Abstract
This study presents a novel layered double hydroxide (LDH) composite consisting of Mg/Fe and Ca/Fe-LDH layers, synthesized via a co-precipitation method, aimed at enhancing hydrogen production from solar energy. The composites have bandgaps of 2.01 eV and 2.81 eV, respectively, making them ideal for photoelectrochemical (PEC) water splitting. The study demonstrates that using Mg/Fe-LDH can significantly increase the rate of hydrogen generation, achieving a catalytic H2 production rate of 2542.36 mmol/h·cm2 and a catalytic efficiency of 56.39% at 460 nm and 59.85% at 490 nm. ABPE achieves maximum peaks of 5.75% at 0.92 V and 5.33% at 0.4 V, setting new standards for LDH-based photocatalysts. The research also examines the effects of temperature and monochromatic light on PEC performance, thermodynamic parameters, electrochemical surface area, Tafel slopes, and hydrogen evolution kinetics. The LDH photoelectrode’s stability and reusability reveals its long-term application potential, providing fresh insights on improving PEC methods for industrial-scale water splitting in sunlight. Also, this is further corroborated by Density Functional Theory (DFT). This distinctive methodology was selected for the design of LDH nanomaterials for hydrogen-generation applications.
Similar content being viewed by others
Introduction
Owing to the huge decline of fossil fuels and the associated environmental issues brought on by human activity and economic growth, much effort has been made to find unlimited and environmentally acceptable energy sources to replace traditional fossil fuels. Consequently, the sustainable development of clean energy can successfully address the issue of environmental contamination, which is seen as a major concern1,2. It is common knowledge that solar energy is one of the cleanest and safest energy sources and that its robust development may significantly reduce environmental pollution. To optimize solar energy resources for water splitting and hydrogen production, the electrochemical water splitting process powered by sustainable energy has been identified as a highly promising alternative to the existing hydrocarbon economy3. Photoelectrochemical (PEC) water-splitting technology is a promising method for converting solar energy into sustainable hydrogen fuel. Many semiconductors with non-noble metals were recommended for large light absorption and H2 generation, among other materials. Layered double hydroxides (LDHs) offer numerous advantages as catalysts in photoelectrochemical (PEC) water splitting, enhancing the PEC activity of wide-bandgap semiconductors4,5,6. They can diminish charge recombination and enhance the kinetics of the water oxidation reaction. Moreover, their elevated layer charge density and unique electrical Characteristics. LDHs consist of divalent and trivalent metal cation layers that are covered with hydroxide ions7,8,9. In particular, the synergistic impact was fundamentally amplified by the existence of flexible chemical structures, a greater number of exposed active sites on the layered surface, and an acceleration of the photocatalytic hydrogen evolution reaction10,11. Currently, the use of bifunctional electrocatalysts is a highly promising approach in the field of industrial hydrogen production. Combining various efficient electrocatalysts into a hierarchical framework to create bifunctional catalysts has the potential to streamline the development and design of electrolytic devices while also decreasing operational expenses12,13. Layered double hydroxides (LDHs) exhibit favorable characteristics for photocatalysis and are regarded as superior alternatives to TiO2-based photocatalysts because of their layered structure, compositional flexibility, controlled dimension, cost-effectiveness, and straightforward preparation methods14. Of the many LDHs, the characteristics of Fe-based LDHs like Ni-Fe-LDH include easy production, low-cost growth, a high specific surface area, and a dense concentration of active centers that enhance interaction with various catalytic materials, making them very attractive. Harvesting these distinct pathways of layered double hydroxides and depositing Ni-Fe layered double hydroxides can enhance their electroactivity15,16,17,18. Iron metal hydrolysis for on-board hydrogen generation is gaining significant interest because of its quick and uncomplicated nature19. High quantities of NaOH and alloying Fe with common transition metals (Fe and Cu), alkaline earth metals (Ca and Mg), and other electrochemically noble metals (Ga, Li, and Bi) are applied to improve the hydrolysis reaction to alleviate this problem. The creation of Fe(OH)3 and Mg(OH)2 photocatalysts impedes hydrolysis reactions19, and reusing solid hydrolysis products may be challenging because of the presence of doped metals. This issue has been overlooked in literature, and it is evident that this practice is not linked with the concepts of green and sustainable development. This work rigorously examines the influence of various cations in LDH on the efficiency of photoelectrochemical (PEC) water splitting. Magnesium ferrite layered double hydroxide (Mg/Fe-LDH) and calcium ferrite layered double hydroxide (Ca/Fe-LDH) were produced using a straightforward and economical co-precipitation technique. LDH was comprehensively assessed using energy dispersive X-ray spectroscopy (EDXS), UV-vis spectroscopy, scanning electron microscopy (FE-SEM), and X-ray diffraction (XRD). The performance of LDHs was assessed using the incident-photon-to-current efficiency (IPCE) and bias potential conversion (ABPE). In addition, the stability, reusability, and the amount of hydrogen moles.
Synthesis of LDHs
Synthesis of Mg/Fe-LDH
A 1:1 molar ratio of Mg/Fe-LDH was synthesized via co-precipitation. Iron sulphate (0.1 M) and magnesium nitrate (0.1 M) were dissolved in 100 ml of H2O before the pH was adjusted with 2 N NaOH applied dropwise at 60 °C while swirling vigorously until the pH reached 10. The LDH that was created after 24 h of stirring was collected, washed many times with tepid distilled water until the pH reached 7, and subsequently dried overnight at 50 °C.
Synthesis of Ca/Fe-LDH
Ca/Fe-LDH with a molar ratio of 1:1 was synthesized utilizing the co-precipitation technique. A solution of calcium nitrate (0.1 M) and iron nitrate (0.1 M) was made by dissolving them in 100 ml of water. The pH of the solution was adjusted to 10 by gradually adding 2 N NaOH solution while rapidly swirling at a temperature of 60 °C. After being stirred for 24 h, the generated LDH was collected and treated with numerous washes using warm distilled water until attaining a pH of 7. It was then dried at a temperature of 50 °C overnight17,20.
Theoretical study
A deep analysis of the Ca/Fe and Mg/Fe-LDH at the molecular level will reveal the causes for the structure-activity relationship. This part exhibited the improvement of HER activity on the Ca/Fe and Mg/Fe-LDH by simulating the geometric structures and electrostatic potential characteristics using DFT. The Gaussian 09 software suite was utilized to conduct the chemical computations outlined in this study, and drawing structures of Ca/Fe-LDH and Mg/Fe-LDH by Gauss view and VESTA illustrated in Fig. 10 (a and d), then take one Molecule for Study DFT and electrostatic potential(ESP). Computational data is produced via the density functional theory (DFT) method with the B3LYP/6-31G(d, p) model chemistry, and illustrated SCF and unit cell Parameters in Tables S1 and S2, respectively. Density functional theory (DFT) approaches have been employed utilizing the B3LYP functional and the 6-311G basis set. These approaches have been selected to determine the band gap of the species under inquiry, as they have been successfully implemented in analogous systems. DFT calculations yield data on the energy gap (Egap = ELUMO − EHOMO), facilitate the identification of the molecular electrostatic potentials (MEP) plot, which visualizes the whole density, and delineate the highest occupied and lowest unoccupied molecular orbitals, EHOMO and ELUMO, respectively.
Characterization of LDH photocatalysts
The produced photocatalysts underwent several characterisation procedures to examine their properties. The crystal structure and phase purity were measured using X-ray diffractometry (XRD, Rigaku D/Max 2500). The XRD investigation was conducted at 30 mA and 40 kV, covering a scan range from 5 to 80°. The morphology of the photocatalysts was studied using a field emission scanning electron microscope (FE-SEM, Zeiss Sigma 500 VP). To assess the chemical composition, energy-dispersive X-ray (EDXS) spectroscopy was done in conjunction with the FE-SEM. Fourier transform infrared spectroscopy (FTIR, NICOLET 6700) was performed to analyze the functional groups contained in the produced materials. The optical characteristics were studied using a double-beam spectrophotometer (PerkinElmer Lambda 950) in the range of 200–1000 nm. 3D image created by ImageJ software, and the roughness was determined by Gwyddion.
Electrodes Preparation and electrochemical measurements
The electrode fabrication process began with the cleaning of the graphite substrate using methanol and ethanol. Approximately 2.0 mg of the LDH photocatalyst was then mixed with 0.20 ml of a 5 wt% Nafion solution and 0.40 ml of isopropanol. This mixture underwent 120 min of ultrasonic treatment to achieve a homogeneous suspension. The 1 mg of the resulting suspension was loaded onto a graphite sheet and dried at 50 °C to create the electrode. The assessment of hydrogen gas generation was conducted in a 0.3 M Na2SO3 aqueous electrolyte solution with a pH of 7.0. For the photoelectrochemical (PEC) studies, a two-electrode OrigaFlex potentiostat (OrigaLys ElectroChem) was utilized. The experimental setup included a solar simulator equipped with an Xe-lamp emitting light (AM 1.5 G, 100 mW/cm2). A platinum electrode is used as the auxiliary electrode to evaluate the photoelectrode’s catalytic activity. The working electrode employed was the fabricated LDH/graphite electrode. To record the photocurrent density (Jph) responses, a scanning voltage (V) ranging from ‒1 to + 1 V was applied at room temperature. The photocurrent density was measured under dark conditions as well as under the illumination of the white Xe lamp and monochromatic light.
Results and discussion
The ionic radii, the most common oxidation states, and electronegativity for Mg, Ca, and Fe can take different values. The ionic radius of Fe(II), Fe(III), Ca (II), and Mg (II) is 70 pm, 60 pm, 100 pm, and 72 pm, respectively. According to the Pauling scale, the electronegativity of Fe, Mg, and Ca atoms is 1.83, 1.31, and 1.00, respectively. These factors affect the number of carrier charges, structure morphology, and crystallite size and therefore modify the chemical and physical properties of photocatalysts21,22.
FE-SEM and surface roughness analysis
The morphologies of Ca/Fe and Mg/Fe-LDH are presented in Fig. 1. The Ca/Fe-LDH nanomaterial has a platelet-like structure with rough flake fragments on its surface (Fig. 1(a)). There are a large number of nanoplatelets with very small sizes. The range of nanoplatelet sizes is from 36 up to 179 nm. The FE-SEM characterization of Mg/Fe-LDH photocatalyst shows agglomerated large particles composed of a well-developed layered structure with sheet-like structures (Fig. 1(b)). These sheets have variable shapes and sizes. Also, the surface of the sheets is wavy and wrinkled, which indicates a large surface area with an average size of 103 nm. Figure 1 (c) and (d) illustrate the surface roughness measurements of Ca/Fe and Mg/Fe-LDH as analyzed using Gwyddion software, and a 3D image using ImageJ software. The roughness of Mg/Fe-LDH is higher than that of Ca/Fe due to the large number of small-sized nanoplatelets. The Ca/Fe surface roughness decreased due to the agglomeration and the formation of large nanoplates21,23. The root-mean-square (RMS) roughness, written as Rq, is often used to measure the height difference. Mg/Fe-LDH has an RMS value of 7.98 nm, and Ca/Fe-LDH has an RMS value of 3.5 nm. Depending on the surface roughness of the Mg/Fe-LDH nanomaterial, which is rougher than that of the Ca/Fe-LDH nanomaterial, this led Mg/Fe-LDH has high performance in hydrogen evolution reactions (HER), and it has better catalytic performance than Ca/Fe-LDH. This enhanced activity can be attributed to the increased surface roughness, which inherently provides a greater number of active sites for catalysis24,25,26. The Mg/Fe-LDH nanomaterial’s complex network and the rougher surface are one of the main reasons why it has better electrocatalytic properties for making hydrogen more efficient24,27,28.
XRD analysis
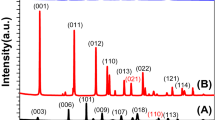
Figure 2(a) depicts the X-ray diffraction (XRD) patterns of the Mg/Fe-LDH and Ca/Fe-LDH photocatalysts. The XRD analysis confirms the presence of the LDH structure in both photocatalysts, as evidenced by distinct peaks in their XRD patterns. These LDH photocatalysts exhibit well-crystallized characteristics and possess a hexagonal structure. In the case of Ca/Fe-LDH, the observed peaks at 2θ = 11.62, 23.12, 34.56, 45.48, 60.08, and 61.52 degrees correspond to the (003), (006), (114), (027), (112), and (113) reflections, respectively. These peak positions align with previous studies conducted by29,30,31. It is worth noting that the photocatalysts also contain some Ca species, such as Ca(OH)2 and CaO impurities. Similarly, for Mg/Fe-LDH, the observed peaks at 2θ = 11.38, 22.62, 33.59, 38.25, 47.33, and 60.03 degrees can be attributed to the (003), (006), (009), (015), (018), and (110) reflections, respectively. These peak positions align with previous studies32,33,34. The XRD peaks Mg/Fe-LDH and Ca/Fe-LDH are mostly from Mg /Fe-LDH JCPDS (#96-153-7460) and for JCPDS Ca/Fe-LDH (#96-434-0356) and (#96-901-3961) according to crystallography open database (COD). To determine the crystallite size (D) and microstrain (ɛ) of the photocatalysts, the Williamson-Hall (W-H) model was utilized. The W-H model is represented by the following Eq(1).32,35,36,37:
where k represents the shape factor for photocatalysts, λ denotes the XRD wavelength, and β corresponds to the broadening of the diffraction lines in radians. Figure 2(b) and(c) and Table 1 provide comprehensive details of the data and the W-H plot. In the W-H plot, the x-axis represents the values of 4 sin(θ), while the y-axis displays β cos(θ). A linear fit was applied to the plot to extract the crystallite size (from the y-intercept) and strain (from the slope). Microstrain refers to local lattice distortions or disorders within the crystal structure, which can arise from defects or impurities present in the crystal. The low microstrain observed suggests minimal distortion within the crystal lattice. Table 1 reveals that the crystallite sizes of Mg/Fe-LDH and Ca/Fe-LDH are 87.0 nm and 27.7 nm, respectively. Moreover, Mg/Fe-LDH (28 × 10‒3) exhibits a higher microstrain compared to Ca/Fe-LDH (25 × 10‒3). The negative strain observed in the Mg/Fe-LDH photocatalyst indicates lattice shrinkage.
FTIR analysis
The FTIR spectra of the Mg/Fe-LDH and Ca/Fe-LDH photocatalysts displayed different absorption peaks. Notably, Mg/Fe-LDH revealed absorption bands at particular wavenumbers: broad bands between 3100 and 3600 cm− 1 were ascribed to the metallic hydroxide layer’s ‒OH stretching and interlayer water molecules38. Additionally, a strong band at 1377.99 cm− 1 indicates the existence of NO3 anions in the interlayer area, signifying carbonate groups, and 480 cm‒1, suggestive of metal-oxygen vibrations (Fig. 3). The appearance of these absorption peaks clearly supports the presence of important functional groups within the photocatalyst structure39. These functional groups contain hydroxyl groups, carbonates, and metal-oxygen linkages, A related observation was made in the FTIR spectra of Ca/Fe-LDH photocatalyst, which revealed analogous absorption peaks (Fig. 3). This closeness in functional groups emphasizes the potential universality in the interaction mechanisms between these nanoparticles and plant systems40.
Notably, all LDH photocatalysts revealed common absorption bands in the FTIR spectra, including a broad absorption band in the 3300–3500 cm‒1 area that was ascribed to the OH stretching mode of the interlayer water and basal layer. The bending mode of interlayer water and anion groups was also detected as a band in the 1620–1650 cm‒1 range. Additionally, a unique band associated with MO and MOH vibrations was identified in the 400–800 cm‒1 range41.
EDXS analysis
A photocatalyst’s elemental composition and chemical analysis can be detailed using the analytical approach known as energy-dispersive X-ray spectroscopy (EDXS)42. The EDXS spectrum shown in Fig. 4 (a, b) indicates the presence of iron, magnesium, oxygen, and calcium elements. No impurity signals were detected in the EDXS spectra, which matches the XRD results. The EDXS analysis supported presence of the intended cations of Mg/Fe-LDH and Ca/Fe-LDH. and confirmed Success synthesis of them by XRD and FTIR analysis.
UV-vis spectroscopy analysis
The absorption spectra of LDH had a significant impact on the PEC performance. The visible region, with wavelengths ranging from 400 to 600 nm, is critical for photocatalytic applications due to its dominance in solar irradiation43. The absorbance spectra of Mg/Fe-LDH and Ca/Fe-LDH as a function of wavelength from 200 to 1000 nm are displayed in Fig. 5(a). Figure 5(b) (red line) shows the Mg/Fe-LDH photocatalyst has a broad and intense absorption band. The Ca/Fe-LDH photocatalyst presented an absorption band within the UV region, as seen in Fig. 5(c) (black line). Above 350 nm, the absorption intensity of the Ca/Fe-LDH photocatalyst decreased. This indicates the Ca/Fe-LDH has very low photo-absorption in the visible region. The absorption edge of Mg/Fe-LDH shifts towards the higher wavelength side (visible region) compared to Mg/Fe-LDH. Also, Mg/Fe-LDH absorbs more photons in the UV and visible regions than Ca/Fe-LDH. Mg/Fe-LDH has two primary peaks at about 320 and 400 nm. The large peak at 386 nm and the weak peak at roughly 320 nm is attributable to π-π* transition of C = C. The faint absorption peak that emerges at 400 nm is due to n-π* concerning the Mg/Fe-LDH44. This suggests a decrease in the band gap energy, which can lead to more electron/hole pair generation under visible light irradiation, resulting in improved photocatalytic hydrogen activity. The optical bandgap energy for direct transition can be assessed by using the Tauc Eqs as illustrated in eq(2).45,46,47:
where \(\:{\upalpha\:}\) is the absorption coefficient, \(\:{\text{E}}_{\text{p}\text{h}}\) is the energy of an incident photon, \(\:\text{E}\text{g}\) is the energy bandgap, and K is the constant. Using Fig. 5(b, c), the \(\:\text{E}\text{g}\) value of the Mg/Fe-LDH (2.01 eV) is low compared to the Ca/Fe-LDH (\(\:\text{E}\text{g}\) = 2.81 eV) value. The decrease in the bandgap of Mg/Fe-LDH agrees with data obtained from the crystalline nature and nanostructured morphology as observed in the FE-SEM and XRD analyses. The electronic modification led to a high absorption spectrum and a decreased band gap48,49,50. This indicates that Mg/Fe-LDH is suitable for solar energy applications.
Photoelectrochemical (PEC) measurements
The PEC behavior of LDH photoanodes
Potentiometry, cyclic voltammetry, and amperometry measurements were performed in the 0.3 M Na2SO3 electrolyte under illumination of white and monochromatic lights to evaluate the photoelectrode’s catalytic activity. Figure 6(a) shows the Jph-E curves of photoanodes in the cases of dark and white lighting at A range of voltages from − 1 to + 1 V. The typical curves for photoelectrochemical current density (Jph) and potential (E) reveal that the maximum Jph values may be seen in the positive voltage range. This indicates that the electrodes are used as photoanodes51. When compared to the Ca/Fe-LDH photoanode, the photoactivity of the Mg/Fe-LDH photoanode is much higher. This shows that the combination of iron and magnesium has a substantially greater impact on the photocatalyst performance than iron and calcium LDH. In light of existence, the photocurrent density for the Mg/Fe-LDH photoanode, which is 31.55 mA/cm2 at 1 V, is almost 3.9 times higher than the photocurrent density for the Ca/Fe-LDH photoanode, which is about 8.2 mA/cm2 at 1 V. The activity of the Mg/Fe-LDH in the dark was less than in the case of light exposure, with a maximum current of around 2.49 mA/cm2 at 1 V. This can be attributed to several factorsPEC performance is substantially influenced by the photoanode material’s band gap energy. A reduced band gap permits the material to absorb a greater range of the solar spectrum, making it more efficient at converting light into electrical energy. A semiconductor photocatalyst utilized in a PEC water-splitting cell requires to have an optimum band gap of about 2.0 eV52. Mg/Fe-LDH has a smaller band gap of 2.01 eV than Ca/Fe-LDH at 2.81 eV; that’s why it is much better at harvesting solar energy. Additionally, efficient charge separation and transport are essential for high PEC performance. Mg/Fe-LDH has superior charge separation and transport properties than Ca/Fe-LDH due to its electronic structure and charge carrier mobility, resulting in better photoconversion efficiency53,54. The modified Mg/Fe-LDH thin photocatalysts that are located on the surface of the Mg/Fe-LDH photoanode contribute to an increase in the estimated carrier concentration32,55. This is because Mg/Fe-LDH is reliant on photogenerated holes occupying the surface of the photoanode to function properly. The oxidation of water is increased as a result, which results in a greater release of hydrogen56,57. This indicates that the hydrogen evolution reaction’s (HER) kinetics are improved upon the deposition of Mg/Fe-LDH. Based on the J-time (amperometry measurements), the following Eq. (3) was applied to determine the total number of moles of hydrogen produced by the process of photoelectrochemical water splitting58.
where Jph is photocurrent density, F is the Faraday constant (96,500 C/mol), and t is the period. Figure 6(b) depicts this relationship between the number of hydrogen moles and time. The estimated hydrogen output rate was about 2542.36 µmole/h.cm2.
The consistency and stability of the Mg/Fe-LDH photoelectrode are examined regarding the total number of runs (5 runs). The results are presented in Fig. 6(c), which demonstrates that the Mg/Fe-LDH maintains 97.46% photocurrent even after five runs have been completed. This shows very clearly that the optimized LDH photocatalyst, Mg/Fe-LDH, is very stable and can be used as a photoanode for a long time in hydrogen production. A chronoamperometric current density vs. time curve was utilized to investigate the stability of the Mg/Fe-LDH, revealing a constant photocurrent density of 0.5 mA cm− 2 sustained for 1200 s at 1 V. As illustrated in Fig. 6 (d), this supports the endurance of the Mg/Fe-LDH and shows its potential for the long term.
Cyclic voltammetry (CV) is a well-suited electroanalytical technique to study the electrochemical performance of layered double hydroxide (LDH) photocatalysts for photoelectrochemical (PEC) hydrogen production59. Figure 7 shows the CV of Mg/Fe-LDH and of Ca/Fe-LDH in dark and light conditions. The superior performance of the LDH photocatalysts in the positive potential range can be correlated with a more effective Hydrogen evolution reaction (HER), which is essential for balanced water splitting and enhanced hydrogen production60. An important part of CV analysis is the onset voltage, which shows the potential at which a noticeable current starts61. This shows the start of electrochemical activity, such as the hydrogen evolution reaction (HER). The CV results showed that the Mg/Fe-LDH photocatalyst has better electrochemical behavior in light, with an onset voltage of 0.116 V. This is a lot less than what was seen for the same material when there was no light (0.71 V), which suggests that light has a strong effect on creating charge carriers that speed up the electrochemical reactions62. In contrast, the Ca/Fe-LDH photocatalyst had a higher onset (0.552 V) voltage even when it was subjected to light, which suggests that it would not work as well as an electrocatalyst for making PEC hydrogen. The marked difference in the onset voltages is due to photogenerated charge carriers under illumination, which reduce the energy barrier for the reaction63. The Mg/Fe-LDH photocatalyst’s material structure may also make it easier for hydrogen ions to attach to its surface, which leads to the release of hydrogen gas. This effect is not as strong in the Ca/Fe-LDH photocatalyst55,64. The CV analysis shows that the Mg/Fe-LDH photocatalyst works well as a photo-electrocatalyst. Its low onset voltage in light shows that it could be used for low-energy PEC hydrogen production.
Efficiency of the Mg/Fe-LDH photoanode
Bandpass filtering with different wavelengths between 410 and 636 nm was utilized in 0.3 M Na2SO3 at + 1 V to evaluate the Mg/Fe-LDH photoanode efficiency in the process of water oxidation for the release of hydrogen. Figure 8(a) shows that at 1 V, the photocurrent was at its lowest at 410 nm, and it was determined to be Jph = 16.9 mA/cm2. The greatest photocurrent was measured at 490 nm, and it was determined to be Jph = 23.65 mA/cm2. This demonstrates that the Mg/Fe-LDH photoanode is sensitive to a significant amount of sunlight and is effective at absorbing a significant amount of that sunlight in the visible spectrum. Calculating external quantum efficiency, also known as the incident photon to current conversion efficiency (IPCE), is an important way to illustrate how the Mg/Fe-LDH photoelectrode’s enhanced solar absorption and application to efficient hydrogen production from water oxidation may be applied. The IPCE for each of the different wavelengths can be estimated using Eq. (4) at an applied voltage of 1 V65,66.
Where λ is the wavelength of the photons that were impacted on the Mg/Fe-LDH photocatalyst and P is the illuminating light power density produced by the Xenon lamp as a function of the wavelength of the monochromatic light. The variation in IPCE as a function of wavelength is depicted in Fig. 8(b). The greatest IPCE of the Mg/Fe-LDH photoelectrode was reached when the wavelength was 490 nm, and it was about 59.85%. At 470 nm, it was 56.39%, while at 460 nm, it was 52.8%; the IPCE was 35.09% at 636 nm, which was the lowest value, which indicates that a significant percentage of incident photons are being converted into current, signifying efficient light absorption and charge generation50.The spectral consistency of IPCE data of Mg/Fe-LDH with the Uv-Vis absorption was mentioned in Figure S122. When a low external potential is provided to the photoelectrochemical system, the electrical energy that is first supplied into the system must be removed to determine how well the photoelectrode is functioning. Alternatively, one might make use of the applied bias photon to current conversion efficiency (ABPE). The ABPE may be determined by applying Eq. (5)67,68.
Vapp refers to the potential that is applied to the photocatalyst. The fluctuation in ABPE that occurs for the Mg/Fe-LDH as a function of the applied voltage is seen in Fig. 8(c). This occurs at a variety of wavelengths. Under conditions of monochromatic illumination, the highest possible conversion efficiency was at 490 nm and found at two different voltages: 5.75% achieved at 0.92 V, and 5.33% achieved at 0.4 V, which suggests that the energy levels in our photoanode are well-suited for efficient charge separation and transport, which is crucial for achieving high photoconversion efficiency68. Due to its higher IPCE and ABPE efficiencies, Mg/Fe-LDH outperforms Ca/Fe-LDH in producing hydrogen. This is because surface roughness characterization revealed that it has a larger surface area. This means that there are more active sites on the surface for electrolytes to interact, which, as expected, improves the PEC process for hydrogen production.
Tafel parameters
The Tafel relation is used to explain HER and its rate-limiting phase. It is given by Eq. (6)69:
Excellent HER efficiency, high current exchange rates, and low Tafel slopes are features of excellent PEC catalysts. Some photocatalysts, however, have higher current conversion rates and larger Tafel slopes, and vice versa70. Figure 9(a) depicts both the anodic and cathodic Tafel plots of the electrodes that are being analyzed. Essential parameters of the electrodes include the anodic and cathodic Tafel slopes (βa and βc), corrosion potential (Ecorr), and corrosion current (Icorr). The slopes of the linear segments of the curves in Fig. 9(b) and Fig. 9(c) determine the βa and βc values for the electrodes under study71. The values of Ecorr, Icorr, βa, and βc for all electrodes are presented in Table 1. The βa value decreases from 1.37 V/dec for Ca/Fe-LDH to 0.9 V/dec for Mg/Fe-LDH. The βc value rose from ‒0.297 V/dec for Ca/Fe-LDH to ‒0.31 for Mg/Fe-LDH. Tafel PEC slopes provide PEC reaction processes and rate-limiting steps. With a Tafel slope of roughly 30 mV/decade, the Volmer-Tafel mechanism illustrated in Eqs. (7)-(9) predominates during rate-limiting recombination. When the Tafel slope is roughly 40 mV/decade and PEC desorption is rate-limiting, the Volmer-Heyrovsky hydrogen evolution mechanism is most likely to be in charge72. A slope of ~ 120 mV/decade on the Tafel reveals that reaction pathways rely on the surface laden with adsorbed hydrogen. A βc number represents the overpotential required to enhance the HGR rate by a factor of 10. The low Tafel slopes of the Mg/Fe-LDH photoelectrode and the low energy of the band gaps resulted in low overpotentials for the photoelectrodes. This is because only a small quantity of energy is required to improve HGR performance, and vice versa. While the corrosion rate may be found at Icorr, the corrosion tendency of the solution can be found at Ecorr. Table 1 shows that the Mg/Fe-LDH photoelectrode exhibited nobler behavior than the Ca/Fe-LDH, with an Ecorr of ‒0.51 V, which is lower. One can evaluate the photoelectrode’s resistance to corrosion based on its Icorr. Table 1 illustrates that Mg/Fe-LDH’s Icorr is 0.392 mA/cm2 less than Ca/Fe-LDH’s 0.4677 mA/cm2. We were able to compute the polarization resistance (Rp in Ω.cm2) using the Stern-Geary equation and the straight part of the curves at Ecorr by utilizing the following relation: Rp = βc βa / [2.303 Icorr (βc + βa)]. Table 2 contains a list of the measured Rp for every electrode. For Ca/Fe-LDH, Rp is 2.2 Ω.cm2, whereas for Mg/Fe-LDH, it is 0.5 Ω.cm‒2.
The Volmer–Heyrovsky mechanism from above equations is expected to manifest at catalytic interfaces where the availability of electroactive surface sites is significantly diminished, resulting in a separation between adjacent sites that exceeds the van der Waals radius of two adsorbed hydrogen atoms, thereby precluding the occurrence of the Tafel mechanism. In this scenario, the sole route for the evolution of H2 is the Volmer–Heyrovsky pathway, illustrated below73. The limited number of electroactive sites on the electrocatalyst’s surface necessitates that the hydrogen evolution reaction (HER) proceeds via the mechanism to produce H273.
Table 3 presents a comparison of performance indicators, illustrating the superior performance of prepared electrodes with previously published literature74,75. The results indicate that our Mg/Fe-LDH photoelectrode displays better efficiency and photogenerated current density compared to the electrodes described earlier. These findings underscore the enormous potential of this photoelectrode in the realms of photocatalysis and sustainable energy production.
Mott-Schottky measurements were conducted by varying the applied potential from − 0.5 V to 1 V at a specified frequency of 10 kHz to ascertain the kind of semiconductor; a positive slope indicates n-type, while a negative slope indicates p-type. The flat-band potential (EFB) and the type and concentration of charge carriers in the Mg/Fe-LDH photoanode can be ascertained using the Motte-Schottky Eqs. (10)-(11)82.
Csc denotes the capacitance of the space-charge region, e represents the electron charge, ε0 signifies the dielectric vacuum permittivity, εr indicates the dielectric constant of the semiconductor, Nd refers to the donor density, A is the surface area of the electrode in contact with the electrolyte, EAppl is the applied potential on the reference electrode, k is Boltzmann’s constant, T is the temperature, and c is a constant that varies with the type of reference electrode employed83.
Figure 10 (a) show that The slope of the linear component of the Mott-Schottky plots of Mg/Fe-LDH was utilized to acquire the (d(C− 2)/dV) values. According to calculations, the slope of Mg/Fe-LDH is 0.37 × 109. Since Mg/Fe-LDH has a positive Mott–Schottky slope, it is an n-type semiconductor with electrons as the major carrier. The Mott–Schottky plots are used to determine the carrier densities. The results show that the donor density of Mg/Fe-LDH is 9.502 × 1018cm3. The higher photocurrent for Mg/Fe-LDH can be attributed to the increased donor density with Hydrogen vacancies on the surface. The larger donor density might boost the conductivity, and then the surface electrons can be promptly transferred, which may reduce the recombination of photoexcited holes with electrons84, and prefer the − 0.5 to 1 V. The flat band potentials of the electrode (the intersection of the tangent and X-axis) are determined to be about 0. 31 V and the conduction and valence bands are 0.42 eV and 4.86 eV, respectively. (EIS) The Nyquist diagram is a useful method for characterizing the interfacial characteristics in PEC systems. In Fig. 10 (b), the Nyquist diagram is linear with Capacitance and includes the charge separation and transfer behaviour between the semiconductor and PEC electrolyte interfaces85. Mg/Fe-LDH has an ionic conductivity is 1.43 mS cm-1.
Theoretical analysis
Study Ca/Fe and Mg/Fe-LDH simulations for use in PEC water splitting applications. The HOMO and LUMO gap energy for Ca/Fe and Mg/Fe-LDH are illustrated by the frontier molecular orbitals (FMOs) for HOMO and LUMO.These are key factors in calculations involving quantum chemistry that portray systems under research at the microscopic level. Strong indicators of an interaction system’s electronic characteristics include the HOMO-LUMO energy gaps (Eg), the lowest unoccupied molecular orbital (LUMO), and the highest occupied molecular orbital (HOMO). The border orbitals, generally referred to as the HOMO and LUMO, are key variables that reveal qualitative data on the excitation properties of simulated materials. In the meantime, a convenient instrument for finding out the chemical reactivity of molecules interacting. Furthermore, a molecule’s chemical stability is determined by its hardness; a molecule with a lower chemical stability is softer and more reactive86.
The gap energy values for Ca/Fe and Mg/Fe-LDH are 2.755 eV, and 2.566 eV, respectively illustrated in (Fig. 11) (a) to (f). The chemical reactivity of Mg/Fe-LDH is higher than that of for Ca/Fe-LDH because the former has a smaller gap energy and the largest electronic transitions. These results further support the data gained from the experimental technique, revealing the effect of the chemical composition of Mg/Fe LDH and Ca/Fe LDH on energy stability for PEC water splitting. Structural modifications, especially the replacement of Mg for Ca, affect the ESP properties and Energy gap, which lowers the energy, which can boost charge separation and transport efficiency throughout the water splitting process. gap values. Subsequently, the electronic structures of the optimized geometry were estimated to explicate whether the two LDH are suitable for PEC water splitting. A greater dipole moment is discovered for Mg/Fe-LDH (2.250836 Debye) than Ca/Fe -LDH(0.422814 Debye).
The quantum molecular descriptors applied in the DFT computation are chemical-reactivity descriptors (µ, ̞, σ, ω, ∆N) that describe the utility of Ca/Fe and Mg/Fe-LDH in PEC water splitting. The qualities that are most frequently employed include the maximum charge transfer index (∆N), softness (σ), global hardness (η), chemical potential (µ), and electrophilicity index (ω)87. Global hardness, commonly known as a molecule’s hardness (η), is directly related to how resistant a molecule is to changes in electrical distribution and has been demonstrated to be effective in explaining chemical reactions. On the other side, softness is the inverse of global hardness (σ = 1/η). Electronegativity (χ) is a measure of an element’s capacity to absorb electrons and produce negative ions during the chemical process of a delivery system88. The electrophilicity index (ω) analyzes a molecule’s reactivity by assessing its capacity to absorb electrons, whereas the chemical potential (µ) shows the direction of electron flow from the higher µ to the lower µ until the chemical potential is equilibrated89. The mathematical relationship between the quantum molecular descriptors and electrical properties is illustrated by Eqs. (12)–(17).
Where I(ionization potential ) = -E HUMO and A(electron affinity) = - LUMO
The targeted region’s softness, hardness, electronegativity, and electrophilic index can all be related to the molecular toxicity, polarizability, structural stability, and reaction rate of any chemical molecule in the biological system. These values are 0.726, 1.377, 7.498, and 4.1315 for Ca/Fe and Mg/Fe-LDH, respectively, and 0.7794, 1.283, 9.805, and 4.988 at Mg/Fe-LDH. Additionally, the maximal charge transfer indices (∆N) for Ca/Fe-LDH and Mg/Fe-LDH were 3 and 3.909, respectively.
The reactivity properties reported in and the overall stability of these compounds are supported by the substantially bigger gap between HOMO and LUMO. The enhanced hardness and decreased chemical softness support this. The chemical potential (µ) is the propensity of an electron to escape from a stable molecule. The complex is stable and unable to break down spontaneously into its constituent atoms due to its negative chemical potential. Examine the energy difference between Ca/Fe and Mg/Fe-LDH to evaluate a molecule’s capability. It was revealed that the energy gap ranges between 2.755 eV and 2.566 eV, reflecting changes in the electron distribution of the molecule. According to this, Mg/Fe-LDH worked better for PEC water splitting to generate hydrogen.
When the electrophilic and nucleophilic activities occur in the molecule, the electrostatic potential (ESP) graphing in the iso-surface plot and the electron density mapping in the MEP plot have been analyzed for the charge distribution concepts. Different colors are utilized to illustrate the Ca/Fe-LDH and Mg/Fe-LDH total density mapping. Chemically active sites and atomic-level comparative reactivity have been utilized to depict these molecular graphs.
Figure 12(a) displays the surface plots of molecule electrostatic potentials for Ca/Fe and Mg/Fe-LDH, a significant computation utilized in the analysis of intermolecular characterization and photocatalytic processes. The spatial distribution of MEPs is graphically shown as the chemical activities linked with a chemical reaction and robust binding to the active sites. MEP is integrally linked to the chemical behavior, electronegativity, and dipole moment of Ca/Fe and Mg/Fe-LDH. These graphs depict the molecule’s features in terms of electron densities (ED) and illustrate its relative polarity90. MEPs surface maps have been traced using different colors based on the electrostatic significance: red shows the molecule’s highest -ve potential, blue its most + ve potential, and green its zero potential regions91. The order of MEP increases on surface mapping/arrays is red, orange, yellow, green, and blue. In this situation, the blue color signifies electrophilic reactivity while the red hue denotes nucleophilic reactivity. Although the positive MEP demonstrates the repulsion of the proton/cation in the blue zone owing to the atomic nuclei areas as the low concentration of ED in the molecule, the negative MEP displays the attraction of a proton/light cation in the red region due to concentrated ED in the molecule92. In Fig. 12(a), the Ca-OH region is represented by the blue region, and the positive potential demonstrates that the proton/cation area—where electrophilic reaction activity is found—is repelled. However, a largely green and yellow zone with zero and medium electric potential regions surrounds the other atoms. These graphs reflect the molecule’s characteristics in terms of electron densities (ED) and indicate its relative polarity. MEPs surface maps have been traced using different colors based on the electrostatic significance: red shows the molecule’s highest -ve potential, blue its most + ve potential, and green its zero potential regions. MEP rises in the following order on surface mapping/arrays: red, orange, yellow, green, and blue. Here, the electrophilic reactivity is indicated by the blue color, whereas the nucleophilic reactivity is exhibited by the red hue. While the positive MEP demonstrates the repulsion of the proton/cation in the blue zone due to the atomic nuclei areas as the low concentration of ED in the molecule, the negative MEP displays the attraction of a proton/light cation in the red region due to concentrated ED in the molecule93. The positive potential depicts the repulsion of the proton/cation area, which is where electrophilic reaction activity is located, and the Ca-OH region is positioned with the blue region in Figure. 12(a). The other atoms, meanwhile, are surrounded by a zone that is primarily green and yellow and has zero and medium electric potential zones. However, the area of Mg-OH between red and yellow in figure. 12(b) The proton/cation area, which is the site of nucleophilic reaction activity, is drawn to this -ve potential, as represented by the red hue of the oxygen between Ca, Mg, and Fe. In order to identify probable active spots for the photocatalytic activity, the charge density, electrostatic potential (ESP) surface, and Bader charge were then computed. Their increased electronegativity is intimately linked to the charge densities, as demonstrated in Figure (12). Sites in Ca/Fe-LDH had a lower electrostatic potential value of − 0.77 eV than in Mg/Fe-LDH of − 0.93 eV, according to the ESP analysis shown in Figure. 12(b). This reveals that Ca/Fe-LDH has a stronger nucleophilicity than Mg/Fe-LDH. As a result, the Mg/Fe-LDH often has the best current density for PEC water splitting’s hydrogen generation, which necessitates a narrow energy gap and high ESP.
Conclusion
This study successfully demonstrates a simple method for preparing Mg/Fe and Ca/Fe layered double hydroxides as novel composites for hydrogen photocatalytic production. The Mg/Fe-LDH material shines in performance, boasting a nearly fourfold higher photocurrent density (31.55 mA/cm²) at 1 V compared to its Ca/Fe counterpart (8.2 mA/cm²). Remarkably, Mg/Fe-LDH also exhibits an impressive IPCE of 59.85% at 470 nm and an ABPE of 5.75% at 0.92 V. Furthermore, Mg/Fe-LDH offers notable stability and high resistance to corrosion. These findings provide valuable insights for designing efficient photocatalytic materials based on Mg/Fe-LDH for sustainable hydrogen generation using solar energy. Furthermore, the experimental results were Confirmed by DFT calculations, which enhanced the understanding of the band gap structure and ESP characteristics of LDH nanomaterials.
Data availability
The data will be available upon request, F.M. and O.M.
References
Xie, X. B. et al. Recent advances in hydrogen generation process via hydrolysis of Mg-based materials: A short review. J. Alloys Compd. 816, 152634. https://doi.org/10.1016/J.JALLCOM.2019.152634 (2020).
Algarni, S., Tirth, V., Alqahtani, T., Alshehery, S. & Kshirsagar, P. Contribution of renewable energy sources to the environmental impacts and economic benefits for sustainable development. Sustain. Energy Technol. Assessments. 56, 103098. https://doi.org/10.1016/J.SETA.2023.103098 (2023).
Hardian, R. et al. Waste Mg-Al based alloys for hydrogen storage. Int. J. Hydrogen Energy. 43 (34), 16738–16748. https://doi.org/10.1016/J.IJHYDENE.2017.12.014 (2018).
Abdalla, A. M. et al. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 165, 602–627. https://doi.org/10.1016/J.ENCONMAN.2018.03.088 (2018).
Lian, H. Y. et al. Plasma chain catalytic reforming of methanol for on-board hydrogen production. Chem. Eng. J. 369, 245–252. https://doi.org/10.1016/J.CEJ.2019.03.069 (2019).
Santra, S., Das, D., Das, N. S. & Nanda, K. K. An efficient on-board metal-free nanocatalyst for controlled room temperature hydrogen production, Chem. Sci. 8 (4), 2994–3001. (2017). https://doi.org/10.1039/C7SC00162B
Shahi, R. R., Gupta, A. K. & Kumari, P. Perspectives of high entropy alloys as hydrogen storage materials. Int. J. Hydrogen Energy. 48 (56), 21412–21428. https://doi.org/10.1016/J.IJHYDENE.2022.02.113 (2023).
Padash, R., Jafari, A. H. & Jamalizadeh, E. Experimental and theoretical study of aluminium corrosion in naoh, NaCl and HCl solutions. Anti-Corrosion Methods Mater. 65 (4), 350–360. https://doi.org/10.1108/ACMM-04-2017-1785/FULL/PDF (2018).
Demirtaş, C. & Özcan, A. K. The experi̇mental thermal analysis of aluminum metal melting with concentrated solar energy. Sol Energy Mater. Sol Cells. 222, 110940. https://doi.org/10.1016/J.SOLMAT.2020.110940 (2021).
Kwon, J. Y. et al. Fabrication of Al-Ni Alloys for Fast Hydrogen Production from Hydrolysis in Alkaline Water, Mater. 2023, Vol. 16, Page 7425 16(23), 7425. https://doi.org/10.3390/MA16237425 (2023).
Davies, J., Du Preez, S. P. & Bessarabov, D. G. The hydrolysis of Ball-Milled Aluminum–Bismuth–Nickel composites for On-Demand hydrogen generation. Energies 2022. 15 (7), 2356. https://doi.org/10.3390/EN15072356 (2022).
Nayak, S. & Parida, K. Recent Progress in LDH@Graphene and Analogous Heterostructures for Highly Active and Stable Photocatalytic and Photoelectrochemical Water Splitting, Chem. - An Asian J. 16 (16), 2211–2248. https://doi.org/10.1002/ASIA.202100506 (2021).
Ahmed, A. M. et al. Enhanced photoelectrochemical water splitting activity of carbon nanotubes@TiO2 nanoribbons in different electrolytes. Chemosphere 238, 124554. https://doi.org/10.1016/J.CHEMOSPHERE.2019.124554 (2020).
Zhang, T., Yang, W., Zhang, S., Zhou, J. & Liu, J. Hydrogen production by the reaction of Al-based metals with water vapor. Energy Sources Part. Recover Util. Environ. Eff. 40 (1), 9–14. https://doi.org/10.1080/15567036.2017.1315759 (2018).
Xie, L. et al. Microstructure and improved hydrogen generation performance via hydrolysis of Mg–Ca alloys with tic and Ni addition. Int. J. Hydrogen Energy. 87, 879–889. https://doi.org/10.1016/J.IJHYDENE.2024.09.112 (2024).
Al Bacha, S., Zakhour, M., Nakhl, M. & Bobet, J. L. Effect of ball milling in presence of additives (Graphite, AlCl3, MgCl2 and NaCl) on the hydrolysis performances of Mg17Al12, Int. J. Hydrogen Energy 45 (11), 6102–6109. https://doi.org/10.1016/J.IJHYDENE.2019.12.162 (2020).
Mohamed, F., Bhnsawy, N., & Shaban, M. Reusability and stability of a novel ternary (Co–Cd–Fe)-LDH/PbI2 photoelectrocatalytst for solar hydrogen production. Sci Rep 11 (1), 1–14. https://doi.org/10.1038/S41598-021-85005-Y (2021).
Guo, J. et al. Enhanced hydrogen generation from Al-water reaction mediated by metal salts. Int. J. Hydrogen Energy. 46 (5), 3453–3463. https://doi.org/10.1016/J.IJHYDENE.2020.10.220 (2021).
Eom, K., Cho, E. & Kwon, H. Feasibility of on-board hydrogen production from hydrolysis of Al–Fe alloy for PEMFCs, Int. J. Hydrogen Energy 36 (19), pp. 12338–12342. https://doi.org/10.1016/J.IJHYDENE.2011.06.099 (2011).
Nayak, S. & Parida, K. Superlative photoelectrochemical properties of 3D MgCr-LDH nanoparticles influencing towards photoinduced water splitting reactions. Sci. Rep. 12 (1), 1–23. https://doi.org/10.1038/S41598-022-13457-X (2022).
Janani, F. Z. et al. Effect of ag doping on photocatalytic activity of ZnO-Al2O3 derived from LDH structure: synthesis, characterization and experimental study. Appl. Surf. Sci. Adv. 16 https://doi.org/10.1016/j.apsadv.2023.100430 (2023).
Nayak, S. & Parida, K. Plasmon-induced electron transportation in heterostructure N-rGO/NiFe-LDH@Au with enhanced photoelectrochemical water oxidation. Electrochim. Acta. 500, 144724. https://doi.org/10.1016/J.ELECTACTA.2024.144724 (2024).
Saad, R. et al. Fabrication of zno/cnts for application in CO2 sensor at room temperature. Nanomaterials 11 (11). https://doi.org/10.3390/nano11113087 (2021).
Shandilya, P. et al. Recent progress and challenges in photocatalytic water splitting using layered double hydroxides (LDH) based nanocomposites. Int. J. Hydrogen Energy. 47 (88), 37438–37475. https://doi.org/10.1016/j.ijhydene.2021.08.190 (2022).
Liang, H. et al. Porous Two-Dimensional nanosheets converted from layered double hydroxides and their applications in electrocatalytic water splitting. Chem. Mater. 27 (16), 5702–5711. https://doi.org/10.1021/ACS.CHEMMATER.5B02177/ (2015).
Saad, R. et al. December., Enhanced CO2 gas sensing at room temperature using Ag-plated Na-doped CuO thin films synthesized by successive ionic layer adsorption and reaction technique, Surfaces and Interfaces 44, 103789. https://doi.org/10.1016/j.surfin.2023.103789 (2024).
Hashem, S. et al. Enhancing pelargonium graveolens l’hér. (geranium) growth using Zn–Al and Mg–Al LDH nanomaterials: a biochemical approach. Chem. Biol. Technol. Agric. 11 (1), 1–15. https://doi.org/10.1186/S40538-024-00683-W/FIGURES/8 (2024).
Mohamed, F., Shaban, M. & Salem, O. M. Metal oxides carbon xerogel nanocomposite for methanol oxidation fuel cell. Sci. Rep. 15 (1), 1–13. https://doi.org/10.1038/S41598-025-85579- (2025).
Park, J. Y., Yoo, S. B., Cho, H. B., Lee, H. S. & Choa, Y. H. CaFe-Based Layered Double Oxides With Superior Iron Alloy Corrosion Inhibition Behaviors in Aggressive Seawater Environment, Front. Chem. 10, 1–12. https://doi.org/10.3389/fchem.2022.813008 (2022).
Escudero-Curiel, S., Giráldez, A., Pazos, M. & Sanromán, Á. From waste to resource: valorization of lignocellulosic Agri-Food residues through engineered hydrochar and Biochar for environmental and clean energy Applications—A comprehensive review. Multidisciplinary Digit. Publishing Inst. (MDPI) https://doi.org/10.3390/foods12193646 (2023).
Lu, X. et al. 2D Layered Double Hydroxide Nanosheets and Their Derivatives Toward Efficient Oxygen Evolution Reaction, Apr. 01, Springer. (2020). https://doi.org/10.1007/s40820-020-00421-5
Rybka, K., Matusik, J., Kuligiewicz, A., Leiviskä, T. & Cempura, G. Surface chemistry and structure evaluation of mg/al and mg/fe LDH derived from magnesite and dolomite in comparison to LDH obtained from chemicals. Appl. Surf. Sci. 538, 147923. https://doi.org/10.1016/J.APSUSC.2020.147923 (2021).
Yuan, M. et al. Enhancing phosphate removal performance in water using La–Ca/Fe–LDH: La loading alleviates ineffective stacking of laminates and increases the number of active adsorption sites. J. Clean. Prod. 388, 135857. https://doi.org/10.1016/J.JCLEPRO.2023.135857 (2023).
Chen, J. et al. Peer review under responsibility of Chongqing University, J. Magnes. Alloy. 9, 1019–1027, In situ growth process of Mg-Fe layered double hydroxide conversion film on MgCa alloy-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/) https://doi.org/10.1016/j.jma.2020.05.019 (2021).
Taha, T. A., Saad, R., Zayed, M., Shaban, M. & Ahmed, A. M. Tuning the surface morphologies of ZnO nanofilms for enhanced sensitivity and selectivity of CO2 gas sensor. Appl. Phys. Mater. Sci. Process. 129 (2). https://doi.org/10.1007/s00339-023-06387-6 (2023).
Shaban, M., Abdelkarem, K., El, A. M. & Sayed Structural, optical and gas sensing properties of Cu2O/CuO mixed phase: effect of the number of coated layers and (Cr + S) co-Doping. Phase Transitions. 92 (4), 347–359. https://doi.org/10.1080/01411594.2019.1581886 (2019).
Sahadat Hossain, M. & Ahmed, S. Easy and green synthesis of TiO2 (Anatase and Rutile): Estimation of crystallite size using Scherrer equation, Williamson-Hall plot, Monshi-Scherrer Model, size-strain plot, Halder- Wagner Model, Results Mater. 20, 100492.https://doi.org/10.1016/J.RINMA.2023.100492 (2023).
Ibrahim, A. M., Mohamed, F., Al-Quraishy, S., Abdel-Baki, A. A. S. & Abdel-Tawab, H. Green synthesis of cerium oxide / Moringa Oleifera seed extract nano-composite and its molluscicidsal activities against biomophalaria Alexanderina. J. King Saud Univ. https://doi.org/10.1016/j.jksus.2021.101368 (2021).
Abdelwahab, N., Rabie, W. & Mohamed, F. RESEARCH open access chemical and biological technologies in agriculture fabrication and characterization of novel biocomposite based on sargassum vulgare for controlling sugar beet root diseases. Chem. Biol. Technol. Agric. 10, 52. https://doi.org/10.1186/s40538-023-00418-3 (2023).
Khedr, N., Elsayed, K. N. M., Ibraheem, I. B. M. & Mohamed, F. New insights into enhancement of bio-hydrogen production through encapsulated microalgae with alginate under visible light irradiation, Int. J. Biol. Macromol. 253, https://doi.org/10.1016/j.ijbiomac.2023.127270 (2023).
Ragab, E., Shaban, M., Khalek, A. A. & Mohamed, F. Design and characterization of pani/starch/fe 2 O 3 bio composite for wastewater remediation. Int. J. Biol. Macromol. https://doi.org/10.1016/j.ijbiomac.2021.03.043 (2021).
Saad, R., Ahmed, A. M., Abdelkarem, K., Zayed, M. & Faidey, Z. M. SILAR-Deposited CuO nanostructured films doped with zinc and sodium for SILAR-Deposited CuO nanostructured films doped with zinc and sodium for improved CO 2 gas detection. Nanomaterials https://doi.org/10.3390/nano13202793 (2023).
Shaban, M., Ahmed, A. M., Shehata, N., Betiha, M. A. & Rabie, A. M. Ni-doped and ni/cr co-doped TiO 2 nanotubes for enhancement of photocatalytic degradation of methylene blue. J. Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2019.07.070 (2019).
Dhanasekaran, T. et al. Fabrication of Ag@Co-Al Layered Double Hydroxides Reinforced poly(o-phenylenediamine) Nanohybrid for Efficient Electrochemical Detection of 4-Nitrophenol, 2,4-Dinitrophenol and Uric acid at Nano Molar Level, Sci. Rep. 9 (1), 1–17. https://doi.org/10.1038/s41598-019-49595-y (2019).
Rabia, M. et al. TiO 2 /TiO x N Y Hollow mushrooms-like nanocomposite photoanode for hydrogen electrogeneration. J. Porous Mater. 27, 133–139. https://doi.org/10.1007/s10934-019-00792-0 (2020).
Ahmed, A. M. et al. Enhanced photoelectrochemical water splitting activity of carbon nanotubes@tio 2 nanoribbons in different electrolytes. Chemosphere https://doi.org/10.1016/j.chemosphere.2019.124554 (2019).
Abdelkarem, K. et al. Efficient room temperature carbon dioxide gas sensor based on barium doped CuO thin films. J. Mater. Sci. 58 (28), 11568–11584. https://doi.org/10.1007/S10853-023-08687-X/TABLES/3 (2023).
Ahmed, A. M., Abdalla, E. M. & Shaban, M. Simple and Low-Cost Synthesis of Ba-Doped CuO Thin Films for Highly Efficient Solar Generation of Hydrogen, J. Phys. Chem. C 124 (41), 22347–22356. https://doi.org/10.1021/acs.jpcc.0c04760 (2020).
Shaban, M., Binsabt, M., Ahmed, A. M. & Mohamed, F. Recycling Rusty iron with natural zeolite heulandite to create a unique nanocatalyst for green hydrogen production. Nanomaterials 11 (12). https://doi.org/10.3390/nano11123445 (2021).
Ahmed, A. M., Rabia, M. & Shaban, M. The structure and photoelectrochemical activity of Cr-doped PbS thin films grown by chemical bath deposition. RSC Adv. 10 (24), 14458–14470. https://doi.org/10.1039/c9ra11042a (2020).
Altowyan, A. S., Shaban, M., Abdelkarem, K., El, A. M. & Sayed The impact of Co doping and annealing temperature on the electrochemical performance and structural characteristics of SnO2 nanoparticulate photoanodes. Mater. (Basel). 15 (19), 6534. https://doi.org/10.3390/MA15196534 (2022).
Sreekantan, S., Saharudin, K. A., Basiron, N. & Wei, L. C. New-generation titania-based catalysts for photocatalytic hydrogen generation. Nanostructured Funct. Flex. Mater. Energy Convers. Storage Syst. 257–292. https://doi.org/10.1016/B978-0-12-819552-9.00008-7 (2020).
Makwana, D., Polisetti, V., Castaño, J., Ray, P. & Bajaj, H. C. Mg-Fe layered double hydroxide modified montmorillonite as hydrophilic nanofiller in polysulfone-polyvinylpyrrolidone blend ultrafiltration membranes: separation of oil-water mixture. Appl. Clay Sci. https://doi.org/10.1016/j.clay.2020.105636 (2020).
Al-Jaberi, M., Ebastien Naille, S., Dossot, M. & Ruby, C. Interlayer interaction in CaeFe layered double hydroxides intercalated with nitrate and chloride species. J. Mol. Struct. https://doi.org/10.1016/j.molstruc.2015.08.064 (2015).
Hudcová, B., Fein, J. B., Tsang, D. C. W. & Komárek, M. Mg-Fe LDH-coated biochars for metal(loid) removal: surface complexation modeling and structural change investigations. Chem. Eng. J. 432, 1385–8947. https://doi.org/10.1016/j.cej.2021.134360 (2022).
Bai, S. et al. An integrating photoanode of WO3/Fe2O3 heterojunction decorated with NiFe-LDH to improve PEC water splitting efficiency. ACS Sustain. Chem. Eng. 6 (10), 12906–12913. https://doi.org/10.1021/ACSSUSCHEMENG.8B02267 (2018).
Hubetska, T., Demchenko, V. & Kobylinska, N. Surface engineering: binary Mg,Fe-LDH· x Fe 3 O 4 nanocomposites for improved magnetic solid-phase extraction of pharmaceuticals from aqueous solution, Mater. Adv. 5 (20), 8145–8163. https://doi.org/10.1039/D4MA00609G (2024).
Altowyan, A. S. et al. Design and characterization of zeolite/serpentine nanocomposite photocatalyst for solar hydrogen generation. Mater. (Basel). 15 (18). https://doi.org/10.3390/ma15186325 (2022).
Wang, F. et al. Activating lattice oxygen in high-entropy LDH for robust and durable water oxidation. Nat. Commun. 141 (14, 1), 1–11. https://doi.org/10.1038/s41467-023-41706-8 (2023).
Yang, L., Liu, Z., Zhu, S., Feng, L. & Xing, W. Ni-based layered double hydroxide catalysts for oxygen evolution reaction. Mater. Today Phys. 16, 100292. https://doi.org/10.1016/J.MTPHYS.2020.100292 (2021).
Batchelor-McAuley, C. Defining the onset potential. Curr. Opin. Electrochem. 37, 101176. https://doi.org/10.1016/J.COELEC.2022.101176 (2023).
Peng, H., Ye, B., Luo, M. & Zheng, X. Mg–Fe layered double hydroxides/polyacrylonitrile nanofibers for Solar-Light induced peroxymonosulfate elimination of Tetracycline hydrochloride. Water (Switzerland). 16 (10), 1345. https://doi.org/10.3390/W16101345/S1 (2024).
Cen, J., Wu, Q., Liu, M. & Orlov, A. Developing new Understanding of photoelectrochemical water splitting via in-situ techniques: A review on recent progress. Green. Energy Environ. 2 (2), 100–111. https://doi.org/10.1016/J.GEE.2017.03.001 (2017).
Hudcová, B., Fein, J. B., Tsang, D. C. W. & Komárek, M. Mg-Fe LDH-coated biochars for metal(loid) removal: surface complexation modeling and structural change investigations. Chem. Eng. J. 432, 134360. https://doi.org/10.1016/J.CEJ.2021.134360 (2022).
Devadiga, D., Selvakumar, M., Shetty, P. & Santosh, M. S. The integration of flexible dye-sensitized solar cells and storage devices towards wearable self-charging power systems: A review. Renew. Sustain. Energy Rev. 159, 112252. https://doi.org/10.1016/J.RSER.2022.112252 (2022).
Altowyan, A. S., Shaban, M., Abdelkarem, K. & El Sayed, A. M. The influence of electrode thickness on the structure and water splitting performance of iridium oxide nanostructured films. Nanomaterials 12 (19), 3272. https://doi.org/10.3390/NANO12193272 (2022).
Hamid, S. B. A., Teh, S. J., Lai, C. W., Perathoner, S. & Centi, G. Applied bias photon-to-current conversion efficiency of ZnO enhanced by hybridization with reduced graphene oxide. J. Energy Chem. 26 (2), 302–308. https://doi.org/10.1016/J.JECHEM.2016.11.006 (2017).
[69], L., Liu, M., Ruan, C., Wang & Liu, Z. Optimization of the BiO8 Polar group of BiVO4 by Cl–embedded modification to manipulate bulk-surface carrier separation for achieving efficient Piezo-PEC water oxidation. Appl. Catal. B Environ. Energy. 354, 124117. https://doi.org/10.1016/J.APCATB.2024.124117 (2024).
Shaban, M., Almohammedi, A., Saad, R. & El Sayed, A. M. Design of SnO2:Ni,Ir nanoparticulate photoelectrodes for efficient photoelectrochemical water splitting. Nanomater. 12 (3), 453. https://doi.org/10.3390/NANO12030453 (2022).
Shaban, M., Saad, R. & El Sayed, A. M. Influence of chromium and lanthanum incorporation on the optical properties, catalytic activity, and stability of IrOx nanostructured films for hydrogen generation. Int J. Hydrogen Energy No January. https://doi.org/10.1016/j.ijhydene.2022.12.294 (2023).
Mohamed, F., Rabia, M. & Shaban, M. Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J. Mater. Res. Technol. 9 (3), 4255–4271. https://doi.org/10.1016/j.jmrt.2020.02.052 (2020).
Shaban, M., Kholidy, I., Ahmed, G. M., Negem, M. & Abd El-Salam, H. M. Cyclic voltammetry growth and characterization of Sn–Ag alloys of different nanomorphologies and compositions for efficient hydrogen evolution in alkaline solutions, RSC Adv. 9 (39), 22389–22400. https://doi.org/10.1039/C9RA03503F (2019).
Anantharaj, S. et al. Apr., Precision and correctness in the evaluation of electrocatalytic water splitting: revisiting activity parameters with a critical assessment, Energy Environ. Sci. 11 (4), 744–771. https://doi.org/10.1039/C7EE03457A (2018).
Hussain, M. K. et al. Enhanced visible light-driven photocatalytic activity and stability of novel ternary ZnO/CuO/MoO3 nanorods for the degradation of rhodamine B and alizarin yellow, Mater. Sci. Semicond. Process. 155, https://doi.org/10.1016/J.MSSP.2022.107261 (2023).
Zhu, Y. et al. Interface engineering of 3D BiVO4/Fe-based layered double hydroxide core/shell nanostructures for boosting photoelectrochemical water oxidation. J. Mater. Chem. A. 5 (20), 9952–9959. https://doi.org/10.1039/C7TA02179H (2017).
Mustafa, E. et al. Efficient Ni–Fe layered double hydroxides/zno nanostructures for photochemical water splitting. J. Solid State Chem. 273, 186–191. https://doi.org/10.1016/J.JSSC.2019.03.004 (2019).
Zheng, S. et al. Jul., An Inexpensive Co-Intercalated Layered Double Hydroxide Composite with Electron Donor-Acceptor Character for Photoelectrochemical Water Splitting, Sci. Reports 5 (1), 1–8. https://doi.org/10.1038/srep12170 (2015).
Zhang, G. et al. Highly efficient photocatalytic hydrogen generation by incorporating cds into ZnCr-layered double hydroxide interlayer. RSC Adv. 5, 5823–5829. https://doi.org/10.1039/C4RA11757C (2015).
Bao, J. et al. Nov., The CoMo-LDH ultrathin nanosheet as a highly active and bifunctional electrocatalyst for overall water splitting, Inorg. Chem. Front. 5 (11), 2964–2970, https://doi.org/10.1039/C8QI00867A (2018).
Eisenberg, D., Ahn, H. S. & Bard, A. J. Enhanced photoelectrochemical water oxidation on bismuth vanadate by electrodeposition of amorphous titanium dioxide, J. Am. Chem. Soc. 136 (40), 14011–14014. https://doi.org/10.1021/JA5082475 (2014).
Bai, S. et al. Photoanode of LDH catalyst decorated semiconductor heterojunction of BiVO4/CdS to enhance PEC water splitting efficiency. Int. J. Hydrogen Energy. 44, 24642–24652. https://doi.org/10.1016/J.IJHYDENE.2019.07.214 (2019).
Tang, Y. et al. Photo-induced synthesis of nanostructured Pt-on-Au/g-C3N4 composites for visible light photocatalytic hydrogen production, J. Mater. Sci. 55 (32), 15574–15587. https://doi.org/10.1007/S10853-020-05120-5/FIGURES/12 (2020).
Shuai, L. et al. Enhanced charge carrier separation and stable photoelectrochemical water splitting via a high-performance BiVO4/BiOBr Type-II heterojunction, Int. J. Hydrogen Energy 88, 19–28. https://doi.org/10.1016/J.IJHYDENE.2024.09.166 (2024).
Xu, D., Zhang, S. N., Chen, J. S. & Li, X. H. Design of the synergistic rectifying interfaces in Mott-Schottky catalysts. Chem. Rev. 123 (1), 1–30. https://doi.org/10.1021/ACS.CHEMREV.2C00426 (2023).
Kim, J. et al. Size effect of a piezoelectric material as a separator coating layer for suppressing dendritic Li growth in Li metal batteries. Nanomaterials 13 (1), 90. https://doi.org/10.3390/NANO13010090/S1 (2023).
Adekoya, O. C., Adekoya, G. J., Sadiku, E. R., Hamam, Y. & Ray, S. S. Application of DFT Calculations in Designing Polymer-Based Drug Delivery Systems: An Overview. MDPI. https://doi.org/10.3390/pharmaceutics14091972 (2022).
Bhatia, M. An overview of conceptual-DFT based insights into global chemical reactivity of volatile sulfur compounds (VSCs). Comput. Toxicol. 29, 100295. https://doi.org/10.1016/j.comtox.2023.100295 (2024).
Mehmood, A. & Janesko, B. G. Extending the Marcus µ-Scale of solvent softness using conceptual density functional theory and the orbital overlap distance: method and application to ionic liquids. J. Solut. Chem. 49 (5), 614–628. https://doi.org/10.1007/s10953-020-00973-5 (2020).
Chakraborty, D. & Chattaraj, P. K. Conceptual density functional theory based electronic structure principles. Royal Society of Chemistry. https://doi.org/10.1039/d0sc07017c (2021).
Chu, Y. et al. Donor-acceptor type conjugated porous polymers based on benzotrithiophen and triazine derivatives: Effect of linkage unit on photocatalytic water splitting, Int. J. Hydrogen Energy 64, 109–119. (2024). https://doi.org/10.1016/J.IJHYDENE.2024.03.260
Ojha, J. K., Ramesh, G. & Reddy, B. V. Structure, chemical reactivity, NBO, MEP analysis and thermodynamic parameters of pentamethyl benzene using DFT study. Chem. Phys. Impact. 7, 100280. https://doi.org/10.1016/J.CHPHI.2023.100280 (2023).
Demircioğlu, Z., Kaştaş, G., Kaştaş, Ç. A. & Frank, R. Spectroscopic, XRD, Hirshfeld surface and DFT approach (chemical activity, ECT, NBO, FFA, NLO, MEP, NPA& MPA) of (E)-4-bromo-2-[(4-bromophenylimino)methyl]-6-ethoxyphenol, J. Mol. Struct. 1191, 129–137. (2019). https://doi.org/10.1016/J.MOLSTRUC.2019.03.060
Kinaytürk, N. K. & Characterization, S. Calculation, and Docking analysis for Understanding molecular interaction mechanism of Propiconazole and DNA. J. Appl. Spectrosc. 90 (6), 1334–1345. https://doi.org/10.1007/S10812-024-01671-6/METRICS (2024).
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization, F.M., K.A., M.S., and A.M.A.; methodology, F.M. and A.M.A.; validation, F.M., K.A., and A.M.A.; formal analysis, F.M., K.A., and A.M.A.; investigation, F.M., K.A., and A.M.A.; resources, F.M., M.S., and A.M.A.; data curation, F.M., M.S., and A.M.A.;F.M. and O.M. conducted the computational calculation and wrote the relevant section.: writing—original draft preparation, F.M., O.M., K.A., and A.M.A.; writing—review and editing, F.M., O.M., K.A., M.S., and A.M.; visualization, F.M., O.M., K.A., M.S., and A.M.A.; project administration, A.M.A.; funding acquisition, A.M.A. and M.S. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, F., Salem, O.M., Abdelkarem, K. et al. Experimental and theoretical insights into LDH based on iron for photoelectrochemical water splitting. Sci Rep 15, 35801 (2025). https://doi.org/10.1038/s41598-025-17648-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17648-0