Abstract
Shipwreck ceramics are rarely analysed in situ since conventional mineral tests require sampling and jeopardise conservation. This study present the first application of full-range visible–near-infrared (VIS–NIR, 350–2500 nm) reflectance spectroscopy to shipwreck ceramics, analysing 30 sherds from the 16th-century Bidong wreck off Peninsular Malaysia. CIELAB colour space reveals the a*–b* linearity improves markedly from R² ≤ 0.50 on uncleaned surfaces to 0.80 in clean-powder subsamples, providing an objective metric for de-encrustation. High chromaticity and pervasive hematite document dominantly oxidising, whereas a minority of grey fabrics signal reduction firing conditions. Varimax-rotated PCA (VPCA) resolves 18 spectral components in intact sherds but only 11 after cleaning, demonstrating how surface alteration inflates apparent mineral diversity. Diagnostic loadings for illite and Fe-rich pyroxene (hedenbergite) tie the ceramic body to Sukhothai red clays, while copper sulphides and marine carbonates fingerprint post-burial interaction with Cu fittings and seawater. A weak 600–620 nm shoulder tentatively matches lazurite, a blue pigment rarely reported in Southeast Asian wares thus requires micro-analytical confirmation. This rapid, micro-destructive VIS–NIR method provides preliminary information about the salvaged artifacts which can be further correlated with another scientific study for provenance and production techniques interpretation.
Similar content being viewed by others
Introduction
Shipwrecks serve as time capsules, preserving ancient artifacts in underwater environments. The inherent value of shipwrecks extends beyond their historical importance, encompassing the cultural artifacts hidden beneath the seafloor. Notable instances like the Mary Rose warship (16th-century) and the Uluburun (14th-century) have provided invaluable insights into historical maritime technology and trade dynamics, underscoring the academic and public interest in such archaeological discoveries1,2,3,4,5.
Ceramics retrieved from shipwrecks have captured the interest of archaeologists and natural scientists due to their potential to provide insights into craftsmanship, trade patterns, and the civilizations of ancient societies. Conventional ceramic analysis has traditionally encompassed visual inspection, typological classification, and chemical testing. Although methods such as the Munsell Colour Chart provide a standardized visual colour identification system6,7, these traditional methods are often subjective, labour-intensive, and risk damaging fragile artefacts. Therefore, there is an urgent need for more advanced, micro-destructive analytical techniques in maritime archaeology.
The excavation and recovery of artefacts from underwater environments involve a range of physical, chemical, and biological challenges8. The inherent porosity of ceramics renders them vulnerable to moisture absorption, potentially leading to cracking and discoloration. Consequently, the implementation of micro-destructive methodologies is imperative for guaranteeing both the accuracy of scientific analyses and the long-term preservation of these valuable items.
Reflectance spectroscopy offers a robust, micro-destructive technique for analysing ancient ceramics. Few studies have successfully applied near-infrared reflectance spectroscopy in terrestrial archaeological contexts, including Etruscan pottery9 and painted archaeological ceramics10. Nonetheless, its application in underwater archaeological settings remains largely unexplored. This study constitutes a pioneering effort to utilise reflectance spectroscopy across a wavelength range of 350 to 2500 nm to investigate the colour variation, preservation status, and mineralogical composition of ceramics recovered from the Bidong Shipwreck. Consequently, it provides significant methodological contributions to the discipline of maritime archaeology.
In general, the visible (VIS, 400–700 nm) and near-infrared (NIR, 700–2500 nm) regions each provide complementary information. Within the VIS range, the raw reflectance curve itself carries diagnostic absorption bands, which persist beyond its conversion to CIE L*a*b* coordinates. Chromophoric minerals, such as chlorite (green, ∼550 nm shoulder) and hematite (red, characteristic doublet near 535 nm plus a trough at 650 nm), imprint distinctive features that can be used to fingerprint clay sources or to evaluate reducing versus oxidising firing conditions11,12,13,14,15. Conversion of VIS data to CIE L*a*b* remains a valuable method for quantitative colour comparison: L* gauges glaze brightness, while the a* tracks iron-oxide driven redness and post-firing alteration, offering an objective proxy for the artefact’s original appearance.
Meanwhile, the NIR spectrum is less affected by surface pigments or glaze and exhibits heightened sensitivity to fundamental OH, Al-OH, and Mg-OH vibrations. Clay minerals exhibit distinct absorptions between 1400 and 2200 nm, facilitating the evaluation of raw material origin, firing temperature, and post-burial hydration conditions. The combination of VIS chromophore bands with NIR lattice-vibration characteristics offers a comprehensive, micro-destructive compositional and technical signature.
Overall, reflectance spectroscopy analysis provides an efficient and micro-destructive method for the rapid examination of ceramics from archaeological sites. This study aims to pioneer the application of the reflectance spectroscopy technique in shipwreck studies to investigate the colour variation and mineral composition of ceramics retrieved from the Bidong Shipwreck.
Results
CIELAB colour statistics
A total of 210 reflectance spectra (30 sherds × 3 treatments × 2 faces + 30 powders) were converted to CIELAB space (Table S2). Summary statistics are listed in Table 1, and bivariate relationships are visualised in Fig. 1. They delineate three fundamental characteristics of chromatic variability, as explained below.
Colorimetric values for exterior, interior, and powder samples. Scatter plots show the relationship between CIELAB coordinates for exterior (a,d), interior (b,e), and powdered (c,f) samples. (a–c) display b* versus a* values with corresponding linear regression lines: exterior (R2 = 0.4), interior (R2 = 0.5), powder (R2 = 0.8). (d–f) display L* versus a* (red dots) and b* (blue dots) values highlighting the variation in chromaticity and lightness among the different sample conditions.
Distribution of L*, a*, b*
The lightness coordinate L* spans ~ 40 units in every treatment, reflecting the great variety of surface finishes and burial stains (Fig. 1). Both chromatic coordinates, a* (green → red) and b* (blue → yellow), are mostly positive throughout virtually all measurements, indicating that the sherds are situated in the reddish–yellow quadrant typical of Fe-rich oxidising firings (Table 1).
Red–yellow chromatic coupling (Fig. 1a-c)
Panels 1 a–c plot b* against a* for the exterior, interior, and powder fractions, respectively. All three datasets display a positive linear trend, indicating that the yellowness increase as redness intensifies; however, the strength of this relationship varies markedly with surface treatment (Table 1).
The minimal difference in R² between the exterior and interior fractions (ΔR² ≈ 0.10) demonstrates that both surfaces of the sherds are substantially affected by surface weathering, with the interior experiencing only a slight decrease in alteration intensity. Conversely, de-encrustation followed by grinding results in a pronounced increase in linearity (R² = 0.80), confirming that post-depositional coatings obscure the primary firing colour, whereas clean-powder samples best preserve the original chromatic relationship imposed during firing.
Lightness decoupling(Fig. 1d–f)
Scatterplots of L* versus a* / b* (Fig. 1d–f) yield regression slops are statistically indistinguishable from zero (p > 0.10). Lightness is therefore not a reliable proxy for compositional or firing variations; instead, it is dominated by surface roughness, micro-porosity, and residual carbonate crusts. Consequently, L* is excluded from the multivariate mineralogical analysis.
Implications for archaeology and conservation
The colourmetric patterns described above have direct consequences for both archaeological interpretation and conservation practice. First, the coefficient of determination between a * and b * provides a quantitative quality-control metric for surface preparation: values below 0.6 (exterior and interior fractions) reveal that heterogeneous encrustations continue to mask the primary firing colour, whereas an R² of 0.8 (clean-powder fraction) demonstrates that complete removal of post-depositional coatings restores the original chromatic relationship. The interior face differs from the exterior by only ΔR² ≈ 0.10, thus both surfaces must be considered equally susceptible to marine alteration; relying on the apparently “shielded” reverse side would therefore give a misleading impression of the vessel’s original appearance.
Second, colorimetric screening can guide conservation strategies. By monitoring the evolution of the a*–b* R² value during successive cleaning steps, conservators can decide objectively when additional mechanical or chemical treatment is warranted and can document the degree to which surface interventions recover primary fabric colour. Such documentation is particularly valuable for collections management, where low-impact powder sampling is preferred.
Furthermore, enhanced linearity in the clean-powder fraction improves the signal-to-noise ratio of first-derivative spectra, thereby increasing the reliability of the VARIMAX-rotated PCA used for mineral identification, as detailed in the following section on Mineral composition. Since the a * and b * value are closely linked to Fe- and Ti-oxide absorptions, the recovered red–yellow coupling confirms that spectral features diagnostic of firing atmosphere and raw-material provenance have not been obscured by post-depositional staining. Thus, the colour data not only illuminate ancient ceramic technology but also validate the sample-preparation protocol that underpins subsequent mineralogical analysis.
Mineral composition
VPCA was applied separately to the sherd (S) and clean-powder (P) data sets for three wavelength windows: visible (VIS, 400–700 nm), near-infrared (NIR, 700–2500 nm), and the combined VIS + NIR range (350–2500 nm). The resulting component–mineral associations are summarized in Table 2, detailed eigenvalues and cumulative variance percentages are provided in Table S3, and the corresponding component loadings are compared with USGS reference spectra in Figs. 2, 3, 4, 5 and 6.
Component loading and mineral fit of sherds and powder sample for visible range (400–700 nm). (a) VPCA loading 1 for sherd sample (representing 32.01% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of illite. (b) VPCA loading 2 for sherd sample (representing 29.38% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of diopside. (c) VPCA loading 3 for sherd sample (representing 28.71% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of chrysocolla. (d) VPCA loading 1 for powder sample (representing 66.02% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of pyroxene. (e) VPCA loading 2 for powder sample (representing 26.21% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of chalcopyrite.
Component loading and mineral fit of sherds sample for near-infrared range (700–2500 nm). (a) VPCA loading 1 (representing 26.80% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of hematite. (b) VPCA loading 2 (representing 21.69% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of ferrihydrite. (c) VPCA loading 3 (representing 15.77% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of sodium bicarbonate. (d) VPCA loading 4 (representing 9.83% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of lazurite. (e) VPCA loading 5 (representing 7.63% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of sphalerite. (f) VPCA loading 6 (representing 5.26% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of illite. (g) VPCA loading 7 (representing 4.01% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of ammonio-smectite.
Component loading and mineral fit of powder samples for near-infrared range (700–2500 nm). (a) VPCA loading 1 (representing 33.09% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of hematite. (b) VPCA loading 2 (representing 27.51% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of gaylussite. (c) VPCA loading 3 (representing 21.01% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of sodium bicarbonate. (d) VPCA loading 4 (representing 8.55% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of illite.
Component loading and mineral fit of sherd samples for full range (350–2500 nm). (a) VPCA loading 1 (representing 24.25% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of hematite. (b) VPCA loading 2 (representing 17.19% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of ferrihydrite. (c) VPCA loading 3 (representing 13.81% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of sodium bicarbonate. (d) VPCA loading 4 (representing 11.53% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of hedenbergite. (e) VPCA loading 5 (representing 7.03% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of sphalerite. (f) VPCA loading 6 (representing 6.90% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of zeolite. (g) VPCA loading 7 (representing 5.89% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of illite. (h) VPCA loading 8 (representing 4.37% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of chlorite.
Component loading and mineral fit of powder samples for full range (350–2500 nm). (a) VPCA loading 1 (representing 34.22% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of hematite. (b) VPCA loading 2 (representing 25.30% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of hedenbergite. (c) VPCA loading 3 (representing 20.31% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of sodium bicarbonate. (d) VPCA loading 4 (representing 7.63% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of sphalerite. (e) VPCA loading 5 (representing 5.18% of the spectral variance in the calibration data set) is similar in shape to the first derivative spectrum of illite.
VPCA of the sherd spectra resolved 3 components in the VIS window, 7 in the NIR, and 8 in the full range (Table 2), accounting for ≥ 90% of the spectral variance (Table S3). The powder spectra required markedly fewer components: 2 (VIS), 4 (NIR) and 5 (VIS + NIR)-reflecting the removal of surficial noise introduced by encrustations, as discussed in the previous section on CIELAB colour statistics. In most cases, the first two components captured > 50% of the variance, indicating that a limited number of mineralogical drivers dominate the reflectance variability.
Visible-range mineral components
Principal component analysis (400–700 nm; Fig. 2) identifies three predominant eigenvectors for the sherd dataset. PC1 has a broad absorption minimum centred at near 580 nm, which roughly corresponds to the crystal-field band of illite11,12 (R²=0.87). PC2 has the distinctive monotonic increase from 400 to 700 nm associated with Fe-rich diopside/clinopyroxene (R² = 0.96); this VIS slope originates from Fe²⁺⇆Fe³⁺ intervalence charge transfer in the pyroxene lattice16,17. PC 3 has a narrow band around 530 nm corresponding to chrysocolla (R² = 0.96), produced by Cu²⁺ crystal-field absorptions18.
After surface cleaning, the powder spectra accentuate these features: the 580 nm band sharpens, aligning most closely with a generic pyroxene spectrum (R² = 0.95), while a subordinate component aligns with chalcopyrite (R² = 0.91), characterised by a broad absorption band centred near 550 nm linked to Cu-Fe charge transfer19. The emergence of sulphide signals after grinding suggests that copper-rich inclusions were masked by surface coatings in the unaltered sherds.
Near-infrared mineral components
Analysis of the 700–2500 nm window (Figs. 3 and 4) requires seven components for sherds, but only four for powders (Table 2). PC1 in both datasets corresponds to hematite, with diagnostic Fe³⁺ ligand-field bands at ~ 900 nm and a broad charge-transfer complex near 1900 nm typical of well-crystallised hematite20. Subsequent sherd components identify ferrihydrite (R² = 0.62) and sodium bicarbonate (R² = 0.74); ferrihydrite displays a weak 950 nm band plus hydration features at 1400 and 1900 nm21; whereas sodium bicarbonate (and gaylussite) show CO₃ overtones at 1450–1500 nm and combination bands in 1950–2000 nm22.
Additional vectors indicate a provisional lazurite component, expressed as a shoulder at 600–620 nm12 that continues into minor NIR oscillations and yields an overall match of R² = 0.59. Further components identify sphalerite (R² = 0.33) and ammonio-smectite (R² = 0.07). Sphalerite has a muted Fe²⁺ band at around 1000 nm19; ammonio-smectite provides extensive OH–NH combinations throughout the range of 1400 to 2100 nm.
In powders, ferrihydrite disappears and is substituted by gaylussite (Na₂Ca(CO₃)₂·5 H₂O) (R² = 0.36) whose carbonate absorption bands at 1450 nm and 1950 nm do not exhibit the ferrihydrite characteristic at 950 nm, so validating its classification as a burial precipitate22. The remaining powder components-hematite, sodium bicarbonate and illite, retain their sherd counterparts but with higher R² values after surface cleaning.
Integrated VIS-NIR components
Combining the full 400–2500 nm range resolves eight components for sherds and five for powders (Figs. 5 and 6). Hematite (R² = 0.37–0.38) and sodium bicarbonate (R² ≈ 0.74) re-emerge as dominant factors. A pronounced hedenbergite (Fe-rich pyroxene) component appears only after the VIS and NIR windows are combined (R² = 0.82 for sherds; 0.64 for powders), underscoring the benefit of integrating VIS and NIR wavelengths for detecting ferromagnesian silicates; hedenbergite is distinguished by coupled Fe²⁺ bands at 1000–1200 nm and a steep VIS slope16,17.
Additional sherd components include laumontite-zeolite (R² = 0.36), identified by a strong H₂O combination band at 1910 nm and an Al–OH stretch at 2305 nm13, and chlorite (R² = 0.52) with Mg–Fe–OH absorptions at 2250 and 2340 nm plus a VIS shoulder near 680 nm23. These factors are absent or muted in powders, underscoring their confinement to surface alteration layers.
Archaeological and technologic significance
The VPCA results imply that the Bidong ceramics were produced from an Fe-rich clay matrix containing detrital pyroxenes and minor copper sulphides, subsequently fired in an oxidising atmosphere that stabilised hematite and fixed the red-yellow body colour documented in the previous section on CIELAB colour statistics. Sodium bicarbonate and gaylussite are interpreted as burial-derived evaporite precipitates, whereas ferrihydrite and sphalerite are confined to alteration rinds removed during powder preparation. The systematic reduction in component number from 18 in sherds to 11 in powders demonstrates that surface contamination inflates apparent mineral diversity; careful de-encrustation is therefore essential before spectroscopic provenance studies.
Discussion
The results show that all Bidong sherds occupy the red–yellow quadrant of CIELAB space (a* > 0; b* > 0) and that the linear coupling between these two chromatic coordinates strengthens dramatically once surface accretions are removed (R² = 0.80 in the clean-powder fraction versus ≤ 0.50 on the intact surfaces). Based on the colour classification scatter plot (Figure S1), since large |a*| and |b*| values denote high chromaticity, clusters 1 and 2 which lie at the upper right of the a*-b* plot-correspond to vivid reddish-brown hues. In contrast, cluster 3 and 4 represents more neutral greys and buffs24. The chromatic distribution is readily explained by the valence state of iron, the principal chromophore in earthen ceramics: hematite formed under oxidising conditions imparts red-brown tones, whereas reduced Fe²⁺ phases yield grey bodies25.
The negligible difference in R² between exterior and interior surfaces (ΔR² ≈ 0.10) demonstrates that both sides are equally overprinted by marine biofilms and mineral crusts or probably affected by the post-depositional alteration after burial; the pristine firing colour is recovered only after complete de-encrustation and homogenisation of the ceramic fabric. While a*-b* linearity is characteristic of Fe³⁺-controlled red–yellow chromaticity in oxidised stoneware, we emphasise that its utility as a cleanliness index remains sample-specific and should be independently verified for ceramics fired under reducing atmospheres or containing non-iron chromophores.
Lightness (L*), by contrast, shows no systematic relationship to hue or chroma, confirming earlier observations that L* is an unreliable proxy for firing atmosphere or raw material composition26. We therefore propose the a*-b* coefficient of determination as an objective metric for conservators: values below 0.6 flag residual surface contamination, whereas values ≥ 0.8 indicate that cleaning has reached the unaltered body.
VPCA reveals that variability in the VIS spectrum is determined not by applied pigments but by clay-silicate absorptions (illite, diopside) and by copper sulphides such as chrysocolla and chalcopyrite. The latter are most plausibly derived from corrosion of Cu-bearing fittings or cargo on the wreck27,28 and illustrate how post-depositional processes can masquerade as technological signals if surface films are not removed.
In the NIR, the predominance of hematite supports the oxidising firing atmosphere indicated by colour data, whereas a provisional lazurite match (R² = 0.59; 600–620 nm shoulder) warrants cautious interpretation. The VIS-NIR spectra alone cannot unequivocally separate lazurite from other Cu- or S-bearing blue phases; hence, targeted micro-analytical techniques-such as spot Raman spectroscopy, SEM-EDS, or µ-XRD on the blue particles are therefore required. Confirming lazurite would be significant because the mineral is virtually unreported in Southeast Asian ceramic assemblages and may indicate the use of imported ultramarine pigments. Lazurite has served as an expensive blue pigment in ceramics and paintings since the 13th century29,30,31, yet it remains rare in ceramics owing to its limited geological sources (Afghanistan, Russia, Iran, Tajikistan, Chile, the United States, and Myanmar)32.
Surface-specific phases such as ferrihydrite and sphalerite disappear in the powder spectra. In contrast, gaylussite and sodium bicarbonate remain, indicating that carbonate salts recrystallised within pore spaces during prolonged seawater immersion.
Full-range analysis isolates hedenbergite, linking the ceramic matrix to Fe-rich pyroxenic clays documented in Sukhothai deposits33, and detects laumontite-zeolite, an alteration product consistent with low-temperature hydrothermal overprint. Collectively, the spectral evidence implies that the Bidong Shipwreck ceramics were manufactured from a locally sourced, Fe-rich clay, fired predominantly under oxidising conditions, and subsequently modified by marine diagenesis that introduced secondary carbonates and sulphides.
Conclusion
We demonstrate that reflectance spectroscopy, applied across the 350–2500 nm range, is a rapid and micro-destructive tool for evaluating both chromatic and mineralogical attributes of the Bidong Shipwreck ceramics. Quantitative colour analysis revealed that all sherds fall within the red–yellow quadrant of CIELAB space, and the a*–b* coefficient of determination increases from ≤ 0.50 on intact surfaces to 0.80 after de-encrustation, providing an objective metric for assessing cleaning efficacy. This R² index has thus far been confirmed just for oxidised, Fe-rich Bidong ceramics; hence, it should be applied to other technological groups with appropriate caution. These chromatic trends, together with the pervasive occurrence of hematite, indicate that most vessels were fired under oxidising conditions, whereas a minority of grey bodies reflect locally reduced firing.
VPCA isolated 18 significant spectral components in uncleaned sherds but only 11 after homogenisation, demonstrating that surface alteration layers inflate apparent mineral diversity. Core constituents include illite and an Fe-rich pyroxene (hedenbergite) that link the fabric to locally documented red clays from the Sukhothai region, while minor copper-sulphide signals (chrysocolla, chalcopyrite) and marine carbonates (sodium bicarbonate, gaylussite) attest to post-depositional interaction with Cu-bearing wreck material and seawater. A tentative lazurite component, rare in Southeast-Asian ceramics, suggest the use of high-value blue pigment but requires confirmation by micro-XRD or SEM-EDS.
Although reflectance spectroscopy effectively discriminates firing atmosphere, raw-material provenance, and burial alteration, it offers limited structural and elemental specificity. We therefore recommend future work integrating full-range VIS-NIR spectroscopy with complementary techniques, including XRD for crystalline phases, XRF for bulk chemistry, and SEM-EDS for micro-scale mineral-pigment mapping to obtain a comprehensive, multi-proxy reconstruction of the production technology and post-burial history of the Bidong Shipwreck assemblage. Such a combined approach will refine provenance attribution, guide conservation strategies, and enhance our understanding of ceramic exchange networks in late-medieval Southeast Asia.
Methodology
Site background
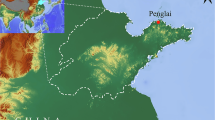
The Bidong Shipwreck is located off the east coast of Peninsular Malaysia, an area of considerable historical significance within the broader framework of Southeast Asian maritime trade (Fig. 7). Academic interest in the shipwreck was piqued in March 2012, when a local fisherman repeatedly found fragments of stoneware in his gear34. This ceramic assemblage is predominantly composed of stoneware, and initial analyses have attributed these artefacts to the Sukhothai and Ayutthaya Kingdoms of Thailand. These findings highlight the potential of this site to shed light on cross-cultural trade dynamics.
(a) Map of Peninsular Malaysia, (b) The yellow square represents the location of the Bidong Shipwreck archaeological site (adapted from Ref.35).
In addition to its archaeological significance, the underwater habitat of the Bidong Shipwreck presents distinct preservation challenges. Submersion can accelerate both the physical degradation and chemical alteration of ceramics, especially in the face of fluctuating salinity, temperature, and biological activity. These challenges underscore the necessity of employing modern, micro-destructive analytical techniques, such as reflectance spectroscopy, to accurately characterize the artefacts without compromising their integrity.
Sample preparation
The salvaged artifacts underwent a year-long desalination treatment to remove salt content35. From 306 artifacts in the collection, 30 ceramic sherds were selected for analysis while the remainder were preserved for potential future study. Sample selection was done based on the artifacts physical condition: sherds with sufficient size and representation of the colour range observed in the collection. A limitation of the selection is that some fragments were too small and incomplete to determine their original structure (e.g.: rim, base, or body). Detailed information about the selected samples is provided in Table S1.
Calcium carbonate deposits, caused by prolonged seawater exposure, were manually removed with a laboratory spatula before grinding to avoid interfering with ceramic compositional analysis. Powder sample preparation adhered to published protocols36. To minimise damage, a single transverse slice (~ 5 × 5 mm) was removed from an existing fracture edge, spanning the full cross-section from exterior to interior. The slice was then gently ground in an agate mortar and subsequently stored in the 1.5 × 1.5 cm plastic boxes. The powder surface was smoothed using a glass slide before measurement. All equipment used during the process was cleaned with water and then wiped with ethanol after each cycle to minimize potential contamination between samples.
Spectra measurement
Reflectance spectra were collected in the VIS-NIR region (350–2500 nm; 1 nm step) using an ASD LabSpec 4 spectrometer equipped with the standard contact probe (8°/0° bidirectional geometry)37. The annular light source and fixed collection angle suppress mirror-like “glint” while still recording the diffuse component, yielding spectra representative of both glazed and unglazed surfaces. Spectra were recorded from 350 to 2500 nm at 1 nm resolution in the Earth Environment and Climate Change Laboratory, Institute of Earth Sciences, National Taiwan Ocean University.
For each sherd, the exterior (front) and interior (back) surfaces were first measured in their original state, retaining any marine encrustations or deposits (e.g., calcium carbonate). Subsequently, the surfaces were scraped clean, and the sherds were ground into powder. The powdered samples in the plastic boxes were levelled with a glass slide before additional spectral measurements. Fifty spectra measurements were automatically averaged per run (~ 60 s), yielding two datasets per sample: (i) untreated surfaces and (ii) cleaned powder, thereby isolating the optical contribution of surface deposits.
Data processing
Spectral data processing steps are outlined in the flowchart (Fig. 8). For colorimetric analysis, the visible range of reflectance spectra (400–700 nm) was re-sampled at 10 nm intervals before conversion to CIELAB colour coordinates system, using illuminant D65 at 10° viewing angle through Microsoft Excel spreadsheet obtained from website developed by Lindbloom38. In the three-dimensional CIELAB space, the L* value signifies lightness, ranging from 0 (black) to 100 (white). The a* axis spans from green (negative) to red (positive), whereas the b* axis extends from blue (negative) to yellow (positive)39,40,41. These coordinates offer a standardized way to compare colour variations across samples42.
K-means clustering has been applied to cluster ceramics based on their colour properties using SPSS software. This approach aims to maximize the differences across clusters while minimizing the variance of data points within a cluster43. K-means clustering method uses k points in space as the centre, group the objects closest to them, and update the value of each cluster centre until the best clustering result is achieved44. Number of clusters chosen at the point where the reduction within the sum of squares starts to level off (Fig. 9).
For statistical approach, spectral data was first subjected to numerical derivative analysis. Prior research has demonstrated that unprocessed spectral curves, particularly those from marine environments, tend to exhibit considerable smoothness, obscuring compositional distinctions. Thus, computing the first derivative is a proficient method to amplify these incremental changes45,46. First-order derivatives of reflectance spectra were calculated using a central difference algorithm. This approach amplifies spectrum changes, facilitating the distinct identification of each spectral characteristic. The first-derivative spectra were standardized by mean-standard deviation normalization, transforming the data to a comparable scale. This step reduces the potential biases caused by measurement or instrumental errors.
Normalized derivatives spectra were subjected to Principal Component Analysis (PCA) using SPSS software with Varimax rotation. This method reduces data dimensionality by grouping correlated spectral information into fewer, orthogonal, statistically independent components. Principal components (PCs) with eigenvalues larger than one (as determined by Kaiser’s criterion) were maintained to ensure the selection of significant components showing compositional variations. In Varimax‑rotated PCA, each principal component is characterised by (i) a loading pattern that represents diagnostic spectral features and (ii) a component score that quantifies how strongly each sample expresses that component (i.e., a proxy for relative abundance). The present study focuses on the loadings for mineral identification; interpretation of the component scores is deferred to forthcoming work that will combine the PCA results with geochemical data.
The resulting component loadings yielded distinct spectral patterns which were subsequently compared with the mineral reference spectra in the USGS Spectral libraries47 to interpret the compositional significance of each component. Our interpretations rely on the positions and depths of Fe- and OH-related absorption bands, not on absolute reflectance. These band parameters are governed by the same electronic/vibrational transitions in both intact and powdered states and are therefore directly comparable. This comparison enabled more accurate identification of materials and provides insight into the archaeological and conservation context of the Bidong Shipwreck artifacts.
Data availability
The raw data of this study is available by the corresponding author up on request.
References
Barker, S. The mary rose revisited: Tudor myth, popular history and the tears that England owes. J. Popular Cult. 29, 51–68. https://doi.org/10.1111/j.0022-3840.1996.51867978.x (1996).
McBride, P. W. J. The mary, Charles II’s yacht. Int. J. Nautical Archaeol. 2, 61–70. https://doi.org/10.1111/j.1095-9270.1973.tb00490.x (1973).
Pulak, C. The Uluburun shipwreck: an overview. Int. J. Nautical Archaeol. 27, 188–224. https://doi.org/10.1111/j.1095-9270.1998.tb00803.x (1998).
Pulak, C. Uluburun shipwreck. Oxf. Handb. Bronze Age Aegean. 862–876 https://doi.org/10.1093/oxfordhb/9780199873609.013.0064 (2012).
Scorrer, J. et al. Diversity aboard a Tudor warship: investigating the origins of the Mary Rose crew using multi-isotope analysis. R. Soc. Open. Sci. 8, (2021).
Indow, T. Global color metrics and color-appearance systems. Color. Res. Application. 5, 5–12. https://doi.org/10.1002/col.5080050103 (1980).
Wright, W. D. Color science, concepts and methods. Quantitative data and formulas. Phys. Bull. 18, 353–353 (1967).
Elkin, D., Edwards, H. G. & Maier, M. S. The application of analytical archaeometry in underwater cultural heritage—a case study from patagonia, Argentina. Anal. Archaeometry. 532–549 https://doi.org/10.1039/9781849732741-00532 (2012).
Bruni, S. Etruscan fine ware pottery: Near-infrared (NIR) spectroscopy as a tool for the investigation of clay firing temperature and atmosphere. Minerals 12, 412. https://doi.org/10.3390/min12040412 (2022).
Bruni, S., Guglielmi, V., Della Foglia, E., Castoldi, M. & Bagnasco Gianni, G. A non-destructive spectroscopic study of the decoration of archaeological pottery: from matt-painted bichrome ceramic sherds (southern italy, VIII-VII B.C.) to an intact Etruscan cinerary urn. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 191, 88–97. https://doi.org/10.1016/j.saa.2017.10.010 (2018).
Clark, R. N., King, T. V., Klejwa, M., Swayze, G. A. & Vergo, N. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Research: Solid Earth. 95, 12653–12680. https://doi.org/10.1029/jb095ib08p12653 (1990).
Hunt, G. R. & Salisbury, J. W. Visible and near-infrared spectra of minerals and rocks—I. Silicate minerals. Mod. Geol. 1, 283 (1970).
Clark, R. N., King, T. V. V., Klejwa, M. & Swayze, G. A. High-spectral-resolution reflectance spectra of minerals in the visible and near infrared (0.2–3.0 µm). U.S. Geological Survey Open-File Report 90–404, 128 (1990).
Jiang, Z. et al. The magnetic and color reflectance properties of hematite: from Earth to Mars. Rev. Geophys. 60 https://doi.org/10.1029/2020RG000698 (2022).
Lepre, C. J., Yazzie, O. M. & Klaus, B. R. Visible-light spectroscopy and rock magnetic analyses of iron oxides in mixed-mineral assemblages. Crystals 14, 644. https://doi.org/10.3390/cryst14070644 (2024).
Burns, R. G. Group theory nomenclature for crystal field states. Mineral. Appl. Cryst. Field Theory 467–467 (1993).
Sunshine, J. M. & Pieters, C. M. Determining the composition of olivine from reflectance spectroscopy. J. Geophys. Research: Planet. 103, 13675–13688. https://doi.org/10.1029/98je01217 (1998).
Clark, R. N. & Williams, P. Visible and near-infrared reflectance spectra of minerals: Chrysocolla. U.S. Geological Survey Open-File Report 91 – 086, 7 (1991).
Clark, R. N., Swayze, G. A., Gallagher, A. J., King, T. V. V. & Calvin, W. M. The U. S. Geological Survey, Digital Spectral Library: Version 1 (0.2 To 3.0um) (Open-File Report, 1993).
Sherman, D. M. & Waite, T. D. Electronic spectra of Fe3+ oxides and oxide hydroxides in the near IR to near UV. Am. Mineral. 70, 1262–1269 (1985).
Scheinost, A. C., Chavernas, A., Barrón, V. & Torrent, J. Use and limitations of second-derivative diffuse reflectance spectroscopy in the visible to near-infrared range to identify and quantify Fe oxide minerals in soils. Clays Clay Miner. 46, 528–536. https://doi.org/10.1346/CCMN.1998.0460506 (1998).
Gaffey, S. J. Spectral reflectance of carbonate minerals in the visible and near infrared (0.35–2.55 um): anhydrous carbonate minerals. J. Geophys. Research: Solid Earth. 92, 1429–1440 (1987).
Bishop, J. L., Lane, M. D., Dyar, M. D. & Brown, A. J. Reflectance and emission spectroscopy study of four groups of phyllosilicates: smectites, kaolinite-serpentines, chlorites and micas. Clay Miner. 43, 35–54. https://doi.org/10.1180/claymin.2008.043.1.03 (2008).
Wang, Y. et al. Color design decisions for ceramic products based on quantification of perceptual characteristics. Sensors 22, 5415. https://doi.org/10.3390/s22145415 (2022).
Ion, R. M. et al. Compositional and microstructural investigations of prehistoric ceramics from Southern Romania (middle neolithic pottery). (2024). https://doi.org/10.20944/preprints202406.0343.v1
Gebremariam, A. Z. et al. The contribution of colour measurements to the archaeometric study of pottery assemblages from the archaeological site of Adulis, Eritrea. Acta Imeko. 11, 8 (2022).
Frost, R. L. & Xi, Y. Is Chrysocolla (Cu, Al) 2H2Si2O5 (OH)4· nH2O related to spertiniite Cu(OH)2?—A vibrational spectroscopic study. Vib. Spectrosc. 64, 33–38. https://doi.org/10.1016/j.vibspec.2012.10.001 (2013).
Tang, Y. et al. Chalcopyrite geochemistry: advancements and implications in ore deposit research. Ore Geol. Rev. 179, 106528. https://doi.org/10.1016/j.oregeorev.2025.106528 (2025).
Catalano, I. M. et al. Lapis lazuli usage for blue decoration of polychrome painted glazed pottery: A recurrent technology during the middle ages in Apulia (southern Italy). J. Archaeol. Sci. 34, 503–511. https://doi.org/10.1016/j.jas.2006.05.010 (2007).
Koochakzaei, A., Hamzavi, Y. & Mousavi, M. A. Characterization of the mural blue paintings in ornamental motif of Ali Qapu palace in isfahan, iran, using spectroscopic and microscopic methods (A case study). J. Archaeol. Science: Rep. 45, 103632. https://doi.org/10.1016/j.jasrep.2022.103632 (2022).
Mangone, A., Caggiani, M. C., Forleo, T., Giannossa, L. C. & Acquafredda, P. A possible natural and inexpensive substitute for Lapis lazuli in the Frederick II era: the finding of Haüyne in blue lead-tin glazed pottery from Melfi castle (Italy). Molecules 28, 1546. https://doi.org/10.3390/molecules28041546 (2023).
Gambardella, A. A., Patterson, S., Webb, C. M., Walton, M. S. & S. M. & Sulfur K-edge XANES of lazurite: toward determining the provenance of Lapis lazuli. Microchem. J. 125, 299–307. https://doi.org/10.1016/j.microc.2015.11.030 (2016).
Singtuen, V., Phajuy, B., Pongsaisri, N. & Pailoplee, S. Georesource distribution impacts the prosperity of the Sukhothai Kingdom and anthropological civilization in Thailand. Sci. Cult. 10, 1–19. https://doi.org/10.5281/zenodo.11250791 (2024).
Mustapa, B. et al. Discovery and excavation of artifacts from the Bidong Shipwreck, Malaysia. Adv. Archaeol. Pract. 11, 246–257. https://doi.org/10.1017/aap.2022.45 (2023).
Chen, K. S. et al. The preliminary investigation on the origin and the sailing route of Bidong Shipwreck, Malaysia. J. Maritime Archaeol. 19, 247–264. https://doi.org/10.1007/s11457-024-09398-3 (2024).
Pan, H. J., Yu, P. S. & Chen, M. T. Testing the uncertainty of diffuse reflectance spectroscopy methods for estimating marine sediment components by full-resolution spectrophotometry and principle component analyses. J. Asian Earth Sci. 114, 526–533. https://doi.org/10.1016/j.jseaes.2015.06.029 (2015).
Malvern Panalytical. ASD FieldSpec® 4 Hi-Res NG spectroradiometer: Integrating sphere accessory description [Product support page]. (2025). https://www.malvernpanalytical.com/en/support/product-support/asd-range/fieldspec-range/fieldspec-4-hi-res-ng-spectroradiometer
Lindbloom, B. J. RGB Working Space Information. Welcome to Bruce Lindbloom’s web site (2017). Available at: http://www.brucelindbloom.com/. (Accessed: 2024).
Blum, P. Physical Properties Handbook: A Guide To the Shipboard Measurement of Physical Properties of deep-sea Cores (Ocean Drilling Program Technical Notes, 1997).
Nederbragt, A. J. & Thurow, J. W. Digital sediment colour analysis as a method to obtain high resolution climate proxy records. Dev. Paleoenvironmental Res. 105–124. https://doi.org/10.1007/1-4020-2122-4_6 (2005).
Nederbragt, A. J. et al. Sediment colour analysis from digital images and correlation with sediment composition. Vol. 267, 113–128 (Geological Society, Special Publications, 2006).
Jin, X. et al. Celadon Colour Data Association classification and its dynasty-kiln site characteristics. Ceram. Int. 47, 29567–29575. https://doi.org/10.1016/j.ceramint.2021.07.124 (2021).
Xie, J. Subclassification of chemical composition of glass based on principal component analysis and K-means clustering. Highlights Sci. Eng. Technol. 84, 171–179. https://doi.org/10.54097/tvpw9x28 (2024).
Yang, J., Wang, Z. & Gao, S. Component analysis and classification model of ancient glassware based on k-means clustering and random forest. Highlights Sci. Eng. Technol. 42, 397–405. https://doi.org/10.54097/hset.v42i.7120 (2023).
Ortiz, J., Mix, A., Harris, S. & O’Connell, S. Diffuse spectral reflectance as a proxy for percent carbonate content in North Atlantic sediments. Paleoceanography 14, 171–186. https://doi.org/10.1029/1998pa900021 (1999).
Pan, H. J. & Chen, M. T. Evaluating color reflectance spectral methods for estimating biogenic contents of South China sea sediments. J. Asian Earth Sci. 69, 121–132. https://doi.org/10.1016/j.jseaes.2012.11.039 (2013).
Kokaly, R. F. et al. USGS Spectral Library version 7. Data Series. https://doi.org/10.3133/ds1035 (2017).
Acknowledgements
This work was funded by (1) State Key Laboratory of Marine Geology Open Project under Grant number MGK202415 and (2) National Taiwan Ocean University and grants to HJP from the Institute of Earth Sciences, College of Ocean Science and Resource, National Taiwan Ocean University, Keelung, Taiwan (NSTC 112-2611-M-019-015). The permit references for the first and second phases of the excavation are JWN.PP.600/3/3(3). We are grateful to Hsuan-Cheng Liu (Institute of Earth Sciences, NTOU) for his valuable assistance with spectral acquisition and data processing.
Author information
Authors and Affiliations
Contributions
N.K.M.Y, H.J.P and H.S conceived the ideas and designed methodology; N.K.M.Y collected the data; N.K.M.Y and H.J.P analyzed and visualized the data; H.J.P and E.I contributed to validation; N.K.M.Y, H.J.P, and H.S led the writing of the manuscript; H.J.P, S.Y, E.I, M.N.I.A.R, E.H.A , and H.S contributed to supervision, manuscript review, and provided critical revisions; H.J.P and H.S acquired the funding and provided resources. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yunos, N.K.M., Pan, HJ., Yang, S. et al. Reflectance spectroscopy reveals mineralogy and preservation of ceramics from the Bidong Shipwreck. Sci Rep 15, 33178 (2025). https://doi.org/10.1038/s41598-025-17661-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17661-3