Abstract
The growing population of older adults necessitate built environment strategies that support sleep and overall health. This pilot study investigates whether dynamic electric lighting (intensity and CCT programmed to resemble day–night cycles) improves sleep among residents of a Jordanian care facility. Utilizing a pre–post within-subject design at Darat Samir Shamma, eight participants (mean age = 71.5 years; range = 60–82) experienced standard light followed by a dynamic lighting system. Objective sleep parameters were recorded using the Withings Sleep Analyzer, and subjective measures were assessed using the Pittsburgh Sleep Quality Index and Geriatric Depression Scale. Dynamic lighting was associated with marked gains in sleep consolidation: sleep quality increased by + 43.4% points (p < 0.001), total sleep time by + 3 h 08 min (p < 0.001), and sleep efficiency by + 16.4% points (p < 0.001). Bedtime advanced by − 2 h 39 min (earlier; p = 0.001) and time in bed increased by + 2.26 h (p < 0.001). WASO decreased by − 1 h 12 min (p = 0.002), and awakenings by − 1.39/night (p = 0.033). Snoring duration declined by − 13.6 min (p < 0.001). PSQI total scores changes significantly, decreasing from 7.00 under standard lighting to 4.00 under dynamic lighting (p = 0.017), reflecting better perceived sleep quality. No significant changes were observed in physiological markers or depressive symptoms. These findings support the potential of dynamic lighting as a non-pharmacological approach for enhancing sleep in institutional care settings. This research contributes context-specific insights from Jordan, where static electric lighting are common and related studies remain limited. Given the small sample size and pilot nature, larger-scale studies are recommended to confirm these preliminary results.
Similar content being viewed by others
Introduction
Sleep is a fundamental biological process essential for physical, cognitive, and psychological health. In older adults, its significance becomes even more pronounced due to its direct impact on health outcomes and overall quality of life1,2. Sleep disorders in this population increase the risk of developing severe health issues, such as cognitive decline, depression, and cardiovascular disease3,4,5,6. This highlights the critical need for targeted research and interventions to enhance sleep quality in this vulnerable population, particularly in care facilities where residents experience more disrupted sleep compared to home-dwelling peers7,8. With the increasing number of such facilities, ensuring their design supports residents’ health and well-being become essential.
The aging population is growing globally and regionally. Projections by the United Nations9 and the World Health Organization10 estimate that the number of older adults will more than double, reaching 2.1 billion by 2050. This demographic shift is projected to be especially pronounced in the Arab region, with the proportion of older adults is expected to rise from 6.7% in 2015 to 15.1% by 205011. In Jordan, individuals aged 60 and above currently make up 6.1% of the population12,13. The demand for long-term care facilities in Jordan is increasing14, yet little evidence links specific architectural choices in Jordanian facilities to measurable health outcomes, leaving practitioners without local design guidance.
A growing body of literature supports the importance of “healthy buildings”—environments that promote physical, mental, and social well-being throughout the building’s life cycle15. The concept extends beyond the absence of harmful elements, encompassing indoor air quality (IAQ), natural and artificial lighting, thermal comfort, acoustic control, biophilic design, and user agency over the environment15,16. For aging populations, healthy buildings prioritize access to daylight, control over lighting and temperature, and spaces that reduce stress while support circadian alignment—key strategies increasingly integrated into contemporary architectural practice15,16,17,18,19. Light plays a vital role in synchronizing the body’s internal clock, enhancing daytime alertness and nighttime sleep onset20,21. Research shows that morning light exposure improves sleep parameters, including latency, duration, and efficiency, while reducing dependence on sleep aids22,23. However, physiological aging alters light sensitivity: the crystalline lens yellows, pupils constrict, and retinal dopamine declines, reducing light input to intrinsically photosensitive retinal ganglion cells (ipRGCs). Older adults may require two- to four-fold higher melanopic light levels for equivalent circadian responses compared to younger individuals24,25. These limitations are particularly relevant in senior care facilities, where natural light access is often restricted and electric lighting is typically designed for visibility rather than biological impact26,27. Static lighting lacks spectral and intensity composition necessary for circadian regulation, leading to adverse effects such as delayed sleep onset, fragmented sleep, and reduced sleep efficiency26,27. This concern has prompted interest in integrative lighting that vary light spectrum and intensity over the day28.
Research on tunable and biodynamic lighting systems has shown promising results. Studies by Elliott, Tinsley29 and Collier, Durmus30 found improvement on sleep patterns, mood, and alertness in in older adults. Biodynamic systems, which replicate the natural light-dark cycle, have enhanced sleep continuity and daytime activity in care settings31,32. However, findings are not universal; outcomes vary based on lighting configuration, exposure timing, and participant health, necessitating further investigation into long-term effects and regional adaptability33. Cultural and environmental factors—such as conservative architectural practices, limited window sizes, and daytime indoor behavior due to heat or mobility—can reduce natural light exposure in Middle Eastern contexts. These conditions moderate the effects of lighting interventions and may explain variations in results across regions.
Despite growing research on the relationship between lighting and sleep, few studies explored lighting interventions in Jordanian care facilities. Current research lacks localized data on how electric lighting impact sleep health in these settings. This study compares dynamic lighting, which replicates natural daylight patterns, with standard static lighting to assess their effects on sleep disturbances in residents of a Jordanian senior care facility. The research aims to offer evidence-based recommendations for elderly care facilities, addressing the unique needs of this region’s growing aging population.
This study integrates insights from circadian biology, environmental psychology, and architectural design. It is anchored in the two-process model of sleep regulation, which describes how homeostatic sleep pressure interacts with the circadian pacemaker; inappropriate light exposure can disrupt this balance and trigger sleep disturbances. Dynamic lighting aims to realign circadian rhythms, shorten sleep latency, and improve sleep continuity. Environmental-psychology research supports this strategy, demonstrating that lighting and spatial conditions shape behaviour and well-being. Contemporary architectural frameworks likewise prioritise “healthy buildings,” where strategies like dynamic lighting are integral to creating spaces that support physical and psychological well-being—a guiding principle that guided the design and evaluation of the present study.
Methodology
Case study
This pilot study was conducted in Darat Samir Shamma, a non-profit senior care facility located in North Amman, Jordan. The facility was selected due to its comprehensive healthcare system, including regular medical evaluations, psychiatric and social support staff, and stable residential environment conducive to environmental interventions. The facility comprises 61 apartments. Each unit is 57 m2 and contains a living room, dining area, kitchen, sleeping space, and bathroom, plus a private garden, parking space, and balcony (Fig. 1). Because all apartments share an identical layout and are insulated from external noise, they provide a controlled environment for assessing the impact of dynamic lighting on sleep.
Study sample and participant selection
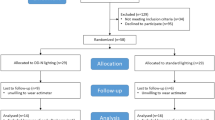
A purposive sampling approach was used to recruit participants based on strict inclusion and exclusion criteria tailored to the study’s focus on sleep outcomes. Eligible individuals were aged 60 or older and physically healthy. Exclusion criteria included cognitive impairment (Montreal Cognitive Assessment (MoCA) score < 25)34, partial or complete visual impairment, and psychiatric conditions such as schizophrenia, schizoaffective disorder, or paranoia, due to their known impact on sleep regulation. To further reduce potential confounding factors, individuals cohabiting with someone diagnosed with mental illness were also excluded.
Out of 50 residents at the facility, 31 expressed interest in participating. Following screening interviews and MoCA assessments,23 were excluded: 11 for cognitive impairment, 5 for schizophrenia, 1 for schizoaffective disorder, 2 for paranoia, 2 for other mental illnesses, and 2 for visual impairment. Ultimately, eight participants (mean age = 71.5 years; range = 60–82; 3 females) met all inclusion criteria and proceeded to the experimental phase.
Although the sample size is small, this was a pilot study designed to explore feasibility and initial effects. A priori power analysis using G*Power (version 3.1) based on a Wilcoxon signed-rank test for matched pairs. Assuming large effect size (Cohen’s d ≈ 1.1), α = 0.05 (two-tailed), and a desired power of 0.80, the analysis confirmed that a minimum of eight participants would be sufficient to detect a statistically significant effect, thereby justifying the sample size for this pilot investigation.
Ethical considerations
Ethical approval (Ref: 23/161/2023; 08 June 2023) was obtained from the Institutional Review Board (IRB) at Jordan University of Science and Technology (JUST) and the administrative board of Darat Samir Shamma. Participants provided informed consent, understanding the study’s purpose, scope, and their right to withdraw at any time. All data and documents were kept confidential, adhering to Darat Samir Shamma’s privacy policies. Researchers had exclusive access to the data, ensuring compliance with ethical and institutional guidelines.
Study design
The study used a within-subject experimental design with pre- and post-test evaluations, exposing participants first to standard electric lighting and then to dynamic lighting to assess effects on sleep quality.
The independent variable was the lighting system (standard vs. dynamic light), and dependent variables included both subjective and objective sleep parameters and depressive symptoms. Objective sleep data were collected using the Withings Sleep Analyzer (WSA), a non-wearable, clinically validated device that records sleep duration, latency, efficiency, heart rate, apnea, and snoring. Subjective assessments included the Pittsburgh Sleep Quality Index (PSQI)35 and the Geriatric Depression Scale (GDS)36. A demographic form captured participant characteristics, including age, gender, education, and health status. Appendix Table 1 summarizes the measured variables, their definitions, and corresponding measurement methods. The integration of subjective and objective measures allowed for a comprehensive assessment of the lighting intervention’s impact on sleep and well-being in older adult residents.
Light intervention
The lighting intervention employed a dynamic electric lighting system designed to reflect natural day–night cycles. Light levels and correlated color temperature (CCT) were programmed to change gradually across the day: beginning with cool, bright light in the morning (500 lx, 6500 K), moderating in the afternoon, and transitioning to dim, warm light in the evening (< 100 lx, 2700 K). This profile aimed to stimulate morning alertness and promote melatonin production at night. To quantify the circadian effectiveness of the lighting, both Circadian Stimulus (CS) and Equivalent Melanopic Lux (EML) values were calculated at key time points across the day, using spectral data and vertical illuminance measurements taken at eye level (1.2 m). CS, developed by Rea, Figueiro37, estimates melatonin suppression potential based on spectral sensitivity, while EML, recommended by the International WELL Building Institute and CIE, quantifies biologically effective light aligned with ipRGC sensitivity. The lighting design targeted CS values > 0.3 during the morning and < 0.1 during the evening, with midday EML values reaching ~ 485 to account for the reduced light sensitivity in older adults27.
Light simulation
Baseline lighting measurements were collected using calibrated meters and entered into DIALux Evo 10.1, an industry-standard software compliant with CIE and IES validation protocols, to simulate and optimize fixture placement, quantity, and spectral settings in participant apartments. The simulation incorporated:
-
3D Revit modeling of apartments, considering room dimensions, window orientation, furniture layout, and daylight exposure.
-
Simulation parameters: Using the DIALux library, Philips Hue Smart LED luminaires were selected for their tunable spectrum and dimming capabilities. Simulations incorporated surface reflectance and material characteristics (e.g., curtains, wall paint, and furniture), with windows modeled at 75% transmittance based on daylight analysis. Each apartment was divided into functional zones (living, dining, and sleeping areas).
-
Optimization: The system was designed to peak at 500 lx between 8:00–12:00 p.m. decrease to < 100 lx by 8:00 p.m., and drop below 20 lx one hour before bedtime. Table 1 presents the full daily temporal lighting profile, including corresponding EML and CS values at key intervals.
-
Installation: Based on simulation outcomes (Fig. 2), existing lighting was replaced with Philips Hue LEDs in all areas except kitchens and bathrooms. The system was controlled via Philips Hue Bridge with automated scheduling of light levels and CCT adjustments throughout the day. Figure 3 shows the visual results post-installation.
Implementation was validated through post-installation measurements, confirming that target light levels were achieved at vertical eye height (1.2 m) across key zones. The lighting protocol aligned with natural daylight patterns, automatically dimming electric light when ambient light was sufficient—particularly during morning hours near windows. Continuous monitoring and periodic spot checks ensured that the programmed temporal lighting profile was maintained throughout the study. Participants had access to smart switches allowed limited brightness adjustments for personal comfort, these adjustments remained within the constraints of the automated system. No manual overrides were observed during critical measurement periods, preserving the integrity of the intervention. Self-reported activity logs were used to estimate participants’ daily light exposure in different settings. On average, participants spent 3.5 to 4 h per day in their private apartments during daylight hours, where the dynamic lighting was active. Outdoor exposure (e.g., walks or garden visits) averaged 45 min to 1 h daily. Time spent in communal indoor areas (e.g., dining or recreation rooms) ranged from 1 to 2 h daily.
Withings sleep analyzer
The WSA is a clinically validated, non-wearable device placed under the mattress for contact-free monitoring of sleep patterns, breathing disturbances, and cardiovascular parameters. It uses ballistocardiography (BCG) sensors and machine learning algorithms to estimate total sleep time (TST), sleep efficiency, latency, interruptions, and potential apnea events. WSA has shown good agreement with polysomnography (PSG) and actigraphy, with comparable TST and efficiency values (e.g., 392.4 ± 67.2 vs. 366.6 ± 61.2 min) and predictive accuracy of 92.6% for moderate-to-severe obstructive sleep apnea38,39,40,41. Studies also report systematic biases: WSA may overestimate sleep time and efficiency and underestimate awakenings42,43.
In this study, WSA devices were placed under participants’ mattresses to collect nightly data over 22 nights (Fig. 4). The choice of a contact-free device was guided by literature and the care facility’s recommendations, as older adults may resist or remove wearable monitors. Data were automatically synced via the Health Mate app. While WSA offered a practical and non-intrusive solution, its limitations were recognized and factored into the interpretation of outcomes.
The WSA recorded nightly data across all study phases for eight participants. Measured variables comprised: core sleep outcomes—sleep quality (%), total sleep time (TST), and sleep efficiency (%); timing and continuity—bedtime, out-of-bed time, time in bed (TIB), sleep-onset latency (SOL), wake after sleep onset (WASO), rise latency (RL), and interruptions (count); sleep stages—light, deep, and REM sleep (% of TST); and physiology/rest—snoring duration, heart rate, apnea index, and in-bed napping. In addition, the WSA-derived global sleep quality score integrates six indicators (duration/TST, depth, interruptions, regularity, sleep latency, and wake-up time). Full definitions and measurement units are provided in (Appendix Table 1).
The Pittsburgh sleep quality index
The PSQI is a self-report tool assessing sleep quality and disturbances through 19 questions across seven components: subjective sleep quality, habitual sleep efficiency, sleep duration, sleep latency, sleep disturbances, use of sleep medications, and daytime dysfunction. Global scores range from 0 to 21, with lower scores indicating better sleep quality (0–4: good sleep; 5–10: poor sleep; >10: severe disturbances)35. A global score above 5 typically identifies poor sleepers. The PSQI has demonstrated validity and reliability in diverse older adult populations44,45, and its Arabic version is validated for Arabic-speaking individuals46.
Aligned with adaptations used in short-term pharmacological sleep trials, participants were instructed to respond to each PSQI item based on the previous week rather than the standard one-month reference period. Prior studies have shown that this modification maintains the instrument’s reliability for short-term assessments (e.g. Figueiro, Plitnick47, Cremascoli, Sparasci48. Snyder, Cai49, and Landry, Best45.
Geriatric depression scale
The GDS is a self-reported tool developed by Yesavage and Sheikh36 to evaluate depression symptoms in older adults. The short form, used in this study, includes 15 yes/no questions36. Ten questions indicate depression with positive responses, while the remaining five do so with negative responses. Scores are categorized as 0–4 (normal), 5–8 (mild depression), 9–11 (moderate), and 12–15 (severe). The Arabic version of the GDS has demonstrated validity comparable to the English version in older adults without dementia50,51.
Study procedures
The study followed five sequential phases:
Recruitment and baseline (Days 1–12): Eligible residents were screened through interviews, cognitive assessment (MoCA). Participants followed their usual routines under standard lighting to establish a baseline.
Standard lighting (Days 13–21): Participants remained under existing electric lighting while sleep was objectively monitored using the Withings Sleep Analyzer (WSA). GDS and PSQI were recorded on Day 21.
Transition (Days 22–23): A brief transition period facilitated installation of dynamic lighting equipment while maintaining default light exposure. This brief interval ensured technical readiness without attempting full circadian resetting.
Dynamic lighting intervention (Days 24–32): Dynamic lighting system—automatically adjusting level and color temperature—was activated in apartment units. Sleep data were collected via WSA. Participants completed the GDS and PSQI at the end of this phase.
Post-intervention (Days 33–34): The original standard lighting was reinstated for a two-day washout period, matching the standard light (Days 13–21). Final GDS, PSQI, and sleep recordings were collected to evaluate sustained improvements in mood and sleep quality under standard lighting.
Controlled variables and study conditions
-
Participant activities: All participants were full-time residents who followed structured daily routines centered on meals, medical checkups, and limited social interactions. They spent most of their time indoors between 8:00 a.m. and 8:00 p.m., with only brief periods of outdoor activity or communal dining.
-
Lighting exposure: Exposure was predominantly to indoor lighting, which was automated and standardized throughout the study. Outdoor light exposure was minimal and self-reported.
-
Room environment: Apartment lighting systems were fully automated and pre-programmed to maintain consistent and controlled lighting conditions.
Statistical analysis
Objective sleep parameters from the WSA were recorded nightly for each of the eight participants across the full 22-night protocol, yielding 22 observations per participant for each of the nine measured variables. Subjective data (GDS and PSQI) were collected at the end of each of the four study phases, resulting in four time-point measurements per participant. Descriptive statistics (means, standard deviations) were reported. Given the small sample size (n = 8) and the non-normal distribution of data, paired comparisons between the standard and dynamic lighting conditions were performed using the Wilcoxon signed-rank test. Statistical significance was set at two-tailed p < 0.05, and effect sizes (r = Z/√N) were calculated. All analyses were conducted using Minitab®52.
Results
A total of eight participants (3 females, 5 males) were enrolled in the study. The mean age was 71.5 years (SD = 10.77), with an age range of 60 to 82 years. Descriptive data across the study’s four key phases—baseline, standard lighting, dynamic lighting, and washout periods—are summarized in Table 2, including cognitive status, depression, subjective sleep quality, and objective sleep parameters.
Sleep measurement assessed via withings sleep analyzer
Core sleep outcomes
-
1.
Sleep quality score (%)
Exposure to dynamic lighting (DL) yielded significant improvements in sleep quality relative to standard lighting (SL). As shown in Table 2, the mean sleep quality score significantly rose from 30.94 ± 13.22% under standard lighting to 74.33 ± 11.46% during dynamic lighting ( p < 0.001, r = 0.89), indicating markedly improved sleep consolidation. Figure 5A plots each night’s quality score for SL1–SL9 versus DL1–DL9, revealing a sustained elevation throughout the dynamic-lighting phase.
-
2.
Total sleep time (TST) (hours and minutes)
TST significantly increased under DL conditions, rising from 4 h 32 min ± 49 min under SL to 7 h 40 min ± 1 h 28 min with DL (p < 0.001, r = 0.89). As shown in Fig. 5B, sleep duration consistently rose across the DL nights (DL1–DL9), indicating a sustained improvement from baseline levels (~ 4.5 h) to approximately 7.5 h.
-
3.
Sleep efficiency (%)
Sleep efficiency also improved markedly, from 73.49 ± 15.61% under SL to 89.9 ± 9.8% with DL (p < 0.001, r = 0.64), indicating better use of time spent in bed for actual sleep.
Sleep timing and structure
-
1.
Bedtime (hh: mm, 24-h)
Participants fell asleep significantly earlier under DL (21:44 pm ± 06:30) than under SL (00:23 am ± 10:39; p = 0.001).
-
2.
Total time in bed (TIB) (hours)
TIB increased from 6.30 ± 1.52 h under SL to 8.56 ± 1.48 h in DL (p < 0.001), reflecting both earlier sleep onset and longer nocturnal rest.
-
3.
Sleep-onset latency (SOL) (minutes)
SOL decreased from 19 ± 12 min under SL to 13 ± 11 min with DL (p = 0.096), suggesting faster sleep initiation under dynamic light.
-
4.
Wake after sleep onset (WASO) (hours and minutes)
Nighttime awakenings were significantly reduced by over an hour: WASO fell from 01 h 48 ± 00 h 57 in SL to 00 h 36 ± 00 h 43 in DL (p = 0.002, r = 0.59).
-
5.
Sleep interruptions (count per night)
The number of nightly awakenings decreased significantly from 3.47 ± 1.86 under SL to 2.08 ± 1.63 during DL (p = 0.033, r = 0.84). As illustrated in Fig. 5C, sleep interruptions declined consistently during the DL nights (DL1–DL9), with group means falling from approximately 3–4 to around 2 or fewer, reflecting enhanced sleep continuity.
-
6.
Out of bed time (hh: mm, 24-h)
Morning out-of-bed time remained stable (06:23 am ± 02:32 SL vs. 06:16 am ± 01:34 DL; p = 0.785), indicating that DL’s primary effect was on sleep onset rather than awakening.
Sleep depth (light, deep, REM %)
Sleep depth, measured by the duration of restorative sleep phases (light, deep, and REM sleep) showed no statistically significant changes between lighting conditions:
-
Deep Sleep: 29.04% ± 21.39 (standard) vs. 25.26% ± 21.89 (dynamic), p = 0.550.
-
REM Sleep: 17.39% ± 14.52 vs. 14.00% ± 12.79, p = 0.448.
-
Light Sleep: 62.40% ± 26.11 vs. 59.65% ± 23.30, p = 0.542.
These results suggest dynamic lighting did not significantly alter sleep microarchitecture, though individual variability was high.
Sleep vitals
The impact of dynamic lighting on physiological sleep parameters—snoring duration, sleep heart rate, and sleep apnea events per hour—was analyzed. The results are summarized in Table 2.
-
1.
Snoring duration (minutes)
Snoring time decreased significantly from 14 ± 18 min under standard lighting to 0.4 ± 11 min under dynamic lighting (p < 0.001), suggesting potential improvements in airway relaxation or breathing patterns. As shown in Fig. 5D, average snoring time dropped markedly during the dynamic lighting phase (DL1–DL9) and increased again during the washout, indicating a potential link to the lighting intervention.
-
2.
Sleep heart rate (beats per minute)
Sleep heart rate showed a slight reduction under dynamic lighting, with the mean decreasing from 56.72 ± 5.71 bpm under standard lighting to 55.76 ± 6.40 bpm under dynamic light. While this minor reduction may suggest improved autonomic regulation and relaxation during sleep, it was not statistically significant (p = 0.091).
-
3.
Sleep apnea events (events per hour)
Contrary to expectations, sleep apnea events increased under dynamic lighting, with the mean apnea index rising from 7.38 ± 11.72 events per hour under standard lighting to 9.25 ± 10.39/hr events per hour. Although this difference was not statistically significant, it suggests dynamic lighting may have limited direct impact on reducing sleep apnea symptoms.
Napping duration (hours and minutes)
Daytime napping, as recorded by the WSA (i.e., naps in bed), decreased slightly from 54 ± 57 min under standard lighting to 47 ± 54 min under dynamic lighting. This change was not statistically significant (p = 0.79). Naps outside the bed (e.g., on chairs or in communal areas) were not captured, and this limitation is acknowledged.
Transitional effects during washout periods
The short washout periods (2 days each) showed partial carryover effects. Washout 1, following standard lighting, showed slight improvements from baseline. Washout 2, after dynamic lighting, retained gains in sleep quality (62.13% ± 14.22), TST (6h03m ± 0:54), low WASO (0:36 ± 0:48), and fewer interruptions (2.44 ± 1.93)—suggesting short-term persistence of DL benefits beyond the intervention phase.
Sleep measurement by the Pittsburgh sleep quality index
PSQI scores presented in Fig. 6, demonstrated a significant improvement in global sleep quality following the dynamic lighting intervention. The mean score decreased from 7.00 ± 0.93 under standard lighting to 4.00 ± 1.93 under dynamic lighting (p = 0.017, r = 0.59), indicating a shift from poor to better sleep quality. After the dynamic phase, scores slightly increased to 6.13 ± 1.55, suggesting a partial loss of benefit but not a full return to baseline (8.25 ± 3.06), reinforcing the observed overall improvement. These results align with objective measures and support the intervention’s impact on subjective sleep quality.
Boxplot of mean global PSQI Values (0–3 scale; lower scores denote better outcome). Pittsburg Sleep Quality Index (PSQI), Baseline (BL), Standard Light (SL), Dynamic Light (DL), Washout 2 (W2). Blue dot (group mean), White dots (individual participant values). Boxes = Interquartile range (25th–75th percentiles) with median line; whiskers = min–max; open circles = individual scores; blue dots = means; gray line = connects means across phases.
As shown in Table 3; Fig. 7, Lighting conditions revealed significant improvements in two in two PSQI components—subjective sleep quality and sleep duration. Subjective sleep quality improved from 0.75 ± 0.46 to 0.13 ± 0.35 (p = 0.025, r = 0.59), and sleep duration improved from 1.75 ± 0.46 to 0.88 ± 0.83 (p = 0.038, r = 0.55), which, using a midpoint-based translation of the PSQI duration rubric, corresponds to an increase from ≈ 5:45 h to ≈ 6:37 h of sleep ( ≈ + 52 min). Other parameters, including sleep latency, efficiency, disturbance, medication use, and daytime dysfunction, showed non-significant changes (p > 0.05), though most were directionally improved. These findings suggest that the intervention notably improved perceived sleep quality and duration while modestly influencing other sleep aspects.
Depressive symptoms by the geriatric depression scale
GDS scores stayed within the minimal-symptom range (0–4) and showed a non-significant decrease from 3.00 ± 0.93 (baseline) to 2.50 ± 0.96 (standard light) and 2.00 ± 0.76 (dynamic light), with a slight rise at Washout 2 (2.13 ± 0.64; p = 0.089).
Discussion
This pilot study explored the effects of dynamic, electric lighting programmed to simulate natural light–dark cycles, on sleep quality among older adults residing in a care facility in Amman, Jordan. With growing evidence that the built environment significantly influences sleep health in aging populations53,54, circadian-aligned lighting has become a critical component of designing supportive living environments. While prior studies have shown that electric lighting strategies can improve sleep outcomes17,22,26,55, our research support those findings by examining dynamic electric lighting intervention in individual apartment units, providing a real-world application in a culturally specific context where such lighting systems are not widely adopted.
Data collected using the WSA showed significant improvements in sleep consolidation under dynamic lighting, including higher sleep quality, longer total sleep time (TST), greater sleep efficiency, earlier bedtimes, longer time in bed (TIB), shorter sleep-onset latency (SOL), reduced wake after sleep onset (WASO), and fewer nocturnal awakenings, while wake-up time remained stable. This pattern aligns with reports that increasing daytime/early-evening melanopic stimulation can strengthen circadian entrainment, advance sleep timing, and consolidate nocturnal sleep in older and institutionalized populations27,56. The concurrent reductions in WASO and awakenings are consistent with literature linking improved continuity to better cognitive and functional outcomes in older adults4,45,57.
Interpretation of these improvements should consider baseline characteristics. Mean TST during the standard-lighting phase was comparatively low relative to objective estimates reported for similar populations using methods such as polysomnography or actigraphy (e.g., 5:46 per Boulos, Jairam58; 5:20–6:48 in institutional settings per Concheiro-Moscoso, Groba59, and Spira, Beaudreau44. This discrepancy may amplify within-subject improvements and limit generalizability. A plausible explanation is either underestimation by the WSA or behavioral shifts under dynamic lighting—for example, higher melanopic light exposure may have reduced out-of-bed napping, thereby consolidating more sleep to nighttime bed rest as captured by the WSA. This aligns with prior findings that circadian-supportive lighting enhances sleep alignment and reduces nocturnal fragmentation27,56.
By contrast, sleep architecture (percent light, deep, REM) did not differ between lighting conditions. Mixed findings for architecture are common: several studies document macro-level gains (longer, less fragmented sleep) without consistent shifts in stage proportions, likely reflecting inter-individual variability, modality differences (bed sensors vs. PSG/actigraphy), and limited power to detect microstructural effects33,56,60,61. Our results fit this pattern, suggesting that dynamic lighting chiefly influences timing and continuity rather than the proportional allocation of stages.
Physiological markers were largely unchanged, except for a marked reduction in snoring duration under dynamic lighting. Although exploratory, this may indicate improved upper-airway stability or posture secondary to more consolidated sleep or earlier bedtimes; related work has noted links between circadian alignment and respiratory stability, though evidence remains heterogeneous4,62. Heart rate and apnea index did not differ significantly, indicating that the principal benefits observed here pertain to behavioral consolidation rather than cardiorespiratory parameters per se.
A slight, non-significant decrease in nap duration was observed. As naps were recorded exclusively by a bed-mounted sensor, out-of-bed sleep episodes—such as naps taken in chairs or lounges—were not captured, potentially underestimating total 24-hour sleep. Given this limitation of the WSA, future studies should incorporate wrist-worn actigraphy to enable a more comprehensive assessment of daytime rest and to validate nocturnal estimates. This approach would capture full sleep–wake cycles, including non-bedtime naps and daytime activity patterns.
Self-reported sleep quality measured via the PSQI paralleled objective sleep outcomes from the WSA. The global PSQI score improved significantly, decreasing from 7 under standard lighting to 4 under dynamic lighting (p = 0.017). Additionally, subjective sleep quality and sleep duration subscales both showed meaningful reductions (p < 0.05), reflecting a shift from poor to improved sleep quality. Other PSQI components (sleep latency, efficiency, disturbances, and duration) did not differ significantly (ps > 0.05). Descriptively, their means were directionally favorable under dynamic lighting, which may reflect limited power in this small sample. These findings align with previous research demonstrating that circadian-aligned lighting interventions improve self-reported sleep quality in older adults23,27. However, the impact of dynamic lighting on mood and depressive symptoms was less pronounced.
The mean GDS decreased slightly from 2.50 under standard lighting to 2.00 following dynamic lighting exposure, but this change was not statistically significant (p = 0.54). This modest reduction may be attributed to the relatively short duration of the intervention. Previous studies have shown that meaningful mood improvements through light therapy—particularly with blue-enriched light—often require several weeks of sustained exposure55,63,64. Additionally, participants in this study began with minimal depressive symptoms, which may have limited the potential for further improvement.
This study highlights the need for context-sensitive design solutions tailored to the lifestyle, social norms, and environmental conditions of older adults in Jordan. Based on promising preliminary findings, we recommend several design improvements for senior care facilities. These include the integration of dynamic, tunable lighting systems that adjust intensity and color temperature throughout the day to reinforce circadian rhythms. Smart, user-centered lighting controls (e.g., dimmers, programmable scenes) can empower residents to customize their lighting environment, promoting autonomy and comfort. Additionally, combining natural and electric lighting through smart systems is crucial for creating adaptable environments responsive to external light variations. Although derived from a small-scale pilot study, these recommendations offer a valuable framework for future research and practical applications aimed at enhancing the quality of life in Jordanian senior care facilities.
Limitations
The primary limitation of this study is its small sample size (n = 8), which restricts statistical power and generalizability. This limitation also affects the internal consistency of findings and highlight the need for cautious interpretation. Measurement limitations also warrant consideration. The Withings Sleep Analyzer, while practical for unobtrusive data collection, may underestimate total sleep duration by failing to capture naps taken outside the bed. Additionally, by focusing on selected variables and outcomes, our study may have overlooked other influential factors—such as, daytime light exposure (including melanopic EDI), medication use, comorbidities, room/environmental conditions (noise, temperature), physical activity, and staff routines—that could modulate sleep. Finally, to directly assess whether dynamic lighting stabilizes and enhances circadian rhythmicity, future work should include objective circadian phase and amplitude measures, such as 24-h urine collections at 2–3-h intervals to quantify 6-sulfatoxymelatonin (aMT6s) profiles (phase and amplitude). Larger, more diverse samples will be important to confirm the robustness and generalizability of these preliminary findings.
Conclusion
This pilot study demonstrates the potential of circadian-supportive lighting systems to enhance sleep quality among older adults residing in Darat Samir Shamma in Amman, Jordan. Significant improvements in both objective and subjective metrics of sleep under dynamic lighting were observed, particularly in sleep quality, sleep duration and interruptions. While mood changes were minimal, the overall findings highlight the importance of integrating lighting design into health-supportive architecture. Given the exploratory nature and limited sample size, replication with larger samples and more rigorous environmental and behavioral monitoring is recommended to inform design guidelines and lighting standards in eldercare environments.
Data availability
The data supporting this study are available from the corresponding author, B.O., upon request.
References
Foley, D. J. et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep 18 (6), 425–432. https://doi.org/10.1093/sleep/18.6.425 (1995).
Irwin, M. R. Sleep and inflammation: partners in sickness and in health. Nat. Rev. Immunol. 19 (11), 702–715. https://doi.org/10.1038/s41577-019-0190-z (2019).
Spira, A. P., Chen-Edinboro, L. P., Wu, M. N. & Yaffe, K. Impact of sleep on the risk of cognitive decline and dementia. Curr. Opin. Psychiatry. 27 (6), 478–483. https://doi.org/10.1097/YCO.0000000000000106 (2014).
Yaffe, K., Falvey, C. M. & Hoang, T. Connections between sleep and cognition in older adults. Lancet Neurol. 13 (10), 1017–1028. https://doi.org/10.1016/S1474-4422(14)70172-3 (2014).
Cappuccio, F. P., Cooper, D., D’Elia, L., Strazzullo, P. & Miller, M. A. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J. 32 (12), 1484–1492. https://doi.org/10.1093/eurheartj/ehr007 (2011).
Baglioni, C. et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 135 (1–3), 10–19. https://doi.org/10.1016/j.jad.2011.01.011 (2011).
Lamb, S. E. et al. Development of a common outcome data set for fall injury prevention trials: the prevention of falls network Europe consensus. J. Am. Geriatr. Soc. 53 (9), 1618–1622. https://doi.org/10.1111/j.1532-5415.2005.53455.x (2005).
Martin, J. L., Alam, T., Harker, J. O., Josephson, K. R. & Alessi, C. A. Sleep in assisted living facility residents versus home-dwelling older adults. J. Gerontol. Biol. Sci. Med. Sci. 63 (12), 1407–1409. https://doi.org/10.1093/gerona/63.12.1407 (2008).
Nations, U. World Population Ageing 2017—Highlights2017 accessed.on. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf
Organization, W. H. Aging and Health2021 accessed.on. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
ESCWA UNEaSCfWA. Ageing in the Arab region: Trends, implications and policy options2017 accessed.on. https://www.unescwa.org/publications/ageing-arab-region-trends-implications-policy-options
HelpAge, I. Importance of Care and Protection for Older People in Jordan. 2020:1–18.
AlHalaseh, L. J. Population ageing in the Eastern mediterranean countries: a regional overview of the situation Jordan. Middle East. J. Age Ageing. 16 (1), 36–54 (2019).
Al-Qudah, H. S. S. Planning of nursing home care services in jordan: its reality and challenges. Asian Social Sci. 7 (10), 94. https://doi.org/10.5539/ass.v7n10p94 (2011).
Liu, H., Xu, X., Tam, V. W. Y. & Mao, P. What is the DNA of healthy buildings? A critical review and future directions. Renew. Sustain. Energy Rev. 183, 113460. https://doi.org/10.1016/j.rser.2023.113460 (2023).
Engelen, L., Rahmann, M. & de Jong, E. Design for healthy ageing—the relationship between design, well-being, and quality of life: a review. Build. Res. Inform. 50 (1–2), 19–35. https://doi.org/10.1080/09613218.2021.1984867 (2021).
Figueiro, M. G. Light, sleep and circadian rhythms in older adults with alzheimer’s disease and related dementias. Neurodegener. Dis. Manag. 7 (2), 119–145. https://doi.org/10.2217/nmt-2016-0060 (2017).
Roenneberg, T. & Merrow, M. The circadian clock and human health. Curr. Biol. 26 (10), R432–R43. https://doi.org/10.1016/j.cub.2016.04.011 (2016).
Wright Kenneth, P. et al. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 23 (16), 1554–1558. https://doi.org/10.1016/j.cub.2013.06.039 (2013).
Daurat, A., Foret, J., Benoit, O. & Mauco, G. Bright light during nighttime: effects on the circadian regulation of alertness and performance. Neurosignals. 9 (6), 309–318. https://doi.org/10.1159/000014654 (2000).
Fani, M. & Sharp, N. Exploring methodological considerations: A literature review on how lighting affects the sleep and cognition in healthy older adults. J. Daylighting. 11 (1), 97–118. https://doi.org/10.15627/jd.2024.6 (2024).
Hadi, K., Du Bose, J. R. & Choi, Y-S. The effect of light on sleep and sleep-related physiological factors among patients in healthcare facilities: A systematic review. HERD Health Environ. Res. Des. J. 12 (4), 116–141. https://doi.org/10.1177/1937586719827946 (2019).
Dautovich, N. D. et al. A systematic review of the amount and timing of light in association with objective and subjective sleep outcomes in community-dwelling adults. Sleep. Health. 5 (1), 31–48. https://doi.org/10.1016/j.sleh.2018.09.006 (2019).
Turner, P. L. & Mainster, M. A. Circadian photoreception: ageing and the eye’s important role in systemic health. Br. J. Ophthalmol. 92 (11), 1439–1444. https://doi.org/10.1136/bjo.2008.141747 (2008).
Kessel, L., Siganos, G., Jørgensen, T. & Larsen, M. Sleep disturbances are related to decreased transmission of blue light to the retina caused by lens yellowing. Sleep 34 (9), 1215–1219. https://doi.org/10.5665/sleep.1242 (2011).
Shochat, T., Martin, J., Marler, M. & Ancoli-Israel, S. Illumination levels in nursing home patients: effects on sleep and activity rhythms. J. Sleep Res. 9 (4), 373–379. https://doi.org/10.1046/j.1365-2869.2000.00221.x (2000).
Shishegar, N., Boubekri, M., Stine-Morrow, E. A. L. & Rogers, W. A. Tuning environmental lighting improves objective and subjective sleep quality in older adults. Build. Environ. 204, 108096. https://doi.org/10.1016/j.buildenv.2021.108096 (2021).
Vetter, C. et al. A review of human physiological responses to light: implications for the development of integrative lighting solutions. LEUKOS. 18 (3), 387–414. https://doi.org/10.1080/15502724.2021.1872383 (2021).
Elliott, J. E. et al. Tunable white light for elders (TWLITE): A protocol demonstrating feasibility and acceptability for deployment, remote data collection, and analysis of a home-based lighting intervention in older adults. Sensors 22 (14), 5372. https://doi.org/10.3390/s22145372 (2022).
Collier, L., Durmus, D. & Davis, R. Lighting in Senior Care Centers: Comparing Tunable LED Systems To Conventional Lighting Systems in Four Senior Care Centers (Office of Scientific and Technical Information (OSTI), 2022).
Figueiro, M. G., Pedler, D., Plitnick, B., Zecena, E. & Leahy, S. Tailored lighting intervention (TLI) for improving sleep-wake cycles in older adults living with dementia. Front. Physiol. https://doi.org/10.3389/fphys.2023.1290678 (2023).
Aarts, M. P. J., Aries, M. B. C., Straathof, J. & Hoof, J. Dynamic lighting systems in psychogeriatric care facilities in the netherlands: A quantitative and qualitative analysis of stakeholders’ responses and applied technology. Indoor Built Environ. 24 (5), 617–630. https://doi.org/10.1177/1420326x14532387 (2014).
Baandrup, L. & Jennum, P. J. Effect of a dynamic lighting intervention on circadian rest-activity disturbances in cognitively impaired, older adults living in a nursing home: A proof-of-concept study. Neurobiol. Sleep. Circadian Rhythms. 11, 100067. https://doi.org/10.1016/j.nbscr.2021.100067 (2021).
Nasreddine, Z. S. et al. The Montreal cognitive assessment, moca: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53 (4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x (2005).
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 28 (2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4 (1989).
Yesavage, J. A. & Sheikh, J. I. 9/Geriatric depression scale (GDS) recent evidence and development of a shorter version. Clin. Gerontologist. 5 (1–2), 165–173. https://doi.org/10.1300/J018v05n01_09 (1986).
Rea, M. S., Figueiro, M. G., Bierman, A. & Bullough, J. D. Circadian light. J. Circadian Rhythm. 8 (1), 2. https://doi.org/10.1186/1740-3391-8-2 (2010).
Ding, F. et al. Polysomnographic validation of an under-mattress monitoring device in estimating sleep architecture and obstructive sleep apnea in adults. Sleep Med. 96, 20–27. https://doi.org/10.1016/j.sleep.2022.04.010 (2022).
Edouard, P. et al. Validation of the withings sleep analyzer, an under-the-mattress device for the detection of moderate-severe sleep apnea syndrome. J. Clin. Sleep Med. 17 (6), 1217–1227. https://doi.org/10.5664/jcsm.9168 (2021).
Mantua, J., Gravel, N. & Spencer, R. Reliability of sleep measures from four personal health monitoring devices compared to Research-Based actigraphy and polysomnography. Sensors 16 (5), 646. https://doi.org/10.3390/s16050646 (2016).
Ravindran, K. K. G. et al. Validation of technology to monitor sleep and bed occupancy in older men and women. Alzheimer’s Dement. 17 (S8). https://doi.org/10.1002/alz.056018 (2021).
Manners, J. et al. Performance evaluation of an under-mattress sleep sensor versus polysomnography in > 400 nights with healthy and unhealthy sleep. J. Sleep Res. https://doi.org/10.1111/jsr.14480 (2025).
Ravindran, G. et al. Three contactless sleep technologies compared with actigraphy and polysomnography in a heterogeneous group of older men and women in a model of mild sleep disturbance: sleep laboratory study. JMIR mHealth uHealth. 11, e46338. https://doi.org/10.2196/46338 (2023).
Spira, A. P. et al. Reliability and validity of the Pittsburgh sleep quality index and the Epworth sleepiness scale in older men. J. Gerontol. Biol. Sci. Med. Sci. 67 (4), 433–439. https://doi.org/10.1093/gerona/glr172 (2012).
Landry, G. J., Best, J. R. & Liu-Ambrose, T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front. Aging Neurosci. 7 (SEP), 166. https://doi.org/10.3389/fnagi.2015.00166 (2015).
Al Maqbali, M. et al. Validation of the Pittsburgh sleep quality index (PSQI) with Arabic cancer patients. Sleep. Biol. Rhythms. 18 (3), 217–223. https://doi.org/10.1007/s41105-020-00258-w (2020).
Figueiro M, Plitnick B, Lok A, Jones G, Higgins P, Hornick T, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin. Interv. Aging. https://doi.org/10.2147/cia.s68557 (2014).
Cremascoli, R. et al. Effects of circadian phase tailored light therapy on sleep, mood, and cognition in Alzheimer’s disease: preliminary findings in a pivotal study. Front. Physiol. https://doi.org/10.3389/fphys.2021.755322 (2022).
Snyder, E., Cai, B., DeMuro, C., Morrison, M. F. & Ball, W. A new single-item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J. Clin. Sleep Med. 14 (11), 1849–1857. https://doi.org/10.5664/jcsm.7478 (2018).
Wrobel, N. H. & Farrag, M. F. A preliminary report on the validation of the geriatric depression scale in Arabic. Clin. Gerontologist. 29 (4), 33–46 (2006).
Chaaya, M. et al. Validation of the Arabic version of the short geriatric depression scale (GDS-15). Int. Psychogeriatr. 20 (3), 571–581. https://doi.org/10.1017/S1041610208006741 (2008).
Alin, A. Minitab. WIRE Comput. Stat. 2(6), 723–727. https://doi.org/10.1002/wics.113 (2010).
Yang, E. et al. Multidimensional environmental factors and sleep health for aging adults: A focused narrative review. Int. J. Environ. Res. Public Health. 19 (23), 15481. https://doi.org/10.3390/ijerph192315481 (2022).
Johnson, D. A., Billings, M. E. & Hale, L. Environmental determinants of insufficient sleep and sleep disorders: implications for population health. Curr. Epidemiol. Rep. 5 (2), 61–69. https://doi.org/10.1007/s40471-018-0139-y (2018).
Blume, C., Garbazza, C. & Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 23 (3), 147–156. https://doi.org/10.1007/s11818-019-00215-x (2019).
van Lieshout-van Dal, E., Snaphaan, L. & Bongers, I. Biodynamic lighting effects on the sleep pattern of people with dementia. Build. Environ. 150, 245–253. https://doi.org/10.1016/j.buildenv.2019.01.010 (2019).
Most, E. I., Scheltens, P. & Van Someren, E. J. Prevention of depression and sleep disturbances in elderly with memory-problems by activation of the biological clock with light–a randomized clinical trial. Trials 11, 19. https://doi.org/10.1186/1745-6215-11-19 (2010).
Boulos, M. I. et al. Normal polysomnography parameters in healthy adults: a systematic review and meta-analysis. Lancet Respiratory Med. 7 (6), 533–543. https://doi.org/10.1016/s2213-2600(19)30057-8 (2019).
Concheiro-Moscoso, P. et al. Use of the Xiaomi Mi band for sleep monitoring and its influence on the daily life of older people living in a nursing home. Digit. Health. 8, 205520762211211. https://doi.org/10.1177/20552076221121162 (2022).
Santhi, N. et al. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J. Pineal Res. 53 (1), 47–59. https://doi.org/10.1111/j.1600-079x.2011.00970.x (2011).
Tähkämö, L., Partonen, T. & Pesonen, A-K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 36 (2), 151–170. https://doi.org/10.1080/07420528.2018.1527773 (2018).
Yamauchi, M. et al. Effects of environment light during sleep on autonomic functions of heart rate and breathing. Sleep. Breath. 18 (4), 829–835. https://doi.org/10.1007/s11325-014-0951-7 (2014).
Figueiro, M. G. et al. Effects of a tailored lighting intervention on sleep quality, Rest–Activity, mood, and behavior in older adults with alzheimer disease and related dementias: A randomized clinical trial. J. Clin. Sleep Med. 15 (12), 1757–1767. https://doi.org/10.5664/jcsm.8078 (2019).
Siraji, M. A., Spitschan, M., Kalavally, V. & Haque, S. Light exposure behaviors predict mood, memory and sleep quality. Sci. Rep. 13 (1). https://doi.org/10.1038/s41598-023-39636-y (2023).
Acknowledgements
The authors thank Darat Samir Shamma Senior Residential Care Facility for their support and the participants for their valuable contributions. We also acknowledge Jordan University of Science and Technology for resources and the Institutional Review Board (IRB) for ethical approval. Appreciation is extended to colleagues and reviewers for their insightful feedback.
Author information
Authors and Affiliations
Contributions
B.O., R.A., and A.H. contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Declaration of generative AI and AI-assisted technologies in the writing process
During manuscript preparation, the authors used ChatGPTo4 for language refinement and clarity enhancement. The content was thoroughly reviewed and edited to ensure accuracy, coherence, and alignment with research objectives. The authors take full responsibility for the publication’s content.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Obeidat, B., Abu Hamdan, R.M. & Hayajneh, A.A. Pilot study of dynamic lighting and sleep consolidation among older adults in a Jordanian senior care facility. Sci Rep 15, 31920 (2025). https://doi.org/10.1038/s41598-025-17351-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17351-0