp.2005.06.032 (2005).
Petroni, G., Cantley, L. C., Santambrogio, L., Formenti, S. C. & Galluzzi, L. Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer. Nat. Rev. Clin. Oncol. 19, 114–131. https://doi.org/10.1038/s41571-021-00579-w (2022).
Galluzzi, L. & Buque, A. Paradoxical control of multifocal mammary oncogenesis by radiation therapy. Oncoimmunology 14, 2458886. https://doi.org/10.1080/2162402X.2025.2458886 (2025).
Deng, L. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124, 687–695. https://doi.org/10.1172/JCI67313 (2014).
Pilones, K. A., Aryankalayil, J., Babb, J. S. & Demaria, S. Invariant natural killer T cells regulate anti-tumor immunity by controlling the population of dendritic cells in tumor and draining lymph nodes. J. Immunother Cancer. 2, 37. https://doi.org/10.1186/s40425-014-0037-x (2014).
Romero, X. et al. Differential expression of SAP and EAT-2-binding leukocyte cell-surface molecules CD84, CD150 (SLAM), CD229 (Ly9) and CD244 (2B4). Tissue Antigens. 64, 132–144. https://doi.org/10.1111/j.1399-0039.2004.00247.x (2004).
Laksono, B. M. et al. In vitro measles virus infection of human lymphocyte subsets demonstrates high susceptibility and permissiveness of both Naive and memory B cells. J. Virol. 92 https://doi.org/10.1128/JVI.00131-18 (2018).
Detre, C., Keszei, M., Romero, X., Tsokos, G. C. & Terhorst, C. SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions. Semin Immunopathol. 32, 157–171. https://doi.org/10.1007/s00281-009-0193-0 (2010).
Bleharski, J. R., Niazi, K. R., Sieling, P. A., Cheng, G. & Modlin, R. L. Signaling lymphocytic activation molecule is expressed on CD40 ligand-activated dendritic cells and directly augments production of inflammatory cytokines. J. Immunol. 167, 3174–3181. https://doi.org/10.4049/jimmunol.167.6.3174 (2001).
Yurchenko, M. et al. SLAMF1 is required for TLR4-mediated TRAM-TRIF-dependent signaling in human macrophages. J. Cell. Biol. 217, 1411–1429. https://doi.org/10.1083/jcb.201707027 (2018).
Shi, D., Zhang, Y. & Tian, Y. SLAMF1 promotes methotrexate resistance via activating autophagy in choriocarcinoma cells. Cancer Manag Res. 12, 13427–13436. https://doi.org/10.2147/CMAR.S278012 (2020).
Romanets-Korbut, O. et al. Expression of CD150 in tumors of the central nervous system: identification of a novel isoform. PLoS One. 10, e0118302. https://doi.org/10.1371/journal.pone.0118302 (2015).
Chen, Q. et al. Identification of a tumor microenvironment-related gene signature to improve the prediction of cervical cancer prognosis. Cancer Cell. Int. 21, 182. https://doi.org/10.1186/s12935-021-01867-2 (2021).
Gordiienko, I. M., Lykhova, O. O., Shcherbina, V. M. & Shlapatska, L. M. SLAMF1/CD150 expression and topology in prostate and breast cancer cell lines. Exp. Oncol. 43, 312–316. https://doi.org/10.32471/exp-oncology.2312-8852.vol-43-no-4.17010 (2021).
Calpe, S. et al. The SLAM and SAP gene families control innate and adaptive immune responses. Adv. Immunol. 97, 177–250. https://doi.org/10.1016/S0065-2776(08)00004-7 (2008).
Veillette, A. NK cell regulation by SLAM family receptors and SAP-related adapters. Immunol. Rev. 214, 22–34. https://doi.org/10.1111/j.1600-065X.2006.00453.x (2006).
Schwartzberg, P. L., Mueller, K. L., Qi, H. & Cannons, J. L. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat. Rev. Immunol. 9, 39–46. https://doi.org/10.1038/nri2456 (2009).
Veillette, A., Dong, Z. & Latour, S. Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity 27, 698–710. https://doi.org/10.1016/j.immuni.2007.11.005 (2007).
Chen, C. N. et al. Gene expression profile predicts patient survival of gastric cancer after surgical resection. J. Clin. Oncol. 23, 7286–7295. https://doi.org/10.1200/JCO.2004.00.2253 (2005).
Qi, J. et al. Single-cell transcriptomic landscape reveals tumor specific innate lymphoid cells associated with colorectal cancer progression. Cell. Rep. Med. 2, 100353. https://doi.org/10.1016/j.xcrm.2021.100353 (2021).
Jun, F., Peng, Z., Zhang, Y. & Shi, D. Quantitative proteomic analysis identifies novel regulators of methotrexate resistance in choriocarcinoma. Gynecol. Oncol. 157, 268–279. https://doi.org/10.1016/j.ygyno.2020.01.013 (2020).
Golden, E. B., Pellicciotta, I., Demaria, S., Barcellos-Hoff, M. H. & Formenti, S. C. The convergence of radiation and Immunogenic cell death signaling pathways. Front. Oncol. 2, 88. https://doi.org/10.3389/fonc.2012.00088 (2012).
Green, D. R., Ferguson, T., Zitvogel, L. & Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 9, 353–363. https://doi.org/10.1038/nri2545 (2009).
Manda, K., Glasow, A., Paape, D. & Hildebrandt, G. Effects of ionizing radiation on the immune system with special emphasis on the interaction of dendritic and T cells. Front. Oncol. 2, 102. https://doi.org/10.3389/fonc.2012.00102 (2012).
Bauer, M. et al. Human monocytes are severely impaired in base and DNA double-strand break repair that renders them vulnerable to oxidative stress. Proc. Natl. Acad. Sci. U S A. 108, 21105–21110. https://doi.org/10.1073/pnas.1111919109 (2011).
Wunderlich, R. et al. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin. Exp. Immunol. 179, 50–61. https://doi.org/10.1111/cei.12344 (2015).
Russell, J. S. & Brown, J. M. The irradiated tumor microenvironment: role of tumor-associated macrophages in vascular recovery. Front. Physiol. 4, 157. https://doi.org/10.3389/fphys.2013.00157 (2013).
Schernberg, A., Blanchard, P., Chargari, C. & Deutsch, E. Neutrophils, a candidate biomarker and target for radiation therapy? Acta Oncol. 56, 1522–1530. https://doi.org/10.1080/0284186X.2017.1348623 (2017).
Boivin, G. et al. Anti-Ly6G binding and trafficking mediate positive neutrophil selection to unleash the anti-tumor efficacy of radiation therapy. Oncoimmunology 10, 1876597. https://doi.org/10.1080/2162402X.2021.1876597 (2021).
Xu, J. et al. CSF1R signaling Blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 73, 2782–2794. https://doi.org/10.1158/0008-5472.CAN-12-3981 (2013).
Liang, H. et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 8, 1736. https://doi.org/10.1038/s41467-017-01566-5 (2017).
Garcia-Diaz, A. et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell. Rep. 19, 1189–1201. https://doi.org/10.1016/j.celrep.2017.04.031 (2017).
Dovedi, S. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 Blockade. Cancer Res. 74, 5458–5468. https://doi.org/10.1158/0008-5472.CAN-14-1258 (2014).
Song, K. H. et al. Poliovirus receptor Inhibition in breast cancer cells induces antitumor immunity via T cell activation. Am. J. Cancer Res. 13, 5966–5980 (2023).
Sato, H. et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 8, 1751. https://doi.org/10.1038/s41467-017-01883-9 (2017).
Permata, T. B. M. et al. High linear energy transfer carbon-ion irradiation upregulates PD-L1 expression more significantly than X-rays in human osteosarcoma U2OS cells. J. Radiat. Res. 62, 773–781. https://doi.org/10.1093/jrr/rrab050 (2021).
Du, S. S. et al. Radiation therapy promotes hepatocellular carcinoma immune cloaking via PD-L1 upregulation induced by cGAS-STING activation. Int. J. Radiat. Oncol. Biol. Phys. 112, 1243–1255. https://doi.org/10.1016/j.ijrobp.2021.12.162 (2022).
Deng, L., Liang, H., Fu, S., Weichselbaum, R. R. & Fu, Y. X. From DNA damage to nucleic acid sensing: A strategy to enhance radiation therapy. Clin. Cancer Res. 22, 20–25. https://doi.org/10.1158/1078-0432.CCR-14-3110 (2016).
van Janse, H. J. et al. The Hippo pathway component TAZ promotes immune evasion in human cancer through PD-L1. Cancer Res. 78, 1457–1470. https://doi.org/10.1158/0008-5472.CAN-17-3139 (2018).
Concha-Benavente, F. et al. Identification of the Cell-Intrinsic and -Extrinsic pathways downstream of EGFR and IFNgamma that induce PD-L1 expression in head and neck cancer. Cancer Res. 76, 1031–1043. https://doi.org/10.1158/0008-5472.CAN-15-2001 (2016).
Lailler, C. et al. DNA damage response- and JAK-dependent regulation of PD-L1 expression in head and neck squamous cell carcinoma (HNSCC) cells exposed to 5-fluorouracil (5-FU). Transl Oncol. 14, 101110. https://doi.org/10.1016/j.tranon.2021.101110 (2021).
Marzec, M. et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl. Acad. Sci. U S A. 105, 20852–20857. https://doi.org/10.1073/pnas.0810958105 (2008).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous Non-Small-Cell lung cancer. N Engl. J. Med. 373, 1627–1639. https://doi.org/10.1056/NEJMoa1507643 (2015).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl. J. Med. 373, 23–34. https://doi.org/10.1056/NEJMoa1504030 (2015).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced Renal-Cell carcinoma. N Engl. J. Med. 373, 1803–1813. https://doi.org/10.1056/NEJMoa1510665 (2015).
Postow, M. A. et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl. J. Med. 372, 2006–2017. https://doi.org/10.1056/NEJMoa1414428 (2015).
Andarini, S. et al. Adenovirus vector-mediated in vivo gene transfer of OX40 ligand to tumor cells enhances antitumor immunity of tumor-bearing hosts. Cancer Res. 64, 3281–3287. https://doi.org/10.1158/0008-5472.can-03-3911 (2004).
Gough, M. J. et al. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 68, 5206–5215. https://doi.org/10.1158/0008-5472.CAN-07-6484 (2008).
Song, K. H. et al. Analysis of immune cell populations and cytokine profiles in murine splenocytes exposed to whole-body low-dose irradiation. Int. J. Radiat. Biol. 91, 795–803. https://doi.org/10.3109/09553002.2015.1068461 (2015).
Acknowledgements
The authors would like to thank Dae-Seog Lim (CHA University, Korea) for feedback and discussion on the experimental concept.
Funding
This work was supported by the National Research Foundation of Korea under grant numbers RS-2025-02613113 and RS-2024-00355198 and the Korea Institute of Radiological and Medical Sciences under grant number 50531-2025, which was funded by the Korean government, Ministry of Science, and ICT.
Author information
Authors and Affiliations
Contributions
K.-H.S. and J.-Y.S. contributed to the conceptualization of the study. The original draft was written by K.-H.S. S.-Y.J. was responsible for developing the methodology, while K.-H.S. and J.I.P. conducted the formal analysis and in vivo study. K.-H.S. and D.H.L. performed the in vitro experiments and tissue array study. J.A. and S.-G.H. participated in the review and editing process. J.-Y.S. served as the supervisor of the project and was also responsible for project administration and funding acquisition. All authors approved the final version.
Corresponding author
Ethics declarations
Compeing interests
The authors declare no competing interests.
Ethical approval
All animal care and usage were done in accordance with federal policies and guidelines and approved by IACUC at the Korea Institute of Radiological & Medical Sciences.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, KH., Jung, SY., Park, JI. et al. SLAMF1 expression in breast cancer cells delays tumor growth in vivo. Sci Rep 15, 31247 (2025). https://doi.org/10.1038/s41598-025-17322-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17322-5