Abstract
Temporomandibular joint (TMJ) disorders (TMJDs) are linked to heightened nerve sensitivity in TMJ tissues. To set the groundwork for investigating the mechanisms governing this increased responsiveness, this study aimed to identify the types of nerves in the retrodiscal tissue (retrodisc), anterior disc, and joint capsule of mouse TMJ using immunohistochemistry (IHC) and reporter mice. The pan-sensory neuronal marker pgp9.5 revealed no nerves in the articular disc but identified approximately 70% unmyelinated and 30% myelinated fibers in other TMJ tissues. Nearly all sensory fibers in the joint capsule and anterior disc were CGRP+ peptidergic fibers, while the retrodisc contained about 80% peptidergic fibers. Notably, CGRP-/NFH+ myelinated non-peptidergic nerves were absent, indicating the absence of non-nociceptive fibers (A-LTMRs) in TMJ tissues. Almost all sensory fibers in the joint capsule and anterior disc were Htr3a+, with the retrodisc containing 60–70% Htr3a+ fibers. Additionally, TMJ tissues had minimal to no (< 5%) MrgprD+, MrgprA3+, MrgprC11+, somatostatin+, or parvalbumin+ fibers, except for the retrodisc, which had about 20% Mrgpr+ fibers. Excluding articular discs, TMJ tissues were highly vascularized, with blood vessels surrounded by both sensory and sympathetic (TH+) nerves. Overall, TMJ tissues were predominantly innervated by peptidergic fibers, with a minor presence of other non-peptidergic fibers.
Similar content being viewed by others
Introduction
Temporomandibular joint disorders (TMJDs) represent a diverse group of conditions that disrupt the normal function of the temporomandibular joint (TMJ)1,2. These disorders can present in both painful and non-painful forms, with pain often being the most debilitating and difficult symptom to manage2. Painful TMJDs can be further classified into subtypes such as osteoarthritis, rheumatoid arthritis, and idiopathic disorders3,4. Each subtype is characterized by distinct pathophysiological mechanisms, which contribute to alterations in the sensory processing within the joint2,3. This understanding highlights the critical importance of characterizing the sensory innervation of the TMJ. These nerves transmit nociceptive signals that may be altered or amplified in TMJDs, contributing to persistent orofacial pain.
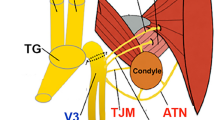
The joint receives its innervation primarily from branches of the mandibular division (V3) of the trigeminal nerve. Specifically, the auriculotemporal nerve (ATN), the masseteric nerve (MN), and the anterior deep temporal nerve are the principal contributors to TMJ sensory input5. Prior studies indicate a predominance of C- and A-fiber nociceptors in TMJ tissues, with TMJ afferents expressing neuropeptides such as calcitonin gene-related peptide (CGRP), substance P (SP), neuropeptide Y (NPY), and nitric oxide synthase (NOS)6,7,8,9. CGRP-positive nerves were found in the capsule and the synovial membrane, but not in the articular disc of sheep10,11. In vivo extracellular recordings from TMJ structures, ATN single nerve preparations, demonstrated that TMJ nerves were classified into the following four subtypes: Adelta-high-threshold mechanonociceptor (Aδ-HTMR) (12.1%), Adelta-polymodal nociceptor (Aδ-POLY) (36.4%), C-HTMR (12.1%), and C-POLY (39.4%)12,13. Overall, despite accumulated data on sensory neurons innervating TMJ, there is limited information regarding the precise make-up of neuronal subtypes innervating the TMJ tissues, such as the joint capsule and associated synovium, articular disc, anterior disc, and retrodisc.
The development of single-cell RNA sequencing technology opened avenues for precise identifications of sensory nerve subtypes in tissues using IHC with well-defined antibody markers and reporter mouse lines highlighting expressions of sensory neuronal markers20. Accordingly, the main aim of this study was to identify nerve subtypes in each TMJ tissue using IHC and reporter mouse lines.
Results
TMJ tissues examined in this study
TMJ tissues exhibit distinct functions and pathophysiological profiles, and are believed to undergo unique nerve plasticity, gene expression changes and non-neuronal cellular plasticity during TMJD. Therefore, this study focused on characterizing the innervation and vascularization of individual TMJ structures8,14,15. We generated serial cryosections that sequentially passed through the mandible, temporal bone, and a thin layer of the joint capsule, which contains synovial tissue in its deeper layers (as shown on the schematic; Fig. 1A). As the sections progressed, they encompassed the lateral pterygoid muscle (LPM), condyle, anterior disc, articular disc, and retrodiscal tissue (aka retrodisc) (as shown on the schematic; Fig. 1B). The morphology of sections passing through the joint capsule was illustrated on a H&E labeled paraffin section (Fig. 1C). The morphology of sections containing condyle, articular disc, anterior disc, and retrodiscal tissue was illustrated on a deeper H&E labeled paraffin section (Fig. 1D). Innervation and vascularization were visualized and quantitatively analyzed in cryo sections containing the joint capsule (Fig. 1E), and in the deeper TMJ sections containing condyle, articular disc, anterior disc and retrodisc (Fig. 1F). After visual analysis of many sections, it remains unclear whether the neurovascular elements observed in the anterior disc and retrodisc extend into and are functionally present within the articular disc itself. Thus, some portions of retrodiscal tissue can overlay the articular disc (Fig. 1D). Therefore, innervation and vascularization were not associated to the articular disc due to inconclusive evidence of nerves or blood vessels in this TMJ tissue.
Schematic for TMJ tissues. (A) Schematic of TMJ tissues on the initial serial sections. (B) Schematic of TMJ tissues in deeper serial sections. Dashed line shows position of the joint capsule overlaying TMJ deeper tissues, such as the anterior disc, articulate disc and retrodiscal tissue. (C) The joint capsula as well as temporal bone and mandible are outlined in an H&E paraffin section. (D) The articulate disc, anterior disc and retrodiscal tissue as well as condyle and temporal bone are outlined in an H&E paraffin section. The temporal bone is outlined with a red line; the anterior disc with a blue line; the articular disc with a black line and the retrodisc with an orange line. (E) The joint capsula is outlined in a cryo-section. (F) The anterior disc and retrodiscal tissue are outlined in a cryo-section. TMJ tissues are labeled and outlined on the panels A-F.
Myelinated and unmyelinated sensory afferents in TMJ tissues
All sensory nerves were identified using the pan-neuronal marker pgp9.5, while myelinated fibers were specifically labeled with NFH antibodies16,17. We did not reliably detect pgp9.5 + nerves in the articular disc (Suppl. Figure 1). Myelinated nerves (pgp9.5⁺/NFH⁺) were 28.1 ± 7.6% (n = 5) of pgp9.5 in the joint capsule; 30.3 ± 6.1% (n = 6) of pgp9.5 in the anterior disc and 30.2 ± 12% (n = 6) of pgp9.5 in the retrodisc (Figs. 2A, 2B). Remaining pgp9.5+ fibers, around 70%, in these TMJ tissues were un-myelinated (pgp9.5⁺/NFH⁻) (Figs. 2A, 2B). This pattern indicates significantly higher innervation by unmyelinated fibers across these TMJ tissues (2-way ANOVA; F (1, 28) = 29.72; P < 0.0001; n = 5–6; Fig. 2B). Overall, in contrast to masticatory muscles, which typically exhibit a higher proportion of myelinated fibers16,17, TMJ structures were predominantly innervated by unmyelinated sensory nerves. The relative proportions of myelinated and unmyelinated fibers were consistent across the joint capsule, anterior disc, and retrodisc.
Pgp9.5 and NFH-positive fibers in the joint capsule, anterior disc and retrodiscal tissues. (A) The left column shows pgp9.5-positive fibers (all sensory fibers), the middle column shows NFH-positive (A-fibers) fibers and the right column shows co-expressions of pgp9.5 and NFH-positive fibers in the mouse joint capsule, anterior disc and retrodiscal tissues. Pictures from the joint capsule, anterior disc and retrodiscal tissues as well as antibodies used and corresponding colors are indicated. Yellow arrows mark pgp9.5+/NFH+ fibers, and blue arrows show relatively thin pgp9.5+/NFH- nerves. (B) Percentages of pgp9.5+/NFH+ and pgp9.5+/NFH- nerves in the joint capsule, anterior disc and retrodiscal tissues. Statistics is 2-way ANOVA Bonferroni’s pot-hoc test (** p < 0.01; n = 5–6).
Peptidergic and Htr3a-positive sensory afferents in TMJ tissues
We next assessed the presence of CGRP⁺ peptidergic nerves in TMJ tissues. The joint capsule (95.9 ± 16.6%; n = 5) and anterior disc (98.9 ± 12.4%; n = 5) were almost entirely populated by peptidergic sensory fibers (Figs. 3A, 3B), while the retrodiscal tissue contained ≈75% CGRP⁺/pgp9.5⁺ nerves (75.4 ± 9.9%; n = 5; Figs. 3A, 3B). Further analysis using CGRP reporter mice and co-labeling with CGRP and NFH antibodies confirmed that roughly 70% of peptidergic fibers were unmyelinated (73.4 ± 2.3%; n = 5 for joint capsule; 70.3 ± 2.4%; n = 5 for anterior disc; 71.6 ± 2.6%; n = 5 for retrodisc), with the remaining ≈30% myelinated (Figs. 4A, 4B). Notably, TMJ tissues showed minimal-to-no (< 5%) CGRP⁻/NFH⁺ fibers (0.8 ± 0.6%; n = 5 for joint capsule; 6.9 ± 3.4%; n = 5 for anterior disc; 2.9 ± 2.9%; n = 5 for retrodisc), non-peptidergic myelinated sensory nerves typically classified as non-nociceptive Aβ low-threshold mechanoreceptors (A-LTMRs)11,18,19.
Peptidergic fibers in the joint capsule, anterior disc and retrodiscal tissues. (A) The left column shows pgp9.5-positive fibers (all sensory fibers), the middle column shows CGRP-positive (peptidergic fibers) fibers in CGRP/tdTom mouse and the right column shows co-expressions of CGRP and pgp9.5-positive fibers in the mouse joint capsule, anterior disc and retrodiscal tissues. Blue arrows mark pgp9.5+/CGRP+ fibers. Pictures from the joint capsule, anterior disc and retrodiscal tissues as well as antibodies used and corresponding colors are indicated. (B) Percentages of CGRP+ nerves relative to mean pgp9.5+ nerves in a selected section for the joint capsule, anterior disc and retrodiscal tissues. Statistics is 2-way ANOVA Bonferroni’s pot-hoc test (n = 5–13).
Peptidergic myelinated and non-myelinated fibers in the joint capsule, anterior disc and retrodiscal tissues. (A) The left column shows NFH-positive fibers (A-fibers), the middle column shows CGRP-positive (peptidergic fibers) fibers in CGRP/tdTom mouse and the right column shows co-expressions of CGRP and NFH-positive fibers in the mouse joint capsule, anterior disc and retrodiscal tissues. Pictures from the joint capsule, anterior disc and retrodiscal tissues as well as antibodies used and corresponding colors are indicated. Yellow arrows mark CGRP+/NFH+ fibers, blue arrows show CGRP+/NFH- nerves and white arrows mark seldomly encountered CGRP-/NFH+ nerves in the anterior disc. (B) Relative percentages for CGRP+/NFH-, CGRP+/NFH- and CGRP-/NFH+ nerves in the joint capsule, anterior disc and retrodiscal tissues. Statistics is 2-way ANOVA Bonferroni’s pot-hoc test (n = 5).
Given that several peptidergic fiber subtypes express Htr3a17, we examined this further using Htr3a/tdTomato reporter mice. In the joint capsule (99.9 ± 13.6%; n = 6) and anterior disc (90.3 ± 14.1%; n = 5), Htr3a⁺ fibers almost completely overlapped with pgp9.5⁺ nerves, whereas the retrodisc (46.6 ± 5.5%; n = 4) showed only ≈45% overlap (Figs. 5A, 5B). As expected, approximately 70% of Htr3a⁺ fibers were unmyelinated (69.7 ± 7.7%; n = 6 for the joint capsule; 68.1 ± 3.0%; n = 5 for the anterior disc; 75.9 ± 0.9%; n = 4 for the retrodisc), with the remainder being myelinated; and with almost no Htr3a⁻/NFH⁺ fibers were detected (Fig. 5C)17. In summary, nearly all sensory nerves in the joint capsule and anterior disc are peptidergic and co-express Htr3a. In contrast, the retrodisc contains a distinct subset (≈20%) of non-peptidergic, Htr3a⁻ fibers17, along with some peptidergic fibers that are likely Htr3a⁻.
Htr3a-positive fibers in the joint capsule, anterior disc and retrodiscal tissues. (A) The left column shows NFH-positive fibers (A-fibers), the middle column shows Htr3a-positive fibers in Htr3a/tdTom mouse and the right column shows co-expressions of Htr3a and NFH-positive fibers in the mouse joint capsule, anterior disc and retrodiscal tissues. Pictures from the joint capsule, anterior disc and retrodiscal tissues as well as antibodies used and corresponding colors are indicated. Yellow arrows mark Htr3a+/NFH+ fibers, and blue arrows show Htr3a+/NFH- nerves. (B) Percentages of Htr3a+ nerves relatively to mean pgp9.5+ nerves in a selected section for the joint capsule, anterior disc and retrodiscal tissues. Statistics is 2-way ANOVA Bonferroni’s pot-hoc test (n = 5–13). (C) Relative percentages for Htr3a+/NFH-, Htr3a+/NFH- and Htr3a-/NFH+ nerves in the joint capsule, anterior disc and retrodiscal tissues. Statistics is 2-way ANOVA Bonferroni’s pot-hoc test (n = 5–6).
Presence of non-peptidergic—MrgprD, MrgprA3, MrgprC11, somatostatin and parvalbumin—sensory afferents in TMJ tissues
Our findings suggest that TMJ tissues are predominantly innervated by peptidergic sensory fibers, with widespread expression of Htr3a. To further validate this, we examined the presence of non-peptidergic nerve subtypes, focusing on MrgprD⁺ fibers, which represent the major class of non-peptidergic neurons (NP-1 group) in the dorsal root ganglia (DRG) and trigeminal ganglia (TG)20. Using MrgprD/tdTomato reporter mice, we found that MrgprD⁺ fibers were scarce in the joint capsule (4.1 ± 1.9%; n = 4 and anterior disc (3.7 ± 2.2%; n = 4), and many sections did not contain MrgprD+ nerves. In contrast, the retrodisc showed a modestly higher presence of MrgprD⁺ fibers (10.8 ± 3.2%; n = 4; Figs. 6A, 6B). As a positive control, facial skin displayed dense MrgprD⁺ innervation, confirming the sensitivity of our detection method (Suppl. Figure 2). These results reinforce the conclusion that TMJ tissues are mainly innervated by peptidergic neurons, while non-peptidergic MrgprD⁺ fibers are limited, particularly outside the retrodisc.
MrgprD-positive fibers in the joint capsule, anterior disc and retrodiscal tissues. (A) The left column shows pgp9.5-positive fibers (all sensory fibers), the middle column shows MrgprD-positive fibers in MrgprD/tdTom mouse, and the right column shows co-expressions of pgp9.5 and MrgprD-positive fibers in the mouse joint capsule, anterior disc and retrodiscal tissues. Pictures from the joint capsule, anterior disc and retrodiscal tissues as well as antibodies used, and corresponding colors are indicated. Yellow arrows show pgp9.5+/MrgprD- nerves. (B) Percentages of MrgprD+, MrgprA3+, MrgprC11+, Sst+ and PV+ nerves relatively to numbers of pgp9.5+ nerves for the joint capsule, anterior disc and retrodiscal tissues. Statistics is 2-way ANOVA Bonferroni’s pot-hoc test (n = 4–5).
Another subset of non-peptidergic fibers, known as the NP-2 group, is marked by MrgprA3⁺ expression18,20. However, some studies have shown that MrgprA3⁺ neurons are heterogeneous, comprising both peptidergic and non-peptidergic subtypes21,22. In MrgprA3/tdTomato reporter mice, MrgprA3⁺ nerves were detected only sporadically on few sections of the joint capsule (4.4 ± 3.2%; n = 5) and retrodiscal tissues (6.4 ± 3.2%; n = 4), with none observed in the anterior disc (0.6 ± 0.6%; n = 4; Figs. 6B, 7). A broader subset, marked by MrgprC11⁺, includes certain TrpV1⁺ peptidergic neurons23. In MrgprC11/tdTomato reporter mice, TMJ tissues displayed only a small number of MrgprC11⁺ nerves on few Sects. (3.7 ± 3.7%; n = 4 for the joint capsule; 2.9 ± 3.0%; n = 4 for the anterior disc; 6.8 ± 3.5%; n = 6 for the retrodisc; Figs. 6B, 8). As with MrgprD⁺ fibers, facial skin, used as a positive control, showed significantly greater innervation by both MrgprA3⁺ and MrgprC11⁺ fibers (Suppl. Figure 2). These results further support the conclusion that TMJ tissues contain very few non-peptidergic sensory fibers, with only limited contributions from MrgprA3⁺ and MrgprC11⁺ subtypes.
MrgprA3-positive fibers in the joint capsule, anterior disc and retrodiscal tissues. The left column shows pgp9.5-positive fibers (all sensory fibers), the middle column shows MrgprA3-positive fibers in MrgprA3/tdTom mouse, and the right column shows co-expressions of pgp9.5 and MrgprA3-positive fibers in the mouse joint capsule, anterior disc and retrodiscal tissues. Pictures from the joint capsule, anterior disc and retrodiscal tissues as well as antibodies used, and corresponding colors are indicated. Blue arrow marks a pgp9.5+/MrgprA3+ fiber, and yellow arrows show pgp9.5+/MrgprA3- nerves.
MrgprC11-positive fibers in the joint capsule, anterior disc and retrodiscal tissues. The left column shows pgp9.5-positive fibers (peptidergic fibers), the middle column shows MrgprC11-positive fibers in MrgprC11/tdTom mouse, and the right column shows co-expressions of pgp9.5 and MrgprA3-positive fibers in the mouse joint capsule, anterior disc and retrodiscal tissues. Pictures from the joint capsule, anterior disc and retrodiscal tissues as well as antibodies used and corresponding colors are indicated. Yellow arrows show pgp9.5+/MrgprC11- nerves.
The final group of non-peptidergic nociceptors in the DRG and TG is marked by somatostatin (Sst⁺), also referred to as the NP-3 cluster11,20. In Sst/tdTomato reporter mice, Sst⁺ fibers were not detected in the anterior disc (0 ± 0%; n = 5) or retrodisc (0 ± 0%; n = 6), while the joint capsule contained a small proportion of Sst⁺ nerves observed on several Sects. (5.7 ± 3.4%; n = 6; Figs. 6A, 9). The dura mater was used as a positive control and showed robust Sst⁺ innervation (Suppl. Figure 2), validating the specificity of our detection. Overall, TMJ tissues exhibited very low levels of non-peptidergic innervation. Among the TMJ regions analyzed, the retrodisc consistently contained the highest proportion of non-peptidergic sensory fibers.
Somatostatin (Sst)-positive fibers in the joint capsule, anterior disc and retrodiscal tissues. The left column shows pgp9.5-positive fibers (all sensory fibers), the middle column shows Sst-positive fibers in Sst/tdTom mouse, and the right column shows co-expressions of pgp9.5 and Sst-positive fibers in the mouse joint capsule, anterior disc and retrodiscal tissues. Yellow arrows show pgp9.5+/Sst- nerves, blue arrows mark pgp9.5+/Sst+ fiber in the joint capsule panels and cyan arrow marks a non-neuronal Sst+ cells in the retrodiscal tissue. Pictures from the joint capsule, anterior disc and retrodiscal tissues as well as antibodies used and corresponding colors are indicated.
Non-peptidergic, non-nociceptive nerves are typically classified as low-threshold mechanoreceptors (LTMRs), which include C-, Aδ-, and Aβ-LTMR subtypes18,20. These subtypes are commonly marked by expression of vGlut3 or tyrosine hydroxylase (TH) for C-LTMRs, TrkB for Aδ-LTMRs, and TrkC or parvalbumin (PV) for Aβ-LTMRs20. While both mouse and primate TG contain few TH⁺ neurons, they show a high prevalence of PV⁺ neurons16,17. Similarly, facial skin, used here as a positive control, exhibited abundant PV⁺ nerve fibers (Suppl. Figure 2). To assess the presence of LTMRs in TMJ tissues, we analyzed PV/tdTomato reporter mice. No PV⁺ nerve fibers were detected in the joint capsule, anterior disc, or retrodisc, despite 6–12 sections were scanned for each tissue (Figs. 6B, 10). In summary, TMJ tissues contain very few, if any, non-peptidergic nociceptors or non-nociceptive mechanoreceptors. Among the TMJ regions examined, the retrodisc consistently exhibited the highest proportion of non-peptidergic nociceptors, accounting for approximately 15–20% of the total innervation.
Parvalbumin (PV)-positive fibers in the joint capsule, anterior disc and retrodiscal tissues. The left column shows pgp9.5-positive fibers (all sensory fibers), the middle column shows PV-positive fibers in PV/tdTom mouse, and the right column shows co-expressions of pgp9.5 and PV-positive fibers in the mouse joint capsule, anterior disc and retrodiscal tissues. Yellow arrows show pgp9.5+/PV- nerves. Pictures from the joint capsule, anterior disc and retrodiscal tissues as well as antibodies used, and corresponding colors are indicated.
Innervation of vasculature in TMJ tissues
Using α-SMA as a marker for vascular smooth muscle, we clearly identified blood vessels in all TMJ tissues except the articular disc (Figs. 11A, B, 12) , Suppl. Figure 3). To determine whether these vessels were innervated by sympathetic or sensory nerves, we used tyrosine hydroxylase (TH) to label sympathetic fibers and pgp9.5 as a pan-sensory nerve marker. Both sensory and sympathetic nerves were detected in the joint capsule, anterior disc, and retrodisc, but not in the articular disc (Figs. 11A, 12, Suppl. Figure 1, Suppl. Figure 3). Nerves were considered to innervate blood vessels if they overlapped or were located within 50 µm of α-SMA⁺ vessels. Approximately 40% of all TH⁺ sympathetic nerves of the entire TMJ were found in close association with blood vessels (Figs. 11A, B), while sensory pgp9.5⁺ fibers showed a slightly lower association at around 35% (Figs. 11B, 12). Overall, these findings indicate that TMJ tissues, excluding the articular disc, are well vascularized and receive comparable innervation from both sensory and sympathetic nerves. The joint capsule, anterior disc, and retrodisc displayed similar patterns of neurovascular pattern.
Innervation of blood vessels with sympathetic nerves in the joint capsule, anterior disc and retrodiscal tissues. (A) The left column shows tyrosine hydroxylase (TH)-positive fibers (sympathetic nerves), the middle column shows α-SMA-positive (blood vessel) cells, and the right column shows relative locations of TH and α-SMA-positive cells in the mouse joint capsule, anterior disc and retrodiscal tissues. Blue arrows show TH+ fibers in vicinity of α-SMA+ blood vessels, and yellow arrows show TH+ fibers distanced from α-SMA+ blood vessels. Pictures from the joint capsule, anterior disc and retrodiscal tissues as well as antibodies used, and corresponding colors are indicated. (B) Percentages of pgp9.5+ and TH+ nerves in a vicinity of the blood vessels (α-SMA+) in the joint capsule, anterior disc and retrodiscal tissues. Statistics is t-test test (n = 4).
Innervation of blood vessels with sensory nerves in the joint capsule, anterior disc and retrodiscal tissues. The left column shows pgp9.5-positive fibers (all sensory fibers), the middle column shows α-SMA-positive (blood vessel) cells, and the right column shows relative locations of pgp9.5 and α-SMA-positive cells in the mouse joint capsule, anterior disc and retrodiscal tissues. Yellow arrows show pgp9.5+ fibers in vicinity of α-SMA+ blood vessels, and blue arrows show pgp9.5+ fibers distanced from α-SMA+ blood vessels. An example of the retrodiscal tissue section does not contain pgp9.5+ fibers distanced from α-SMA+ blood vessels. Pictures from the joint capsule, anterior disc and retrodiscal tissues as well as antibodies used, and corresponding colors are indicated.
Discussion
Studies of painful joint pathologies in the knee have demonstrated that gene expression and cellular plasticity within the synovium play a critical role in the development of hypersensitivity and the persistence of chronic pain24,25,26. Both neuronal and non-neuronal cell plasticity in the knee synovium contribute significantly to the mechanisms underlying chronic pain27. In the TMJ, the synovium is distributed across three major anatomical components: the joint capsule, the anterior disc, and the retrodiscal tissue28. However, it remains unclear whether these distinct synovial regions of the TMJ possess different cellular compositions or patterns of innervation. Therefore, the rationale for this study was to characterize the types and distribution of nerves innervating specific regions of the TMJ; namely, the joint capsule, the articular disc, the anterior disc, and the retrodiscal tissue. A detailed understanding of the regional innervation patterns may provide insights into the mechanisms of TMJ pain and guide the development of targeted therapies for painful TMJ disorders.
We found that TMJ tissues are primarily innervated by peptidergic unmyelinated sensory fibers, with the remaining fibers consisting largely of peptidergic myelinated afferents. These results are consistent with previous anatomical and electrophysiological studies identifying various neuropeptides in TMJ-innervating nerves7,8,9,10 and classifying them as C- and A-fiber nociceptors12,13. While prior research mainly focused on the joint capsule7,8,9,10, our study expanded the anatomical mapping to include the anterior disc and retrodisc. Although the overall innervation pattern was similar across regions, key differences were observed. The retrodisc contained approximately 20% non-peptidergic fibers, including MrgprD⁺, MrgprA3⁺, and MrgprC11⁺ subtypes, fiber types largely absent in other TMJ regions. In contrast, the joint capsule uniquely harbored Sst⁺ fibers. A further distinguishing feature was the expression of Htr3a: nearly all fibers in the joint capsule and anterior disc expressed Htr3a, whereas only about 50% of retrodisc fibers did. These differences suggest that the retrodisc is innervated by a more diverse and molecularly distinct population of sensory neurons.
Electrophysiological studies revealed that TMJ is innervated by C- and A-fiber nociceptors, which could be classified into two main functional subtypes: polymodal nociceptors and high-threshold mechanoreceptors (HTMRs)12,13. Building on this, recent integrative analyses of single-cell RNA sequencing data from mouse and human trigeminal ganglia (TG) have identified several molecularly distinct subtypes of peptidergic nociceptors20. Among C-fiber nociceptors, these subtypes include Oprk1⁺, Adra2a⁺, Sstr2⁺, and Dcn⁺ neurons. A-fiber nociceptors are categorized primarily as Smr2⁺ and Bmpr1b⁺ neurons20. Both Smr2⁺ and Bmpr1b⁺ A-nociceptors express Htr3a, while only the Oprk1⁺ and Dcn⁺ subtypes among C-fiber nociceptors express this receptor20. In light of our findings, the retrodisc, where only ~ 50% of sensory fibers are Htr3a⁺, may contain the full range of peptidergic C- and A-nociceptor subtypes. Conversely, the joint capsule and anterior disc, which show near-complete Htr3a expression among their sensory fibers, are likely innervated mainly by Oprk1⁺ and Dcn⁺ C-fiber nociceptors. These differences point to region-specific specialization in nociceptive innervation within TMJ tissues. A proposed summary of nociceptor subtype distribution across TMJ structures is illustrated in Fig. 13.
Schematic for sensory neuronal types innervating TMJ tissues. Schematic for sensory neuronal types innervating the joint capsule, anterior disc and retrodiscal tissue. Red box shows myelinated peptidergic nociceptors. Blue box shows unmyelinated peptidergic nociceptors. The green box indicates a group of unmyelinated non-peptidergic nociceptors. Markers for sensory neuronal groups are indicated.
One potential limitation of this study is utilized solely female mice for MrgprA3-tdTomato and solely male mice for Htr3a/tdTom, CGRP/tdTom, MrgprD/tdTom, MrgC11/tdTom, and α-SMA. Sst/tdTom consisted of male and female mice. Prior studies utilizing bulk and single-cell RNA sequencing have identified some transcriptomic differences between male and female DRG and TG neurons11,20,29,30. Although these differences were generally modest within neuronal populations, notable sex-specific distinctions were observed, particularly in Aβ-rapid adapting (Aβ-RA) and Aδ-low threshold mechanoreceptors (Aδ-LTMRs) in the naïve state31. Thus, we do not anticipate sex differences in innervations of TMJ tissues, since they do not contain Aβ-RA and Aδ-LTMRs. Another limitation lies in the manual counting of nerve fibers from IHC images. This method does not distinguish between individual nerve branches and closely adjacent fibers, potentially leading to over- or underestimation. Additionally, we did not use specific markers for neuronal terminals, raising the possibility that some counted fibers were merely passing through the tissue rather than terminating within it. Another potential limitation of this study is the exclusive use of conventional two-dimensional IHC for analyzing TMJ innervation. While this method is well-established and effective, it does not capture the full complexity of the 3D neural architecture. Advanced imaging techniques such as light sheet fluorescence microscopy could provide additional spatial information and more comprehensive quantification of nerve distribution within the TMJ. However, the primary objective of this study was to identify and characterize the types of sensory neurons innervating the TMJ. For this purpose, two-dimensional IHC imaging represents a practical and cost-effective approach, particularly suitable for high-throughput analysis across multiple tissue samples. It allows for reliable detection of nerve fiber subtypes while maintaining efficiency in data collection and analysis. Nonetheless, light sheet microscopy offers distinct advantages in visualizing 3D nerve architecture and would be especially valuable in follow-up studies aimed at exploring nerve remodeling and plasticity in various mouse models of TMJ disorders.
In conclusion, despite certain limitations, the data presented in this study reliably reveal key patterns of innervation and establish a foundational map of sensory nerve subtypes within the TMJ. The identification of both predominant and less common nerve fiber types across distinct TMJ tissues holds important implications, as different nerve types possess unique transcriptional signatures, biochemical characteristics, and functional responses to environmental cues. These differences underscore the diverse roles that specific nerve populations play in both normal joint physiology and pathological conditions. For example, nociceptive and non-nociceptive neurons may exhibit differential responses to tissue injury, while peptidergic and non-peptidergic fibers are likely to contribute to pain signaling through separate mechanisms. Understanding these distinctions is a central goal in the field of pain research and is essential for the development of precise, targeted therapeutic strategies for managing TMJ-related disorders.
Materials and methods
Ethical approval
This study was conducted in accordance with the ARRIVE 2.0 guidelines32. All animal care and experimental procedures complied with the U.S. Public Health Service Policy on the Use of Laboratory Animals, as well as ethical standards set by the National Institutes of Health (NIH) and the Society for Neuroscience (SfN), with a commitment to minimizing both animal use and suffering. Mice were euthanized via transcardiac perfusion following intramuscular injection of 100 μL of a 1:1 Ketamine/Dexdormitor cocktail. This method, recommended by the AVMA Guidelines for the Euthanasia of Animals, was chosen to ensure minimal distress. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Health Science Center at San Antonio (UTHSCSA) under protocol number 20220069AR.
Mouse lines
Mice were housed under controlled conditions at approximately 22 °C, with 40–60% relative humidity, and maintained on a 12-h light/dark cycle (lights on at 7:00 AM). Food and water were provided ad libitum in standard home cages. Experiments were performed on adult wild-type C57Bl/6 mice (10–23 weeks old) obtained from The Jackson Laboratory (Bar Harbor, ME). The following transgenic mouse lines were purchased from The Jackson Laboratory: B6.Cg-Gt(ROSA) 26Sortm9(CAG-tdTomato)Hze/J (Ai9; tdTomato; strain 007,909); B6.129P2-Pvalbtm1(cre)Arbr/J (PVcre; strain 008,069); Mrgprdtm1.1(cre/ERT2)Wql/J (MrgprDcre/ER; strain 031,286), and B6N.Cg-Ssttm2.1(cre)Zjh/J (SstCre; stock 013,044). The CGRPcre/ER mouse line was generously provided by Dr. Pao-Tien Chuang (University of California, San Francisco), and the MrgprA3cre and MrgprC11cre/ERT2 lines by Dr. Liang Han (Georgia Institute of Technology). The Htr3acre line was developed by the Mutant Mouse Resource & Research Centers (MMRRC) at UC Davis. In inducible Cre-carrying lines, Cre recombination was induced in 6–8-week-old mice via three intraperitoneal injections of tamoxifen (100 mg/kg in corn oil), administered every other day. Recombination was expected to occur within two to three weeks post-injection.
Tissue collection and processing
All mice were deeply anesthetized via intramuscular injection of 100 μL of a ketamine (75 mg/kg) and dexdomitor (1 mg/kg) cocktail, then transcardially perfused with 25 mL of 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB). Following perfusion, mice were decapitated, and the masseter and temporalis muscles were carefully removed. Bilateral TMJs, along with surrounding bone structures, were then dissected. Facial skin overlying the masseter and TMJ, as well as the TG and dura mater, were collected as positive controls for tdTomato fluorescence in afferent fibers. Dissected TMJs were post-fixed overnight in 4% PFA at 4 °C. The next day, tissues were rinsed twice in 1X PB and transferred to 15 mL of 10% ethylenediaminetetraacetic acid (EDTA; ThermoFisher, Cat# 17,892) in ddH₂O, adjusted to pH 7.2–7.4. Decalcification in EDTA was carried out over two weeks, with solution changes every three days. After decalcification, tissues were cryoprotected in 10% sucrose for 18 h, followed by 30% sucrose for another 18 h. TMJs were then embedded in Neg-50 embedding medium (FisherScientific, Cat# 22–110-617) and cryo-sectioned at a thickness of 20 μm.
Immunohistochemistry (IHC)
Immunostaining was performed as previously described16,33. Briefly, tissue sections were blocked for 90 min at room temperature in a solution containing 4% normal donkey serum (Sigma, St. Louis, MO), 2% bovine gamma-globulin (Sigma-Aldrich, St. Louis, MO), and 0.3% Triton X-100 (Fisher Scientific) in 0.1 M phosphate-buffered saline (PBS). Following blocking, sections were incubated overnight (18 h) at room temperature with primary antibodies. After incubation, sections were washed with 0.1 M PBS to remove unbound primary antibodies, re-blocked, and then incubated for 90 min at room temperature with species-appropriate fluorophore-conjugated secondary antibodies (1:200; Jackson ImmunoResearch, West Grove, PA, USA). Sections were then washed three times for five minutes each with 0.1 M PBS, air-dried, and mounted with Vectashield Antifade Mounting Medium (Vector Laboratories, SKU H-1000–10). The following well-characterized primary antibodies were used on mouse tissue sections: anti-neurofilament heavy chain (NFH) chicken polyclonal antibodies (BioLegend, catalog #PCK-592P, 1:300)34; anti-pgp9.5 (Millipore-Sigma, catalog #AB1761-I, 1:400)35; anti-CGRP rabbit polyclonal (Sigma, C8198, 1:300)36,37,38; anti-tyrosine hydroxylase (TH) rabbit polyclonal (Pel-Freez; Rogers, AR; P40101; 1:400)39,40; and anti-smooth muscle actin (α-SMA) Cy3-conjugated mouse monoclonal antibody (Sigma, C6198, 1:200)16,41. Secondary antibodies were generated in donkey (Jackson Immuno-Research).
Counting and imaging of fibers
Images were acquired using a Keyence BZ-X810 all-in-one microscope (Itasca, IL, USA) or a Nikon AX confocal microscope with Z-stack (“sectioning”) functionality. For nerve fiber counting, all images were captured using a 10 × objective. Representative images in figures are cropped versions of 10 × images to increase visibility and resolution. Control IHC was conducted on tissue sections processed identically, but either lacking primary antibodies or both primary and secondary antibodies. Imaging settings were calibrated to ensure that these negative controls produced no detectable nerve fiber signal. Z-stack images for fiber quantification were obtained. For each condition, at least 3 independent tissue sections were analyzed. Actual numbers are presented in the legends for figures. Sensory fiber quantification was performed manually, following previously established protocols16, to estimate the distribution and density of nerve subtypes innervating TMJ tissues. The rationale and validation for manual fiber counting as a reliable method for detecting and quantifying peripheral nerve fibers have been previously described16,41. Each quantification analysis had a reference point, which was either pgp9.5 (all sensory nerves) or NFH (all myelinated A-fibers). This reference points (defined as 100%) were based on the mean number of pgp9.5+ or NFH+ fibers, which were obtained by counting pgp9.5+ and NFH+ fibers from all sections for each specific TMJ tissue type. This normalization approach was intentionally designed to illustrate inter-animal variability and heterogeneity in nerve innervation.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 10 (GraphPad Software, La Jolla, CA). Data are presented as mean ± standard error of the mean (SEM), with “n” indicating the number of mice analyzed for IHC. For each mouse, fibers were counted from at least 5 randomly selected sections and then the counting was averaged. Group differences were evaluated using appropriate statistical tests, including chi-square analysis with Fisher’s exact test, unpaired t-tests, and one-way or two-way ANOVA followed by Bonferroni’s post hoc test, as applicable. A p-value of < 0.05 was considered statistically significant. Where relevant, interaction F-ratios and corresponding p-values are reported.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Romero-Reyes, M. & Uyanik, J. M. Orofacial pain management: current perspectives. J. Pain Res. 7, 99–115. https://doi.org/10.2147/jpr.S37593 (2014).
TMD (Temporomandibular Disorders), <https://www.nidcr.nih.gov/health-info/tmd#:~:text=A%20recent%20study%20found%20that,35%20and%2044%20years%20old> (2025).
Bouloux, G. F. et al. Guidelines for the Management of Patients With Orofacial Pain and Temporomandibular Disorders. J. Oral. Maxillofac. Surg. https://doi.org/10.1016/j.joms.2024.03.018 (2024).
Facial Pain, <https://www.nidcr.nih.gov/research/data-statistics/facial-pain> (2018).
Greenberg, J. S. & Breiner, M. J. StatPearls (2025).
Uddman, R., Grunditz, T., Kato, J. & Sundler, F. Distribution and origin of nerve fibers in the rat temporomandibular joint capsule. Anat. Embryol. (Berl.) 197, 273–282. https://doi.org/10.1007/s004290050137 (1998).
Tahmasebi-Sarvestani, A., Tedman, R. & Goss, A. N. Distribution and coexistence of neuropeptides in nerve fibres in the temporomandibular joint of late gestation fetal sheep. J. Anat. 191(Pt 2), 245–257. https://doi.org/10.1046/j.1469-7580.1997.19120245.x (1997).
Liu, W. et al. Changes of Trigeminal Ganglion Neurons Innervating the Temporomandibular Joint in Chronic Pain Rat Model. Int. J. Dent. 2024, 7015382. https://doi.org/10.1155/2024/7015382 (2024).
Park, C. K. Maresin 1 Inhibits TRPV1 in Temporomandibular Joint-Related Trigeminal Nociceptive Neurons and TMJ Inflammation-Induced Synaptic Plasticity in the Trigeminal Nucleus. Mediators Inflamm 2015, 275126. https://doi.org/10.1155/2015/275126 (2015).
Tahmasebi-Sarvestani, A., Tedman, R. A. & Goss, A. Neural structures within the sheep temporomandibular joint. J. Orofac. Pain 10, 217–231 (1996).
Yang, L. et al. Human and mouse trigeminal ganglia cell atlas implicates multiple cell types in migraine. Neuron 110, 1806-1821 e1808. https://doi.org/10.1016/j.neuron.2022.03.003 (2022).
Takeuchi, Y., Ishii, N. & Toda, K. An in vitro temporomandibular joint-nerve preparation for pain study in rats. J. Neurosci. Methods 109, 123–128. https://doi.org/10.1016/s0165-0270(01)00405-8 (2001).
Takeuchi, Y. & Toda, K. Subtypes of nociceptive units in the rat temporomandibular joint. Brain Res. Bull. 61, 603–608. https://doi.org/10.1016/s0361-9230(03)00219-3 (2003).
Asaki, S., Sekikawa, M. & Kim, Y. T. Sensory innervation of temporomandibular joint disk. J. Orthop. Surg. (Hong Kong) 14, 3–8. https://doi.org/10.1177/230949900601400102 (2006).
Chung, M. K., Wang, S., Alshanqiti, I., Hu, J. & Ro, J. Y. The degeneration-pain relationship in the temporomandibular joint: Current understandings and rodent models. Front. Pain Res. (Lausanne) 4, 1038808. https://doi.org/10.3389/fpain.2023.1038808 (2023).
Hovhannisyan, A. H. et al. Sensory innervation of masseter, temporal and lateral pterygoid muscles in common marmosets. Sci. Rep. 13, 23062. https://doi.org/10.1038/s41598-023-49882-9 (2023).
Lindquist, K. A. et al. Identification of Trigeminal Sensory Neuronal Types Innervating Masseter Muscle. eNeuro https://doi.org/10.1523/ENEURO.0176-21.2021 (2021).
Sharma, N. et al. The emergence of transcriptional identity in somatosensory neurons. Nature 577, 392–398. https://doi.org/10.1038/s41586-019-1900-1 (2020).
Bhuiyan, S. A. et al. Harmonized cross-species cell atlases of trigeminal and dorsal root ganglia. bioRxiv https://doi.org/10.1101/2023.07.04.547740 (2023).
Bhuiyan, S. A. et al. Harmonized cross-species cell atlases of trigeminal and dorsal root ganglia. Sci. Adv. 10, eadj9173. https://doi.org/10.1126/sciadv.adj9173 (2024).
Vrontou, S., Wong, A. M., Rau, K. K., Koerber, H. R. & Anderson, D. J. Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature 493, 669–673. https://doi.org/10.1038/nature11810 (2013).
Liu, Q. et al. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat. Neurosci. 10, 946–948. https://doi.org/10.1038/nn1937 (2007).
Xing, Y. et al. MrgprC11(+) Jugular Neurons Control Airway Hyperresponsiveness in Allergic Airway Inflammation. Am. J. Respir Cell Mol. Biol. 72, 393–407. https://doi.org/10.1165/rcmb.2024-0153OC (2025).
Adatia, A., Rainsford, K. D. & Kean, W. F. Osteoarthritis of the knee and hip. Part I: aetiology and pathogenesis as a basis for pharmacotherapy. J. Pharm. Pharmacol. 64, 617–625. https://doi.org/10.1111/j.2042-7158.2012.01458.x (2012).
Sofat, N. & Howe, F. A. Bone marrow lesions in osteoarthritis: Characterising genetic and histological changes to understand disease pathophysiology. Osteoarthr Cartil Open 6, 100531. https://doi.org/10.1016/j.ocarto.2024.100531 (2024).
Muramatsu, K., Iwanaga, R. & Sakai, T. Synovial hemangioma of the knee joint in pediatrics: Our case series and review of literature. Eur. J. Orthop. Surg. Traumatol. 29, 1291–1296. https://doi.org/10.1007/s00590-019-02431-5 (2019).
Bai, Z. et al. Synovial fibroblast gene expression is associated with sensory nerve growth and pain in rheumatoid arthritis. Sci. Transl. Med. 16, eadk3506. https://doi.org/10.1126/scitranslmed.adk3506 (2024).
Minervini, G. et al. Stem Cells in Temporomandibular Joint Engineering: State of Art and Future Persectives. J. Craniofac. Surg. 33, 2181–2187. https://doi.org/10.1097/SCS.0000000000008771 (2022).
Mecklenburg, J. et al. Transcriptomic sex differences in sensory neuronal populations of mice. Sci. Rep. 10, 15278. https://doi.org/10.1038/s41598-020-72285-z (2020).
Tavares-Ferreira, D. et al. Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci. Transl. Med. 14, eabj8186. https://doi.org/10.1126/scitranslmed.abj8186 (2022).
Barry, A. M., Zhao, N., Yang, X., Bennett, D. L. & Baskozos, G. Deep RNA-seq of male and female murine sensory neuron subtypes after nerve injury. Pain 164, 2196–2215. https://doi.org/10.1097/j.pain.0000000000002934 (2023).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol 18, e3000410. https://doi.org/10.1371/journal.pbio.3000410 (2020).
Belugin, S. et al. Mechanisms of transient signaling via short and long prolactin receptor isoforms in female and male sensory neurons. J. Biol. Chem. 288, 34943–34955. https://doi.org/10.1074/jbc.M113.486571 (2013).
Zappulo, A. et al. RNA localization is a key determinant of neurite-enriched proteome. Nat. Commun. 8, 583. https://doi.org/10.1038/s41467-017-00690-6 (2017).
Roy, S. et al. Neurogenic tissue nanotransfection in the management of cutaneous diabetic polyneuropathy. Nanomedicine 28, 102220. https://doi.org/10.1016/j.nano.2020.102220 (2020).
Ruparel, N. B., Patwardhan, A. M., Akopian, A. N. & Hargreaves, K. M. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain 135, 271–279. https://doi.org/10.1016/j.pain.2007.06.005 (2008).
Neeb, L. et al. IL-1beta stimulates COX-2 dependent PGE(2) synthesis and CGRP release in rat trigeminal ganglia cells. PLoS ONE 6, e17360. https://doi.org/10.1371/journal.pone.0017360 (2011).
Lorenzo, L. E. et al. Gephyrin clusters are absent from small diameter primary afferent terminals despite the presence of GABA(A) receptors. J. Neurosci. 34, 8300–8317. https://doi.org/10.1523/JNEUROSCI.0159-14.2014 (2014).
Dunkley, P. R., Bobrovskaya, L., Graham, M. E., von Nagy-Felsobuki, E. I. & Dickson, P. W. Tyrosine hydroxylase phosphorylation: regulation and consequences. J. Neurochem. 91, 1025–1043. https://doi.org/10.1111/j.1471-4159.2004.02797.x (2004).
Ma, F. Y., Grattan, D. R., Bobrovskaya, L., Dunkley, P. R. & Bunn, S. J. Angiotensin II regulates tyrosine hydroxylase activity and mRNA expression in rat mediobasal hypothalamic cultures: the role of specific protein kinases. J. Neurochem. 90, 431–441. https://doi.org/10.1111/j.1471-4159.2004.02492.x (2004).
Tram, M., Ibrahim, T., Hovhannisyan, A., Akopian, A. & Ruparel, S. Lingual innervation in male and female marmosets. Neurobiol. Pain 14, 100134. https://doi.org/10.1016/j.ynpai.2023.100134 (2023).
Acknowledgements
We would like to thank Miss. Anahit Hovhannisyan for guidance on IHC. We are grateful to Dr. Pao-Tien Chuang (UC San Francisco, San Francisco, CA) for kindly providing the CGRPcre-ER mouse line; Dr. Liang Han (College of Sciences, Georgia Tech, Atalanta, GA) for kindly providing MrgprA3cre and MrgprC11cre/ER mouse lines generated in Dr. Xinzhong Dong’s laboratory (Johns Hopkins University, Baltimore, MD) and Dr. Liang Han’s laboratory, respectively; and MMRRC at UC Davis for preparing Htr3acre mouse line.
Funding
This research work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH/NIAMS) through the NIH HEAL (https://heal.nih.gov/) Initiative the Restoring Joint Health and Function to Reduce Pain (RE-JOIN) Consortium UC2 AR082195 (to A.N.A.) and by the National Institute of Dental and Craniofacial Research (NIH/NIDCR) training CO-STAR grant T32 DE014318 (to J.J.A.).
Author information
Authors and Affiliations
Consortia
Contributions
J.J.A. and E.W.: methodology, investigation, visualization. J.J.A., E.W., and A.N.A.: analysis, conceptualization. J.J.A. and A.N.A.: research design. J.J.A., E.W., RE-JOIN and A.N.A.: discussion of results. J.J.A. and A.N.A: drafted the manuscript. J.J.A., E.W., RE-JOIN and A.N.A: prepared final version of the manuscript. A.N.A.: resources, supervision, funding acquisition. All authors reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The reporting in the manuscript follows the recommendations in the ARRIVE guidelines (PLoS Bio 8(6), e1000412,2010). All experimental protocols were approved by the UTHSCSA IACUC committee. Protocol numbers is 20220069AR.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alfaro, J.J., Wang, E., R. E.-JOIN Consortium Investigators. et al. Identification of sensory fiber types in mouse temporomandibular joint tissues. Sci Rep 15, 32210 (2025). https://doi.org/10.1038/s41598-025-17508-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17508-x