Abstract
Breast cancer has become one of the most common malignant tumors in women, and the incidence rate is increasing annually in men. This study focused on exploring the prognostic significance of the combined detection of serum ALP and LDH in patients with breast cancer. We enrolled 80 individuals with male breast cancer (MBC), female breast cancers (FBCs) were randomly matched via propensity score matching (PSM). In the MBC cohort, the optimal cutoff values for ALP and LDH were determined to be 114 U/L and 207 U/L, respectively, whereas in the FBC cohort, they were 68 U/L and 133 U/L, respectively. There was a difference in median survival between patient groups classified by the optimal cutoff values of ALP and LDH in the FBC cohort. However, in the FBC cohort, there was no significant correlation between the levels of ALP or LDH and patient prognosis. We developed a “joint score” system that incorporates the values of both ALP and LDH and separates MBCs into three groups. Survival was significantly different among the three groups. In the multivariate analysis, the “joint score” was identified as an independent prognostic factor for both disease-free survival(DFS) and overall survival (OS).
Similar content being viewed by others
Introduction
Male breast cancer (MBC) is a primary malignancy that arises specifically in the male breast1. Despite its rarity, accounting for less than 1% of new breast cancer cases reported in the literature, the incidence of MBC is increasing, with a high incidence at the age of 68–71 years2,3. Compared with female breast cancer (FBC), MBC frequently presents with more advanced clinical staging at the time of diagnosis, a phenomenon that may be associated with the absence of sex-specific screening initiatives3. Given its low incidence, randomized controlled trials on MBC are limited, predominantly small-sample retrospective studies4,5. Consequently, the current guidelines for the diagnosis and management of MBC often rely on extrapolation from research conducted on FBC6,7despite notable discrepancies in their biological characteristics7,8. Numerous studies9,10,11 have assessed the prognosis of MBC and reported that the unadjusted overall survival rate for MBC is lower than that for FBC. Consequently, the development of prognostic metrics specific to MBC that are convenient and accurate is critically important.
In recent years, an increasing number of serum biomarkers, such as circulating microRNAs, inflammatory factors and stem cell markers, have been identified as prognostic factors for a variety of malignant tumors, including breast cancer12,13,14,15,16. In addition, we found that serum alkaline phosphatase (ALP) and lactate dehydrogenase (LDH), two routine diagnostic markers commonly used in clinical practice, also have some prognostic value in patients with a variety of malignant tumors, including breast cancer17,18,19,20. These two indicators can be obtained from routine blood biochemistry tests and are routinely tested for breast cancer patients today. Nevertheless, the prognostic value of the combined detection of serum ALP and LDH in breast cancer remains controversial, especially given the lack of relevant studies on MBC. Thus, this study focused on exploring the prognostic significance of the combined detection of serum ALP and LDH in patients with breast cancer, especially MBC, with the aim of providing new prognostic insights for MBC.
Methods
Data sources and study population
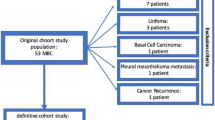
This study enrolled 80 male patients histologically diagnosed with breast or breast-derived malignant tumors at the Third Affiliated Hospital of Kunming Medical University (Yunnan Cancer Hospital) from December 2005 to August 2023. The exclusion criteria included a previous history of other malignancies, a first diagnosis of distant metastasis or bilateral breast cancer, other serious metabolic diseases, and incomplete follow-up data. Moreover, 80 FBCs were randomly matched according to propensity score matching (PSM). FBC controls were required to have undergone radical surgery to align with the primary MBC analysis excluding biopsy-only cases. Figure 1 shows the detailed flow chart of the study. This study was approved by the Ethics Office of Yunnan Cancer Hospital (Project Ethics Trial No. SLKYLX2022236). The Institutional Review Board of Yunnan Cancer Hospital waived the requirement for informed consent because this was a retrospective study. All the procedures performed in our study involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration.
Data collection
Demographic, clinical, pathological, and molecular data, as well as survival information, were collected for the study. Blood levels of ALP and LDH at baseline, prior to treatment (within 30 days preceding the initial treatment), were retrieved from the electronic medical records.
The time of initial diagnosis was defined as the time of the first pathological diagnosis of a breast or breast-derived malignant tumor. TNM staging was conducted in accordance with the American Joint Committee on Cancer (AJCC) eighth edition TNM staging system. Molecular typing was divided into luminal A type, luminal B type, HER-2-overexpressing type and triple-negative breast cancer on the basis of the expression of four markers: estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER-2) and Ki-67. Patient follow-up was managed through an outpatient medical record system and via telephone calls. The primary endpoint of the follow-up was overall survival (OS), which was the time interval between the diagnosis of a malignant tumor of breast or breast-derived origin and the occurrence of death from any cause. The secondary endpoints included disease-free survival (DFS). DFS is measured from the clinical determination of complete remission to the reappearance of new lesions or the development of metastasis. Patients in the study were monitored and followed up until July 2025, which marked the end of the study period. During this time, the researchers meticulously recorded the dates of any disease recurrence, the development of metastases, and the occurrence of death among the participating patients.
Statistical analysis
To minimize baseline confounding, PSM was performed to match MBC patients with FBC controls at a 1:1 ratio (80 MBC vs. 80 FBC). Matching covariates included clinically significant prognostic factors: age (± 5 years), clinical stage (I/II/III), and molecular subtype (Luminal A/B, HER2+, triple-negative). Nearest-neighbor matching with a caliper width of 0.2 times the standard deviation of the propensity score was applied, and matching was conducted without replacement. Post-matching, baseline characteristics (e.g., age, stage, molecular subtype) were well-balanced (all p > 0.05, Table 1).
In this study, all the statistical analyses were conducted via jamovi statistical software version 2.3.21. The significance of the results was determined by a p value of less than 0.05, which is commonly used as the threshold for statistical significance. To determine the optimal cutoff values for the serum biomarkers ALP and LDH, maximal selected rank statistic (MSRS) analysis was employed. This method helps to identify the best threshold values for these markers that maximize the power of the test to detect differences between groups. The relationships between clinical characteristics and ALP and LDH levels were examined via Fisher’s exact test. A “joint score” was developed on the basis of the levels of ALP and LDH, and patients were divided into different groups according to the “joint score”. To investigate the associations between the “joint score” and the clinical outcomes of OS and DFS in patients with breast cancer, a Cox proportional hazard regression model was utilized. Univariate Cox regression analysis was used to investigate the independent influence of various clinical and pathological factors on survival outcomes. Statistically significant factors from this analysis were integrated into a multivariate Cox regression model, which also considered additional recognized prognostic factors relevant to MBC on the basis of clinical experience. The associations between the “joint score” and OS and DFS were subsequently evaluated.
Results
The study included a cohort of 160 patients with comprehensive clinical data, consisting of 80 individuals with MBC and an equal number of patients with FBC, who were carefully matched via PSM. The baseline characteristics of the MBC patients were comparable to those of the FBC patients (Table 2). The median age at diagnosis for MBC and FBC patients were 66 (range: 41–83) and 62 (range: 41–85), respectively. In the MBC cohort, most patients had stage II disease (46.3%, 37/80). With respect to biological markers, the majority of MBCs were ER positive (93.8%, 75/80), particularly the luminal A type (78.8%, 63/80). In terms of systemic therapies, 63.8% (51/80) received chemotherapy, and 52.5% (42/80) received endocrine therapy. Radiotherapy was applied to 21.3% (17/80) of this cohort.
In the MBC cohort, the median follow-up time was 89.6 months (range 18.0-204.6), and the 5-year OS rate was 87.1% [95% confidence interval (CI), 79.5–95.4]. Similarly, the FBC cohort had a median follow-up time of 97.4 months (range 22.6–196.0), and the 5-year OS rate was 92.1% (95% CI, 86.1–98.4).
The optimal cutoff values for ALP and LDH
Serum pretreatment values of ALP and LDH were collected and imported into version 2.3.21 of the jamovi software for statistical analysis. The data were analyzed via the MST method with OS as the endpoint, and the results are presented in Fig. 2. In the MBC cohort, the optimal cutoff values for ALP and LDH were determined to be 114 U/L and 207 U/L, respectively, whereas in the FBC cohort, they were 68 U/L and 133 U/L, respectively. The correlations between clinical characteristics and the levels of ALP and LDH are detailed in Table 3. In the MBC cohort, there is no significant correlation between the levels of ALP/LDH and clinical characteristics.
Maximally selected rank statistics were used to evaluate the optimal cutoff values for ALP and LDH levels. (a) The best cutoff value of ALP for MBC was 114 U/L. (b) The best cutoff value of LDH for MBC was 207 U/L. (c) The best cutoff value of ALP for FBC was 68 U/L. (d) The best cutoff value of LDH for FBC was 113 U/L. ALP, alkaline phosphatase; LDH, lactate dehydrogenase; MBC, male breast cancer; FBC, female breast cancer.
ALP and LDH are risk factors for breast cancer
In the MBC cohort, the median OS significantly differed between patients with pretreatment ALP levels less than 114 U/L and those with ALP levels equal to or greater than 114 U/L (p < 0.0001) (Fig. 3a). The hazard ratio (HR) was 22.75 (95% CI: 6.89–75.10, p < 0.001). When the LDH level was equal to or greater than 207 U/L, the HR was 4.49 (95% CI: 1.74–11.57, p = 0.002) compared with levels less than 207 U/L. (Fig. 3b). Therefore, elevated levels of ALP (≥ 114 U/L) and LDH (≥ 207 U/L) serve as risk factors for the prognosis of MBC. However, in the FBC cohort, there was no significant correlation between the levels of ALP or LDH and patient prognosis (Fig. 3c-d). Consequently, the prognostic implications of these two factors for FBC were not further investigated.
The relationship between ALP/LDH and the prognosis of MBC and FBC. (a) OS according to ALP in MBC. (b) OS according to LDH in MBC. (c) OS according to ALP in FBC. (d) OS according to LDH in FBC. (e) OS according to the score of “Joint Score” in MBC. (f) DFS according to the score of “Joint Score” in MBC. (g) OS according to the score of “Joint Score” in FBC. (h) DFS according to the score of “Joint Score” in FBC. (i) Grouping based on the score of “Joint Score”. DFS, disease-free survival; OS, overall survival; PFS, progression-free survival; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; MBC, male breast cancer; FBC, female breast cancer.
We developed a “joint score” system that incorporates the values of both ALP and LDH and separates MBCs into three groups according to the score of the “joint score” (good, 0 factors; intermediate, 1 factor; poor, 2 factors) (Fig. 3i). Of the 80 MBCs evaluated in our study, 60 (75.0%) were classified as having a good joint score, 15 (18.8%) as having an intermediate joint score, and 5 (6.3%) as having a poor joint score. The 5-year OS rates for patients with good, intermediate and poor “joint scores” were 100.0%, 96.2% and 87.1%, respectively. The risk indices for intermediate and poor “joint scores” were 3.169 and 6.947 times greater than those for good “joint scores”, respectively. The survival curves for OS based on the “joint score” are presented in Fig. 3e and revealed significant differences among the risk groups (p < 0.0001).
Univariate and multivariate Cox proportional hazards regression analyses were performed to examine the prognostic factors associated with DFS and OS in MBC patients. The factors considered in the analysis included the score of the “joint score,” age, BMI, T stage, N stage, and molecular type. In the multivariate analysis, the “joint score” was identified as an independent prognostic factor for both DFS and OS, indicating its significant predictive value for MBC (Table 4).
Discussion
In our study, the median age of MBC diagnosis was approximately 60 years. Over half of the patients were diagnosed with stage I or II disease. The vast majority of tumors are positive for estrogen and progesterone receptors, that is, luminal breast cancer, similar to the findings of previous studies4,21,22.The positive rate of HER-2 receptor expression was 3.1%, which was lower than the rates reported in previous studies23,24. We also observed that the 5-year overall survival rate in our study was comparable to those reported in other studies22,25indicating a generally favorable prognosis.
Previous studies26,27,28 have revealed that the ALP is closely related to cell cycle control, tumor metastasis and proliferation. Clinically, elevated serum levels of ALP have been consistently linked to unfavorable prognoses in various malignancies29,30,31,32. The underlying mechanisms remain incompletely understood. Potential explanations include ALP33 may hydrolyze extracellular matrix proteins and modulate adhesion molecules, facilitating basement membrane penetration and metastatic dissemination. Besides, ALP participates in phosphorylation/dephosphorylation processes34altering tumor microenvironment (TME) pH and immune cell recruitment/polarization, thereby promoting immune evasion. However, the correlation between ALP levels and the prognosis of patients with breast cancer remains unclear. Aqeel et al.35 reported a correlation between low ALP levels and more aggressive breast cancer subtypes, higher tumor stages, and poorer outcomes. Another study36 reported that low ALP activity is associated with an increased risk of recurrence and metastasis, as well as a decreased overall survival rate in breast cancer patients. Some studies hold a contrasting viewpoint, suggesting that elevated ALP levels serve as a prognostic indicator of poor OS and play a significant role in tumor invasion and metastasis. A study37 revealed that serum ALP levels were significantly greater in patients with stage IV disease (who had developed recurrent metastases) than in patients with stages I, II, and III disease (p < 0.001), indicating a potential correlation between advanced disease progression and ALP levels (p < 0.001). In a retrospective analysis by Yazdani et al.38high ALP levels were associated with a poorer prognosis in patients with breast cancer with bone metastases (patients with high ALP had more severe bone-related adverse events and lower OS). A meta-analysis39 encompassing 53 studies and 22,436 patients revealed that high ALP levels were associated with shorter survival, with a hazard ratio of 1.72 (95% CI: 1.37–2.16; p < 0.001). Thus, these findings suggest that further research is needed to explore whether ALP can serve as a sensitive and economic biomarker for the diagnosis of breast cancer, monitor its progression, and serve as a critical indicator of patient prognosis.
The role of LDH as a key rate-limiting enzyme in glycolysis is well documented, and its importance in malignant tumors is particularly notable. Malignant tumor cells utilize LDH to recycle pyruvate and reduce nicotinamide adenine nucleotide (NADH) levels, thereby accelerating ATP production, tumor cell metabolism, and lactate production. This process plays a critical role in promoting malignant tumor cell growth and inhibiting the body’s immune response against tumors40,41. The prognostic value of serum LDH levels has been firmly established in numerous types of cancer42,43,44,45. For example, the International Prognostic Index (IPI)46a widely utilized clinical tool for predicting the prognosis of patients with non-Hodgkin’s lymphoma, incorporates five risk factors, one of which is the serum LDH level. LDH, which is also an important indicator of the second revision of the International Staging System (R2-ISS)19is not only a poor prognostic factor for the first diagnosis of multiple myeloma but also a predictor of a poor prognosis in patients with multiple myeloma at the time of first relapse. Furthermore, serum LDH levels are incorporated into the melanoma tumor node metastasis (TNM) staging system47. Several studies48,49,50,51 have explored the prognostic implications of serum LDH in conjunction with other indicators, such as the neutrophil‒lymphocyte ratio (NLR) and absolute lymphocyte count (ALC), in patients with breast cancer and have revealed a correlation between elevated serum LDH levels and poor prognosis.
Our study also explored the implications of ALP and LDH levels for breast cancer prognosis, revealing that elevated pretreatment ALP (≥ 114 U/L) and LDH (≥ 207 U/L) levels were indicative of a poor prognosis in MBC patients, yet these associations were not observed in FBC patients. This finding conflicts with prior research. The discrepancy observed may be attributed to several factors. First, our study primarily focused on male breast cancer (MBC), with the female breast cancer (FBC) cohort serving as a reference control. Our findings align with the distinct biology of MBC, which exhibits unique molecular characteristics including high ER-positivity (> 90%), lower HER2 over-expression rates, frequent PIK3CA mutations. These biological differences may modulate LDH-driven glycolytic processes, potentially changing LDH’s independent prognostic impact in MBC. Second, our study identified significantly lower optimal cut-off values for ALP/LDH in FBC (68 U/L and 133 U/L, respectively) compared to MBC (114 U/L and 207 U/L), underscoring the importance of sex-specific thresholds in prognostic evaluation. Third, the null prognostic association of ALP/LDH in FBC may be attributed to more standardized screening protocols, wider adoption of adjuvant therapies (e.g., targeted treatments) in female patients. These factors might overshadow ALP/LDH’s predictive value by introducing stronger competing prognostic variables.
In recent years, numerous studies52,53,54 have elucidated the utility of routine blood parameters as prognostic indicators. The development of comprehensive prognostic scores has been instrumental in standardizing and enhancing the predictive value of these parameters in cancer outcomes55,56. As mentioned earlier, prior research has extensively investigated the prognostic implications of ALP and LDH levels across a spectrum of cancers, including breast cancer. To our knowledge, our study is the first to assess the combined significance of ALP and LDH as prognostic indicators in MBC.
In our study, a “joint score” was established on the basis of the levels of ALP and LDH, thereby enabling the stratification of our MBC patient population into three distinct groups. Notably, patients classified within the poor “joint score” category presented a greater likelihood of disease progression and significantly shorter DFS and OS than did those in the intermediate or good score groups. Additionally, pretreatment ALP levels ≥ 114 U/L and LDH levels ≥ 207 U/L were identified as adverse prognostic factors in both univariate and multivariate analyses of DFS and OS. Our concurrent evaluation of ALP and LDH suggested a potential synergistic effect of these serum markers on the prognosis of patients with malignant tumors. Specifically, patients with higher “joint score” values had more severe disease outcomes and earlier metastasis. This observation underscores the importance of considering the combined impact of ALP and LDH levels on the prognosis of MBC patients. Furthermore, the “joint score” may also be applicable to other malignant tumors, and more comprehensive experiments and studies are needed for verification.
In summary, our study established a “joint score” model to demonstrate that ALP and LDH have prognostic significance in MBC. An increase in ALP and LDH levels prior to treatment heralds a poor prognosis, potentially corroborating the utility of these biomarkers as prognostic indicators for MBC. Our primary analysis included biopsy-only cases to reflect real-world scenarios, though sensitivity analysis confirmed results in surgically-treated patients. Future studies should validate these biomarkers in non-operative settings. Furthermore, as medical science advances and our understanding of malignancies deepens, treatment modalities for cancer are becoming increasingly precise, exemplified by therapies such as cyclin-dependent kinase (CDK) 4/6 inhibitors for estrogen/progesterone receptors and anti-HER2 targeted therapies. These developments imply that future strategies could target the modulation of ALP and LDH expression to elicit antitumor effects.
Further detailed subgroup analyses are increasingly challenging due to the low prevalence of MBC and the fact that all the data in our study were collected from a single research institution, which restricts the diversity and generalizability of the sample population. Additionally, as a retrospective study, the potential for recall bias remains, despite our efforts to gather comprehensive information from patients. In addition to the factors discussed in our study, other factors, such as treatment, may also influence the outcome and thus affect the prognosis. Moreover, the determination of optimal cutoff values for serum ALP and LDH levels may require the accumulation of larger datasets to facilitate more precise thresholds. The prognostic value of ALP/LDH in non-surgical FBC patients warrants further study. Despite its limitations, this study is anticipated to serve as a valuable reference for future large-scale, statistically robust, and prospectively designed investigations.
Conclusions
This study suggests that the “joint score”, which is calculated on the basis of pretreatment levels of serum ALP and LDH, has the potential to predict the risk of recurrence, metastasis, and poor prognosis in MBC patients. Nonetheless, the current study’s statistical sample size is limited, and it is confined to a single center. A larger, multicenter, prospective study is needed to validate the utility of the “joint score” in MBC treatment and to elucidate its predictive value.
Data availability
All data are available from the corresponding author on reasonable request.
References
Spreafico, F. S. et al. Breast cancer in men: clinical and pathological analysis of 817 cases. Am. J. Mens Health. 14, 1557988320908109 (2020).
Gao, Y., Heller, S. L. & Moy, L. Male breast cancer in the age of genetic testing: an opportunity for early detection, tailored therapy, and surveillance. Radiographics 38, 1289–1311 (2018).
Ruddy, K. J. & Winer, E. P. Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship. Ann. Oncol. 24, 1434–1443 (2013).
Miglietta, F. et al. Gender minorities in breast cancer - Clinical trials enrollment disparities: focus on male, transgender and gender diverse patients. Breast 75, 103713 (2024).
Giordano, S. H. Breast cancer in men. N Engl. J. Med. 378, 2311–2320 (2018).
Gradishar, W. J. et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 20, 691–722 (2022).
Breast cancer professional committee of the chinese anti. -cancer association, breast tumor group of the oncology branch of the Chinese medical association. Chinese anti-cancer association breast cancer diagnosis and treatment guidelines and norms (2024 Edition). China Cancer J. 33, 1092–1187 (2023).
Bielcikova, Z. et al. Treatment and prognosis of male breast cancer: a multicentric, retrospective study over 11 years in the Czech Republic. Oncologist 29, e750–e762 (2024).
Yu, E. et al. Male breast cancer prognostic factors versus female counterparts with propensity scores and matched-pair analysis. Cureus 7, e355 (2015).
Eskander, M. F. et al. Hormone receptor-positive breast cancer and black race: does sex matter. Breast Cancer Res. Treat. 190, 111–119 (2021).
Wang, B., Wang, H., Zhao, A., Zhang, M. & Yang, J. Poor prognosis of male triple-positive breast cancer patients: a propensity score matched SEER analysis and molecular portraits. BMC Cancer. 21, 523 (2021).
Truffi, M. et al. Prognostic potential of immune inflammatory biomarkers in breast cancer patients treated with neoadjuvant chemotherapy. Cancers (Basel). 14, 5287 (2022).
Savioli, F. et al. Prognostic role of preoperative Circulating systemic inflammatory response markers in primary breast cancer: meta-analysis. Br. J. Surg. 109, 1206–1215 (2022).
Quirico, L. & Orso, F. The power of MicroRNAs as diagnostic and prognostic biomarkers in liquid biopsies. Cancer Drug Resist. 3, 117–139 (2020).
Zografos, E. et al. Prognostic role of MicroRNAs in breast cancer: A systematic review. Oncotarget 10, 7156–7178 (2019).
Nairuz, T., Mahmud, Z., Manik, R. K. & Kabir, Y. Cancer stem cells: an insight into the development of metastatic tumors and therapy resistance. Stem Cell. Rev. Rep. 19, 1577–1595 (2023).
Abu-Talib, M. & Brown, L. R. Albumin-to-alkaline phosphatase ratio as a novel prognostic indicator for patients undergoing minimally invasive lung cancer surgery: propensity score matching analysis using a prospective database. Int. J. Surg. 69, 152 (2019).
Xue, X. et al. Association between alkaline phosphatase/albumin ratio and the prognosis in patients with chronic kidney disease stages 1–4: results from a C-STRIDE prospective cohort study. Front. Med. (Lausanne). 10, 1215318 (2023).
D’Agostino, M. et al. Second revision of the international staging system (R2-ISS) for overall survival in multiple myeloma: a European myeloma network (EMN) report within the HARMONY project. J. Clin. Oncol. 40, 3406–3418 (2022).
Olszewski, A. J. et al. Burkitt lymphoma international prognostic index. J. Clin. Oncol. 39, 1129–1138 (2021).
Masci, G. et al. Clinicopathological and immunohistochemical characteristics in male breast cancer: a retrospective case series. Oncologist 20, 586–592 (2015).
Gao, Y. et al. The features of male breast cancer in china: A real-world study. Breast 76, 103762 (2024).
Lautrup, M. D. et al. Male breast cancer: a nation-wide population-based comparison with female breast cancer. Acta Oncol. 57, 613–621 (2018).
Liu, N., Johnson, K. J. & Ma, C. X. Male breast cancer: an updated surveillance, epidemiology, and end results data analysis. Clin. Breast Cancer. 18, e997–e1002 (2018).
Liu, G. et al. Clinical features and prognoses of patients with breast cancer who underwent surgery. JAMA Netw. Open. 6, e2331078 (2023).
Zhang, K. et al. Prediction of microvascular invasion in HCC by a scoring model combining Gd-EOB-DTPA MRI and biochemical indicators. Eur. Radiol. 32, 4186–4197 (2022).
Zeng, J., Zeng, J., Liu, J. & Zeng, J. Development of pre and post-operative nomograms to predict individual survival for ideal liver resection candidates with hepatocellular carcinoma. Liver Int. 41, 2974–2985 (2021).
Hashem, S. et al. Machine learning prediction models for diagnosing hepatocellular carcinoma with hcv-related chronic liver disease. Comput. Methods Programs Biomed. 196, 105551 (2020).
Ma, X. et al. Blood biomarkers of bone metastasis in digestive tract malignant tumors. Future Oncol. 17, 1507–1518 (2021).
Qiu, G. et al. Preoperative alkaline phosphatase-to-cholesterol ratio as a predictor of overall survival in pancreatic ductal adenocarcinoma patients undergoing radical pancreaticoduodenectomy. Med. Sci. Monit. 27, e931868 (2021).
Sandfeld-Paulsen, B., Aggerholm-Pedersen, N. & Winther-Larsen, A. Pretreatment albumin-to-alkaline phosphatase ratio is a prognostic marker in lung cancer patients: a registry-based study of 7077 lung cancer patients. Cancers (Basel). 13, 6133 (2021).
Slagter, A. E. et al. Prognostic value of tumor markers and ctdna in patients with resectable gastric cancer receiving perioperative treatment: results from the CRITICS trial. Gastric Cancer. 25, 401–410 (2022).
Lou, Z. et al. Alkaline phosphatase downregulation promotes lung adenocarcinoma metastasis via the c-Myc/RhoA axis. Cancer Cell. Int. 21 (1), 217 (2021).
Zheng, Y. et al. Alkaline phosphatase and atg4b sequentially activated fluorescent probe for cancer cell-specific live imaging of autophagy. Anal. Chem., 97 (15), 8370-8377 (2025).
Aqeel Rashid, F., Mahdi, S., Abd-Alkader Mahdy, S. & Thamer Salim, A. Effect of obesity on plasma alkaline phosphatase activity in breast cancer. Rep. Biochem. Mol. Biol. 10, 307–313 (2021).
Tayubi, I. A. & Madar, I. H. Biomineralization associated alkaline phosphatase as a potential marker of bone metastasis in the patients with invasive breast cancer. Saudi J. Biol. Sci. 29, 103340 (2022).
Singh, A. K. et al. Advanced stage of breast cancer Hoist alkaline phosphatase activity: risk factor for females in India. 3 Biotech. 3, 517–520 (2013).
Yazdani, A., Dorri, S., Atashi, A., Shirafkan, H. & Zabolinezhad, H. Bone metastasis prognostic factors in breast cancer. Breast Cancer (Auckl). 13, 1178223419830978 (2019).
Jiang, C., Hu, F., Xia, X. & Guo, X. Prognostic value of alkaline phosphatase and bone-specific alkaline phosphatase in breast cancer: A systematic review and meta-analysis. Int. J. Biol. Markers. 38, 25–36 (2023).
Granchi, C., Bertini, S., Macchia, M. & Minutolo, F. Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Curr. Med. Chem. 17, 672–697 (2010).
Claps, G. et al. The multiple roles of LDH in cancer. Nat. Rev. Clin. Oncol. 19, 749–762 (2022).
Carlino, M. S., Larkin, J. & Long, G. V. Immune checkpoint inhibitors in melanoma. Lancet 398, 1002–1014 (2021).
Cowan, A. J. et al. Diagnosis and management of multiple myeloma: a review. JAMA 327, 464–477 (2022).
Comandatore, A. et al. Lactate dehydrogenase and its clinical significance in pancreatic and thoracic cancers. Semin Cancer Biol. 86, 93–100 (2022).
Wu, M. et al. Prognostic role of serum lactate dehydrogenase in patients with urothelial carcinoma: a systematic review and meta-analysis. Front. Oncol. 10, 677 (2020).
International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl. J. Med. 329, 987–994 (1993).
Balch, C. M. et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 27, 6199–6206 (2009).
Zhang, K. et al. A novel systematic oxidative stress score predicts the prognosis of patients with operable breast cancer. Oxid. Med. Cell. Longev. 2021, 9441896 (2021).
Li, L. et al. High score of LDH plus dNLR predicts poor survival in patients with HER2-positive advanced breast cancer treated with trastuzumab emtansine. BMC Cancer. 22, 29 (2022).
Ma, Y. Y. et al. Prognostic value of combined lactate dehydrogenase, c-reactive protein, cancer antigen 153 and cancer antigen 125 in metastatic breast cancer. Cancer Control. 29, 10732748211053150 (2022).
Chen, K. et al. Clinical outcomes and biomarkers of phyllodes tumors of the breast: A single-center retrospective study. Cancer Med. 12, 11363–11374 (2023).
Cheng, M. L. et al. Circulating tumor DNA in advanced solid tumors: clinical relevance and future directions. CA Cancer J. Clin. 71, 176–190 (2021).
Reichert, Z. R. et al. Prognostic value of plasma Circulating tumor DNA fraction across four common cancer types: a real-world outcomes study. Ann. Oncol. 34, 111–120 (2023).
Dyikanov, D. et al. Comprehensive peripheral blood Immunoprofiling reveals five immunotypes with immunotherapy response characteristics in patients with cancer. Cancer Cell. 42, 759–779e12 (2024).
Dolan, R. D., McSorley, S. T., Horgan, P. G., Laird, B. & McMillan, D. C. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 116, 134–146 (2017).
Zhao, Q., Wang, L., Yang, X., Feng, J. & Chen, Q. Association of the Scottish inflammatory prognostic score with treatment-related adverse events and prognosis in esophageal cancer receiving neoadjuvant immunochemotherapy. Front. Immunol. 15, 1418286 (2024).
Funding
This research was funded by First-Class Discipline Team of Kunming Medical University, grant number 2024XKTDYS08;Biomedical Projects of Yunnan Key Science and Technology Program, grant number 202302AA310046; the Biomedical Projects of Yunnan Key Science and Technology Program, grant number No.202302AA310046; National Natural Science Foundation of China, grant number No.82060481; National Natural Science Foundation of China, grant number No.82260542; Kunming Medical University Breast Cancer Metastasis and Drug Resistance Basic Research Innovation Team, grant number No.K1322137; Yunnan Province Higher Education Collaborative Innovation Center for Research on Breast Cancer Drug Resistance Mechanisms and Transformation Application, grant number No.K1322147.
Author information
Authors and Affiliations
Contributions
ZL and ZHL proposed the overall idea of this study; XSK, CT and QSL carried out preliminary data analysis and first draft writing and revision; ZHL and JW sorted the data and checked the statistical analysis results; YXZ and FHM assisted in sorting and analyzing the data and helped write the first draft; YWW edited the language of the article and corrected the grammar. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kong, X., Tao, C., Liu, Q. et al. Evaluation of serum alkaline phosphatase and lactate dehydrogenase as predictive biomarkers for the prognosis of male breast cancer. Sci Rep 15, 31634 (2025). https://doi.org/10.1038/s41598-025-17563-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17563-4