Abstract
Thrombosis is a life-threatening complication in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. This study aims to conduct a statistical analysis of the incidence of blood clots and lipid concentrations, and to examine the networks of oxylipins in hospitalised patients with SARS-CoV-2. Serum samples of 1731 hospitalised patients with SARS-COV-2 were used to measure six lipid parameters: total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A (apoA), and apolipoprotein B (apoB). Additionally, the lipid profiles and oxidative lipidomics characteristics were examined via liquid chromatography-electrospray ionization-tandem mass spectrometry (LC–MS/MS) in SARS-COV-2-positive patients with and without thrombosis. The mortality rate in the SARS-COV-2 thrombosis group was significantly higher at 29.6% compared to the SARS-COV-2 non-thrombosis group at 12.1% (P < 0.0001). The levels of the lipid parameters were closely associated with both thrombosis and SARS-COV-2 severity. Patients with SARS-COV-2 admitted to the hospital exhibited significant changes in oxidative lipid metabolites, specifically in the arachidonic acid (ARA) and docosahexaenoic acid (DHA) classes, compared with those in the control group. Among the thrombus group, 28 oxidative lipid metabolites were found to be differentially expressed compared to the non-thrombus group, and with the most notable variations observed in 20-hydroxyPGF2α and 14(15)-EpETE. Enrichment analysis using KEGG revealed that differential oxidized lipid metabolites mainly concentrated in the ARA and serotonergic synapses metabolism signaling pathway. Our findings indicate a close association between lipid mediators and both SARS-COV-2 and thrombi. Specifically, ARA and serotonergic synapses metabolism signaling pathway may be an important pathogenic factor for thrombosis caused by SARS-COV-2. Furthermore, 20-hydroxyPGF2α and 14(15)-EpETE show promise as potential biomarkers for SARS-CoV-2-induced thrombosis.
Similar content being viewed by others
Introduction
SARS-CoV-2 virus, as a beta coronavirus with an RNA genome and lipid envelope, has had widespread effects globally1. Thrombus formation has emerged as a significant complication in hospitalised patients with SARS-COV-2. Thrombotic complications, including venous thromboembolism (VTE), arterial thrombosis, and thrombotic microangiopathy, are major contributors to the onset and mortality of patients with SARS-COV-22. Studies have shown that the incidence of thrombotic complications among ICU patients with SARS-COV-2 varies from 16 to 49%3. Despite the use of standard measures to prevent blood clots, the rate of VTE remains elevated at 22.7%4. An examination of patients with SARS-COV-2 who were hospitalised in the United States indicated a notably increased overall occurrence of VTE in both general patients with SARS-COV-2 and those in the ICU, compared with hospitalised patients without SARS-COV-25. Two Chinese cohort studies found that, even with prophylactic anticoagulation, up to 46% of ICU patients with SARS-COV-2 experienced deep vein thrombosis6,7. Post-mortem studies on patients with SARS-COV-2 have identified severe vascular endothelial damage, extensive thrombus formation, and microvascular abnormalities in lung tissues8,9,10. Excessive inflammatory responses following SARS-COV-2 infection can lead to lung damage, including microvascular and endothelial dysfunction, resulting in coagulation disorders and thrombus formation, particularly in critically ill patients3,11. In addition to lung complications, thrombotic complications associated with SARS-COV-2 affect the heart. Numerous studies have indicated a higher incidence of SARS-COV-2 among individuals with underlying heart conditions such as hypertension, diabetes, and various cardiovascular disorders. These complications may result in myocardial damage, leading to conditions such as heart failure, myocarditis, and arrhythmias12,13,14. Furthermore, a post-mortem study of seven patients with SARS-COV-2 revealed the presence of platelet-fibrin microthrombi in various organs, including the lungs, liver, kidneys, and heart, irrespective of anticoagulation status15. Additionally, the presence of microvascular clotting linked to SARS-COV-2 can lead to negative consequences, such as respiratory distress and mortality. Hospitalised patients with SARS-COV-2 face an increased risk of thrombosis, which leads to further adverse outcomes.
Lipids are naturally occurring compounds that perform diverse biological functions, such as cell membrane formation, signalling molecule production, and energy provision. They exist in various forms, such as fatty acids, glycerolipids, glycerophospholipids, and sterols16. Polyunsaturated fatty acids can undergo oxygenation via enzymes or free radical-mediated mechanisms, resulting in the production of bioactive oxygenated lipid mediators, referred to as oxylipins17. Recently, oxylipins have been found to promote or inhibit thrombus formation. Yeung et al. found that Omega-6 DPA, along with its oxylipins from 12-lipoxygenase, effectively hindered platelet activation and blood clot formation following damage to blood vessels18. Cummings et al. found that the activation of mouse platelets in the serum by leukotriene C4 is specifically mediated by the type 2 cysteinyl leukotriene receptor. This activation ultimately results in increased inflammation and thrombus formation19. Prostaglandin E2 is a natural metabolic product of ARA. It exerts anti-thrombotic effects and effectively inhibits platelet activation. It lowers the arterial tension by inhibiting vascular smooth muscle cell proliferation and promoting vasodilation20. Additionally, prostaglandin E2 exhibits anti-inflammatory properties through downregulating the production of pro-inflammatory cytokines IL–1β and IL-6, while enhancing the degradation of von Willebrand factor (vWF) protein21. A recent study found that the oxylipin analogue CS585 prevented platelet activation and thrombus formation by activating prostaglandin E2 receptors without disrupting coagulation or increasing the risk of bleeding in a mouse model22. However, the correlation between oxylipins in hospitalised patients with SARS-COV-2 and their risk of thrombosis remains unexplored.
In this unprecedented study, we conducted a statistical analysis of the types, incidence rates, and mortality rates of thrombosis in patients with SARS-COV-2. Subsequently, 1731 hospitalised patients with SARS-COV-2 were categorised into mild, severe, and critical groups based on disease severity. They were also classified into thrombus and non-thrombus groups based on the presence or absence of thrombosis. Using these two grouping methods, we explored the differences in six lipid parameters. Additionally, employing LC–MS/MS methodology, the study analysed the differential oxidative lipid spectra in the SARS-COV-2 group compared to that of the healthy control group. Furthermore, the study investigated the differential oxidative lipid spectra between SARS-COV-2-positive patients with thrombosis and those without thrombosis. This study indicated that lipid mediators are essentially involved in the SARS-COV-2 infection and thrombi. Oxidative lipids metabolites, in particular, 20-hydroxyPGF2α and 14(15)-EpETE, may be useful in predicting SARS-COV-2-related thrombus risk. And these differential metabolites in patients with SARS-COV-2 with or without thrombus were predominantly clustered within the ARA and serotonergic synapses metabolism signaling pathway, which maybe valuable in revealing the mechanism of thrombosis caused by SARS-COV-2.
Methods
Study population
Between December 2022 and October 2024, a study conducted at Taikang Xianlin Drum Tower Hospital included 1731 adult individuals (≥ 18 years old) diagnosed with SARS-COV-2. The diagnosis was confirmed through a real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis of samples obtained from nasopharyngeal swabs. Patients with a history of thrombosis, those demonstrating signs of chronic thrombosis identified through Doppler ultrasound screening, pregnant women, patients who died within 24 h of admission, individuals who received therapeutic doses of anticoagulants (such as heparin, warfarin, or direct oral anticoagulants) or lipid-lowering medications like statins within the first four weeks of enrollment, as well as those who received the COVID-19 vaccine within four weeks prior to enrollment, were excluded from the study. The severity grading of pneumonia in clinical settings followed the guidelines outlined in the Clinical Classification of Pneumonia Severity established by the National Health Commission of China and Chinese Center for Disease Control23,24. This system categorises individuals as mild, severe, or critical, based on their condition. Mild included non-pneumonia and mild pneumonia cases. Severe was characterized by dyspnea, respiratory frequency ≥ 30/minute, blood oxygen saturation ≤ 93%, PaO2/FiO2 ratio < 300, and/or lung infiltrates > 50% within 24–48 h. Critical cases were those that exhibited respiratory failure, septic shock, and/or multiple organ dysfunction/failure. A healthy control group was also selected, comprising 300 age- and sex-matched individuals who underwent routine health examinations during the same period and were not infected with SARS-COV-2, excluding the hypertension, diabetes, cardiovascular disease and tumor.
Serum collection and lipid detection
Fasting peripheral blood samples were collected from enrolled patients during their initial hospitalization, prior to the initiation of any drug treatment. These samples were subsequently centrifuged at 3,500 rpm for 10 min at 4 °C to isolate the serum, followed by the addition of antioxidant BHT (Butylated Hydroxytoluene). Specifically 1 ul of the antioxidant BHT (100 mM in ethanol) per 1000 ul of serum was added. The samples were then stored at − 80 °C for future research. The six lipid parameters assessed included Total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A (apoA), and apolipoprotein B (apoB), which were analyzed using a Beckman AU5800 chemistry analyzer with the manufacturer’s reagents (Beckman AU5800, Germany). Demographic information and clinical characteristics were extracted from the hospital’s records system for medical and laboratory management purposes. Table 1 provides a summary of the demographic and clinical characteristics of all participants.
Sample pretreatment and extraction
Serum samples were removed from a − 80 °C freezer and thawed in an ice bath. All subsequent procedures were carried out in a cold environment. After thawing, the liquid samples were vortexed uniformly. Subsequently, 100µL of the sample was mixed with 200 µL of an internal standard extraction solution, composed of a 1:1 mixture of methanol (Merck, Germany) and acetonitrile (Merck, Germany). The mixture was vortexed for 5 min and then stored in a − 20 °C freezer for 30 min to precipitate proteins. Following this, the mixture was centrifuged at 12,000 rpm/min for 10 min at 4 °C to collect the supernatant. This process was repeated once, and the supernatants were then combined. For solid-phase extraction, an activated and equilibrated Poly-Sery MAX SPE column (60 mg/3 cc, ANPEL, Shanghai, PRC) was used. The sample was applied to the column, subjected to washing, elution, and collection of the eluate. The collected eluent was dried under vacuum and subsequently dissolved in 100 µL of a 1:1 methanol and water solution. After vortexing for 30 s, the resulting supernatant was prepared for further analysis using Ultra Performance Liquid Chromatography (UPLC) coupled with Tandem Mass Spectrometry (MS/MS).
LC–MS/MS analysis
The sample extracts were analyzed using a liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) system consisting of a UPLC (ExionLC AD, Sciex) and an MS (QTRAP® 6500+ System, Sciex). The UPLC analysis was conducted using a Waters ACQUITY UPLC HSS T3 C18 column (100 mm × 2.1 mm id, 1.8 μm). The solvent system included mobile phase A, a combination of acetonitrile and water (60/40, V/V) with 0.002% acetic acid (Merck, Germany), and mobile phase B, a mixture of acetonitrile and isopropanol (50/50, V/V) (Merck, Germany). The gradient elution program involved a transition from 0.1 to 30% B over 0–2.0 min, followed by a change to 50% B from 2.0 to 4.0 min, further increasing to 99% B from 4.0 to 5.5 min and maintaining this composition for 1.5 min, then decreasing to 0.1% B from 7.0 to 7.1 min and holding for 2.9 min. The flow rate was set at 0.4 ml/min, temperature at 40 °C, and injection volume at 10 µL. Linear ion trap (LIT) and triple quadrupole (QQQ) scans were acquired on a triple quadrupole-linear ion trap mass spectrometer (QTRAP), QTRAP® 6500 + LC–MS/MS System, equipped with an ESI Turbo Ion-Spray interface, operating in negative ion mode and controlled by Analyst 1.6.3 software (Sciex).The parameters for the ESI source operation included an ion source set at ESI- with a temperature of 550 °C, an ion spray voltage (IS) of − 4500 V, and a curtain gas (CUR) set at 35 psi. All extracted samples were prepared as a composite sample regarded as the quality control sample. A quality control sample was run every ten injections during the analytical process to ensure repeatability. Overlapping analysis of total ion chromatography of a quality control sample was used to ensure the repeatability of oxylipin extraction and detection. Figure S1 presents the stacking chart of the total ion chromatogram for the quality control samples, while Fig. S2 illustrates the coefficient of variation (CV) distribution chart of the QC samples. Quantification was conducted using the multiple reaction monitoring (MRM) mode of triple quadrupole mass spectrometry. Following the collection of mass spectrometry data from various samples, chromatographic peaks were integrated and quantitatively analyzed using a standard curve. Standard solutions (Merck, Germany) with concentrations ranging from 0.001 to 400 nmol/L were prepared to establish a correlation between each concentration and the corresponding chromatographic peak intensity. By plotting the concentration ratio of the external standard to the internal standard on the x-axis and the peak area ratio of the external standard to the internal standard on the y-axis, diverse standard curves were generated for different substances. The integrated peak area ratio of all detected samples was then used in the linear equation of the standard curve to determine the substance content present in the actual sample.
Statistical analysis
Continuous variables were reported as medians with interquartile range (IQR), and categorical variables were presented as numbers with corresponding percentages. Statistical analyses were conducted using SPSS software (version 23.0; SPSS Inc., Chicago, IL, USA) and GraphPad Prism 9 (GraphPad Software Inc., La Jolla, CA, USA). Qualitative data between the groups were compared using the chi-squared test. Similarly, the comparison of variables between two groups was performed using the Mann–Whitney U test. Statistical significance was determined at a threshold of P < 0.05. Mass spectrometry data underwent processing using Analyst 1.6.3 and MultiQuant 3.0.3 software. The chromatographic peaks for each metabolite in the different samples were integrated and corrected based on the retention time and peak details. Orthogonal partial least squares discriminant analysis (OPLS-DA) was used to assess the statistical differences between the groups. Additionally, in order to avoid overfitting, a permutation test (200 permutations) was performed. P values of oxylipin data were corrected for multiple testing by the false discovery rate (FDR) of Benjamini-Hochberg. Variable importance in projection (VIP) values from the OPLS-DA model were used for preliminary screening of different metabolites, considering the fold change values for further filtering. Metabolites were differentially selected based on the criteria: VIP > 1 with fold change ≥ 2, or VIP > 1 with fold change ≤ 0.5. More information is provided in the Supplementary Materials.
Results
Population demographics and clinical characteristics of hospitalised patients with SARS-COV-2
This study analysed 1731 hospitalised cases with SARS-COV-2 and categorised them into 1255 mild, 153 severe, and 323 critical cases. Male hospitalisations outnumbered female hospitalisations across all groups. More than half of the patients had underlying health conditions, with hypertension being the most prevalent. Fever and cough were the most frequently reported initial symptoms, with fewer reports of headache and diarrhoea.
Close association between thrombosis and SARS-COV-2 severity
As depicted in Fig. 1A, arterial thrombosis accounted for 0.6% (11/1731) and venous thrombosis accounted for 10.9% (188/1731). As shown in Fig. 1B, superficial vein thrombosis was the least common venous thrombosis at 0.3% (5/1731), followed by pulmonary embolism at 0.6% (10/1731) and deep vein thrombosis at 10.0% (173/1731). Figure 1C shows thrombosis rates of 7.6% (95/1255) in mild patients, 15.7% (24/153) in severe patients, and 24.8% (80/323) in critical patients, with significant intergroup differences (P < 0.05). Figure 1D illustrates that, in the non-thrombosis group, severe patients (severe and critical) accounted for 24.3% (372/1532), whereas in the thrombosis group, severe patients comprised 52.3% (104/199), showing a significant difference (P < 0.0001). Figure 1E shows that when all patients with SARS-COV-2 were categorised into survivors and non-survivors, the rate of thrombosis in the non-survivor group was 24.1% (59 of 245 patients), which was notably higher than the rate in the group of survivors at 9.4% (140/1486) (P < 0.0001). Figure 1F shows a mortality rate of 29.6% (59/199) in the SARS-COV-2 thrombosis group, which was notably higher than the rate of hospitalised patients without thrombosis at 12.1% (186/1532) (P < 0.0001).
Thrombosis incidence bar charts. (A) Proportion bar chart of arterial thrombosis versus venous thrombosis. (B) Proportion bar chart of superficial venous thrombosis, pulmonary embolism, and deep venous thrombosis. (C) Thrombosis incidence bar chart in hospitalized SARS-COV-2 patients across three groups (mild, severe, and critical). (D) Proportion bar chart of mild, severe, and critical cases in thrombotic and non-thrombotic groups. (E) Thrombosis incidence bar chart in overall mortality and survival groups. (F) Death rate bar chart in thrombotic and non-thrombotic groups. (Qualitative data comparison between groups was performed using the chi-square test. ****P < 0.0001, **P < 0.01, *P < 0.05, and ns represents no significance.)
Close association between lipid levels and thrombosis
To investigate the correlation between lipid levels and thrombosis, the participants were categorised based on the occurrence of thrombosis. Individuals in the thrombosis group exhibited decreased serum levels of TC, HDL-C, LDL-C, ApoA, and ApoB compared with those in the non-thrombosis group, with no significant difference in TG levels (Fig. 2). In the mild SARS-COV-2 category, thrombosis cases displayed reduced serum levels of TC, HDL-C, LDL-C, ApoA, and ApoB compared with those of non-thrombosis cases, particularly ApoA levels (P < 0.0001), whereas TG levels showed no notable difference. However, no significant differences were found in lipid levels between patients with and without thrombosis in the severe and critical SARS-COV-2 groups (Fig. 3). More detailed information on the lipid levels can be found in Table S1.
Lipid profile differences cloud plots between thrombotic and non-thrombotic groups in different disease grades (mild, severe, critical). Conducted using Mann–Whitney U test for quantitative data differences between the two groups. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, and ns represents no significance.
Lipid levels and their close association with SARS-COV-2 severity
This investigation was conducted to explore the correlation between SARS-COV-2 severity and blood lipid levels. Hospitalised patients with SARS-COV-2 were divided into mild, severe, and critical categories. As shown in Fig. 4, the serum levels of TC, HDL-C, LDL-C, and ApoA were significantly lower in the three severity groups than in the healthy control group. The critical group did not show a significant difference in ApoB levels compared to the severe group, but there was a significant contrast with the mild group (P < 0.0001). Serum TG levels did not differ significantly among the four groups.
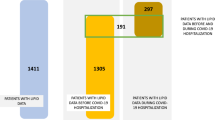
Serum oxylipin metabolomics analysis of patients with SARS-COV-2
In this study, 48 serum samples from patients with SARS-COV-2 and 24 serum samples from the normal control group were randomly selected to detect oxylipin metabolites. These serum samples were analyzed using the LC–MS/MS platform to identify a total of 115 oxidized lipid metabolites. The information on standard curve linear equations, retention times (RT), m/z values, and fragmentation patterns for these metabolites can be found in Table S2. Additionally, Fig. S3 illustrates the individual extracted ion chromatograms (EICs) from a healthy individual, while Figs. S4 and S5 depict the EICs of oxidized lipids extracted from serum samples of SARS-CoV-2-positive patients with and without thrombosis. Utilising the OPLS-DA technique, the VIP value was computed, and distinctions were deemed noteworthy if VIP exceeded 1 and the fold change was either ≥ 2 or ≤ 0.5. Figure S6 presents the OPLS-DA verification diagram for SARS-CoV-2 patients and healthy controls. As illustrated in Fig. 5A and B, differences in oxylipin metabolites were observed between the two groups. Eleven significantly upregulated and one significantly downregulated oxylipin metabolites were identified. As shown in Fig. 5C, the main substance category of the different metabolites was ARA. Figure 5D shows the original intensities of the 12 different metabolites, and detailed information is listed in Table S3. Based on Table S3 and Fig. 5E, the SARS-COV-2 group exhibited the greatest increase in the oxylipin, 20-hydroxyPGF2α, compared to that in the normal control group. Analysis of the pathways enriched with the differential metabolites revealed that these 12 metabolites were predominantly clustered within the ARA metabolism pathway (Fig. 5F).
Serum oxylipin spectrum analysis of SARS-COV-2 group (n = 48) vs. Healthy Control Group (n = 24). (A) The OPLS-DA scores plot comparing SARS-COV-2 and HC groups. (B) The volcano plot illustrating the disparities between SARS-COV-2 and HC groups. Each data point in the volcano plot signifies a metabolite, where red points denote significantly upregulated metabolites (VIP > 1, Fold change ≥ 2), green points signify downregulated metabolites (VIP > 1, Fold change ≤ 0.5), and gray points indicate metabolites with no significant differences. The dot size corresponds to the VIP value. (C) Heatmap showing 12 oxylipins with distinct expression patterns in SARS-COV-2 and HC groups. The color scale reflects standardized expression levels, with red hues indicating higher expression and green hues suggesting lower expression. (D) Raw intensity data of the 12 oxylipins with altered expression in SARS-COV-2 and HC groups. (E) Bar graph demonstrating the fold changes in metabolite levels. Red bars indicate upregulated metabolites (VIP > 1, Fold change ≥ 2), while green bars represent downregulated metabolites (VIP > 1, Fold change ≤ 0.5). (F) Pathway analysis based on KEGG enrichment for the 12 oxylipins with differential expression in SARS-COV-2 and HC groups. The arrow thickness corresponds to the VIP value of the respective oxylipin, with red circles representing upregulated oxylipins and green circles indicating downregulated oxylipins. Circle size indicates the magnitude of Log2 Fold change.
Serum oxylipin metabolomics analysis between SARS-COV-2 thrombosis and non-thrombosis groups
To investigate whether there were differences in lipid levels between the SARS-COV-2 thrombosis and non-thrombosis groups, 24 serum samples from the SARS-COV-2 thrombosis group and 24 serum samples from the SARS-COV-2 non-thrombosis group were randomly selected for the detection of oxylipin metabolites. Using the LC–MS/MS platform, a total of 115 oxidized lipid metabolites were identified. Table S4 contains detailed information on the retention times (RT), m/z values, fragmentation patterns and standard curve linear equations for each of these metabolites. Using the OPLS-DA technique, VIP values were calculated, revealing notable differences when VIP values were > 1 and the fold change was ≥ 2 or ≤ 0.5. Figure S7 illustrates the OPLS-DA verification diagram for SARS-CoV-2 patients with and without thrombosis. As shown in Fig. 6A and B, differences in oxylipin metabolites were observed between the two groups. Seven significantly upregulated (SARS-COV-2 thrombosis group vs. non-thrombosis group) and twenty-one significantly downregulated (SARS-COV-2 thrombosis group vs. non-thrombosis group) oxylipin metabolites were identified, with 87 showing no significant difference. The seven upregulated oxylipins in the COVID-19 thrombotic group include 20-hydroxyPGF2 α, 6-keto-PGF1 α, PGF3 α, PDX, LXB4, PGD2, and 11-keto-TXB2. As shown in Fig. 6C, the main substance categories of different metabolites were α-linolenic acid (ALA), ARA, dihomo-γ-linolenic Acid (DGLA), DHA, eicosapentaenoic acid (EPA), and linoleic acid (LA). Figure 6D shows the original intensities of the specific 28 different metabolites, and detailed information is provided in Table S5. Based on the results presented in Fig. 6E and Table S5, the SARS-COV-2 thrombosis group exhibited the most significant upregulation of 20-hydroxyPGF2α among oxylipins, whereas 14(15)-EpETE showed the most significant downregulation compared to that of the non-thrombosis group. Enrichment analysis using KEGG25,26 revealed that differential oxidized lipid metabolites mainly concentrated in the ARA and serotonergic synapses metabolism signaling pathway (Fig. 6F).
Serum oxylipin spectrum analysis of SARS-COV-2 thrombotic (n = 24) and non-thrombotic (n = 24) groups. (A) The OPLS-DA scores plot comparing No thrombosis with Thrombosis. (B) The volcano plot illustrating the comparison between No thrombosis and Thrombosis. Each data point in the volcano plot corresponds to a metabolite, where red points indicate upregulated metabolites (VIP > 1, Fold change ≥ 2), green points indicate downregulated metabolites (VIP > 1, Fold change ≤ 0.5), and gray points signify metabolites with no significant difference. The size of the data points reflects the VIP value. (C) The heatmap showing the expression levels of 28 oxylipins that are differentially regulated in the comparison between No thrombosis and Thrombosis. Redder colors indicate higher expression levels, while greener colors indicate lower expression levels. (D) The raw intensity values of 28 oxylipins that are differentially expressed in the comparison between No thrombosis and Thrombosis. (E) Bar graph displaying the fold change of differentially expressed metabolites. Red bars indicate upregulated metabolites (VIP > 1, Fold change ≥ 2), while green bars represent downregulated metabolites (VIP > 1, Fold change ≤ 0.5). (F) Analysis of KEGG pathway enrichment for the 28 differentially expressed oxylipins in the comparison between No thrombosis and Thrombosis. Bubble size indicates the number of metabolites enriched in the pathway, whereas bubble color represents the significance level. P value reflect the degree of enrichment significance.
Discussion
This study found that thrombosis formation was frequently associated with the course of SARS-COV-2 hospitalisation, with VTE being more common than arterial thrombosis, and a higher incidence of deep vein thrombosis. As the disease severity increased, the thrombosis rate gradually increased, reaching its highest value in the critical group. Furthermore, a thrombotic state was present throughout the course of SARS-COV-2 hospitalisation, regardless of the severity. In the SARS-COV-2 thrombosis group, severe patients (severe + critical) accounted for 52.3%, whereas in the non-thrombosis group, severe patients accounted for 24.3%. The study found a significantly higher mortality rate in SARS-COV-2-positive patients with thrombosis compared to those without thrombosis, aligning with findings from meta-analyses that included 8,271 individuals27. Additionally, this study investigated the differential oxidative lipid spectra between SARS-COV-2-positive patients with thrombosis and those without thrombosis, and indicated that lipid mediators are essentially involved in the SARS-COV-2 infection and thrombi. Oxidative lipids metabolites, in particular, 20-hydroxyPGF2α and 14(15)-EpETE, may be useful in predicting SARS-COV-2-related thrombus risk. These differential metabolites differential metabolites in patients with SARS-COV-2 with or without thrombus were predominantly clustered within the ARA metabolism and serotonergic synapses, may be valuable in revealing the mechanism of thrombosis caused by SARS-COV-2.
SARS-CoV-2 is encased in an envelope with a lipid bilayer. Lipids are crucial cellular components that serve a range of biological functions, from being foundational building blocks to acting as signalling molecules and energy storage. During viral infection of host cells, the virus interferes with lipid synthesis and signal transduction in host cells to control its entry and replication28. Lipids are essential for viral infection, mediating membrane fusion, viral replication, endocytosis, and the virus’s extracellular effects29. Prior research has noted a considerable build-up of fats in cells infected by SARS-CoV-2, both in laboratory settings and in the respiratory organs of individuals, showing a correlation with the N protein30,31. Toelzer et al. discovered three closely associated LA pockets within the receptor-binding domain of SARS-CoV-2. Further research has confirmed the existence of these LA-binding sites in both SARS-CoV and MERS-CoV32. Moreover, patients with SARS-COV-2 exhibit elevated levels of inflammatory mediators and oxidative stress (OS). In addition to cytokines, oxylipins, essential inflammatory mediators, are intricately linked to SARS-COV-2. Through the cyclooxygenase (COX) pathway, ARA produces prostaglandins and LA produces hydroxyoctadecadienoic acids (HODEs), both of which have dual pro-inflammatory activities. During the early phases of the innate immune response, these compounds prompt the production of pro-inflammatory chemicals and proteins, collaborating with the pro-inflammatory leukotrienes generated by ARA via the LOX pathway to trigger bronchoconstriction, mucin synthesis, and enhanced vascular permeability33. In subsequent immune responses, HODEs, PGD2, and PGE2 exhibit anti-inflammatory effects, especially in the lungs34,35. Regidor et al. discovered that individuals with SARS-COV-2 exhibited increased concentrations of pro-inflammatory and pro-thrombotic lipid mediators in their bloodstream compared with those of individuals without SARS-COV-2. These mediators include prostaglandins, thromboxanes, and leukotrienes36. ARA and its various forms, including thromboxane A2, have platelet-activating properties37. Interestingly, lipids play a crucial role in the initiation and progression of SARS-CoV-2 infection. The findings of this study demonstrated that in comparison to a group of healthy individuals, the levels of TC, HDL-C, LDL-C, and ApoA in the serum were notably reduced across all three severity levels, with no significant difference in ApoB levels between critically ill and severely ill patients, but a marked contrast between critically ill and mildly ill patients. These findings provide strong evidence of a correlation between the severity of SARS-COV-2 and blood lipid levels, consistent with existing literature38,39.
Oxylipins are important bioactive mediators produced during infections that regulate OS and inflammatory responses. A total of 115 oxylipins derived from EPA, ARA, DHA, DGLA, LA, ALA, and γ-linolenic acid were identified in the SARS-COV-2 group in comparison to the normal control group. Notably, 20-hydroxyPGF2α was the most significantly upregulated oxylipin, whereas 15-keto-PGE2 showing the most significant downregulation. Half of the differentially expressed oxylipins belonged to the prostaglandins (PGs) family. PGs are oxygen-containing metabolites produced by the action of COX on ARA, with PGF2α being one of the most stable PGs40. Moreover, it is a potent vasoconstrictor and smooth muscle stimulant that is involved in acute and chronic inflammation. Conversely, PGE2 is primarily a vasodilator in small arteries, capillaries, and veins41. The relevance of the PGs family to SARS-COV-2 pathophysiology has been reported42. Misato Kida et al. discovered a significant rise in the metabolites of pro-inflammatory lipid mediators PGE2, TXA2, as well as PGF2α among individuals with SARS-COV-2 in comparison to healthy controls43. In animal and cellular models of acute ischemic stroke, PGE2 has been shown to exert pro-inflammatory effects, while the active metabolite of PGD2, 15d-PGJ2, exhibits anti-inflammatory properties, thereby enhancing post-infarct cerebral blood flow perfusion and reducing neuronal damage. Furthermore, 15d-PGJ2 can inhibit the production of reactive oxygen species and prevent vascular endothelial cell apoptosis44. Numerous COX-related lipid molecules can lead to endothelial dysfunction, contributing to hypertension45. Liu Yao et al. found that the inhibition of mTORC1 activity in endothelial cells reduces the production of prostaglandin E2 and induces hypertension by decreasing the expression of YAP-mediated COX-2/mPGES-146. Notably, 15-keto-PGE2 is a product of the oxidation of PGE2 catalysed by 15-hydroxyprostaglandin dehydrogenase and has recently been shown to exhibit anti-inflammatory properties47. Protectin DX (PDX) is a derivative of DHA and can alleviate chronic inflammation. It inhibits viral replication by targeting influenza virus RNA metabolism48. LXA4 and LXB4 are important mediators of inflammation-resolving responses, as they inhibit neutrophil recruitment, stimulate vasodilation, and promote efferocytosis49. The increased presence of pro-inflammatory lipids and the decreased presence of anti-inflammatory lipids following SARS-COV-2 infection are significant factors that may contribute to the inflammation observed in individuals.
In the SARS-COV-2 thrombosis and non-thrombosis groups, 115 oxylipins were detected, 28 of which showed significant differences. Among these 28 oxylipins with significant differences, 20-hydroxyPGF2α, LXB4 and 6-keto-PGF1α showed significant differences between the SARS-COV-2 non-thrombosis group and the normal control group. This indicates that these oxylipins not only exhibited significant abnormalities in overall levels in patients with SARS-COV-2 but also became more pronounced when thrombosis occurred in patients with SARS-COV-2. This suggests a close association between oxylipins and SARS-COV-2 infection and thrombosis. COVID-19 predisposes patients to thrombotic and thromboembolic events, owing to excessive inflammation, endothelial cell activation and injury, platelet activation and hypercoagulability50. Recent studies have increasingly demonstrated that oxidized lipids can influence the occurrence and progression of cardiovascular disease. Aleš Kvasnička et al. described a trend in PUFA esterified in phosphatidylcholines (PC) that were systematically increased in surviving patients with heart failure. This trend in low-abundant and rarely identified PUFA phosphatidylcholines (mainly very long chain PUFA containing PC such as PC 42:10 or PC 40:9 containing FA 22:6, FA 20:5 and FA 20:4) suggests candidate biomarkers51. AA, dihomo-γ-linolenic acid (DHGLA), and EPA are all classified as eicosapolyunsaturated fatty acids. In the body, these fatty acids can be further metabolized and converted into a series of compounds collectively known as eicosanoids, which include PG, TX, leukotrienes (LT), and lipoxins (LX). Cardiovascular disease risk factors, such as diabetes, obesity, smoking, are variably associated with elevated levels of PGF2 metabolites, interleukin-6 (IL-6), and C-reactive protein in bodily fluids52. Atherosclerosis is a significant risk factor for cardiovascular and cerebrovascular diseases, with matrix metalloproteinases (MMPs) in vascular smooth muscle cells (VSMCs) playing a crucial role in the vulnerability and rupture of atherosclerotic plaques. LTB4 can increase MMP-2 levels in VSMCs in a dose-dependent manner by binding to the LTB4 receptor53.
In this study, the downregulated oxylipins included hydroxyeicosapentaenoic acids and EPETEs derived from EPA, and 16(17)-EpDPE and 7,8-EpDPE derived from DHA. Studies have indicated that consuming more omega-3 polyunsaturated fatty acids, particularly EPA and DHA, can lower the prevalence of chronic illnesses marked by increased inflammation, such as heart diseases54. In addition to their antioxidant and anti-inflammatory effects, these compounds are believed to inhibit prostaglandin production from endogenous ARA, regulate platelet homeostasis, and reduce thrombosis risk55. Evidence indicates that EPA and DHA exert beneficial effects on cardiovascular disease by reprogramming triglyceride-rich lipoprotein (TRL) metabolism, decreasing inflammatory mediators such as cytokines and leukotrienes, and modulating cell adhesion molecules56,57. Yajin Liu et al. demonstrated that the metabolites of EPA, specifically 18-HEPE and 17,18-EEQ, reverse the activation of endothelial cells and the adhesion of monocytes by inhibiting the NF-κB pathway, thereby exerting an anti-inflammatory effect and mitigating the development of atherosclerosis58. Additionally, 17,18-EEQ and 5-HEPE have been identified as key metabolites of eicosanoids that can suppress the expression of inflammatory factors in macrophages while activating the JNK pathway in response to palmitic acid59. Moreover, 12-HEPE has been shown to activate PPARγ, inhibiting the transformation of macrophages into foam cells, which alleviates carotid plaque atherosclerosis in mice and improves their pulsation and resistance indices60. EPA and DHA are precursors to structurally distinct families of signaling molecules known as specialized pro-resolving lipid mediators (SPMs), which include resolvins (Rvs), protectins, and maresins (MaRs)61. Persistent inflammation is a significant aggravating factor in various cardiovascular diseases, such as atherosclerosis, aneurysms, injury/reperfusion, and thrombosis. The resolution of inflammation is a constructive process orchestrated by SPMs. Evidence indicates that these mediators can inhibit acute inflammatory signals, promote healing, and facilitate the restoration of homeostasis through their anti-inflammatory and pro-resolving activities62. Alnouri MW et al. found that SPMs derived from DHA, including RvD, protectins, and MaRs, exert their anti-inflammatory and pro-resolving effects through the conformational regulation of EP4 receptors63. Similarly, Díaz Del Campo LS et al. demonstrated that RvD2 influences cardiovascular damage in the context of angiotensin II-induced hypertension. Specifically, RvD2 enhances the availability of vascular protective factors, modifies the spectrum of SPMs, reduces cardiovascular fibrosis, and promotes the infiltration of pro-catabolic macrophages64. Numerous prospective cohort studies have confirmed that elevated levels of n-3 PUFAs, specifically EPA and DHA, in the bloodstream of healthy individuals are negatively correlated with all-cause mortality and cardiovascular-related mortality. Manson JE and colleagues investigated the primary preventive effects of n-3 PUFAs on atherosclerotic cardiovascular disease (ASCVD). Their study included 25,871 adults with a median follow-up period of 5.3 years, revealing that n-3 PUFAs can reduce the overall risk of myocardial infarction by 28%65. In a recent study, Mourikis P and colleagues demonstrated that EPA dose-dependently reduced platelet adhesion, degranulation, and aggregation in vitro. Furthermore, oral administration of EPA has been shown to inhibit arterial thrombosis in wild-type mice prior to thrombus formation66. Federica Laguzzi et al. found a significant interaction between biomarkers indicative of low EPA/DHA intake and family history, while no such interaction was observed for other PUFAs. This novel finding suggests the necessity of emphasizing the benefits of consuming oily fish for individuals with a family history of cardiovascular disease67. Collectively, these findings underscore the potential therapeutic applications of EPA and DHA-derived metabolites in cardiovascular disease management. Additionally, targeting key enzymes involved in the biosynthesis pathway of oxidized lipids represents a significant research avenue in this domain. Aspirin’s ability to inhibit cyclooxygenase (COX) regulates the production of oxidized lipids, such as thromboxane A2 (TXA2) and prostacyclin (PGI2), thereby exerting anti-inflammatory and antithrombotic effects, which may help prevent ischemic cerebrovascular disease68.
COVID-19 can elevate the risk of thrombosis through various mechanisms, with thrombosis serving as a direct cause of numerous cardiovascular diseases. By triggering intense inflammatory responses, damaging vascular endothelium, and inducing a hypercoagulable state, COVID-19 infection fosters an environment conducive to thrombus formation within the body. These clots obstruct blood vessels in the heart, brain, lungs, and other parts of the body, directly leading to severe cardiovascular conditions such as myocardial infarction, stroke, and pulmonary embolism69,70. Recent research has affirmed the crucial role of PUFAs and their oxidative metabolites in assessing and intervening in cardiovascular risks. With advancements in metabolomics, artificial intelligence, and molecular biology, our research group’s future endeavors will concentrate on elucidating the mechanisms between oxidized lipids and cardiovascular diseases, with the aim of facilitating precise cardiovascular prevention and treatment strategies targeting the lipid oxidation profile, ultimately enhancing the prognosis and quality of life for patients with cardiovascular diseases.
Study strengths and limitations
This study had several unique advantages. Firstly, the data used in this study were obtained from a diverse group of patients with SARS-COV-2, some with thrombosis and others without thrombosis, leading to representative results. Secondly, this study examined the connection between lipid mediators and SARS-COV-2, specifically investigating the various types of oxylipins identified in this study. Finally, this study provides valuable insights into the pathogenesis of SARS-CoV-2 infection and thrombotic complications, offering potential avenues for preventing SARS-COV-2 thrombosis and reducing mortality.
However, this study has several limitations. Firstly, the sample size is relatively small and is derived from a single medical center, which may introduce geographic and institutional selection biases, thereby affecting the broader applicability of the research findings. Secondly, concerning the selection of serum or plasma samples, literature indicates that serum preparation activates platelets, leading to artificial oxylipin production that could bias the results. Nevertheless, in this study, serum samples were more readily obtainable, and we acknowledge that rapid separation of plasma can reduce in vitro oxidation, thereby providing a more accurate reflection of oxidized lipid levels in the body. Thirdly, this study did not conduct a comprehensive investigation into the temporal changes of lipid mediators during the progression of viral infection in patients. Fourthly, while selecting healthy controls, we considered age, gender, BMI, and comorbidities; however, we did not specify confounding factors such as medication use and lifestyle. Fifth, the LC–MS method was not validated for extraction and recovery in this study, which may impact the measurement accuracy. Therefore, conducting studies with larger sample sizes across multiple centers in the future is essential to validate the findings of this research. Additionally, we plan to undertake longitudinal studies to capture temporal dynamics and accurately reflect disease status. Furthermore, for the selection of healthy controls, we will incorporate various confounding factors that may influence disease-specific lipidomics results in future studies. We will also strive to collect plasma samples for the investigation of oxidized lipids and enhance the LC–MS methodology, including the addition of extraction and recovery validation.
Conclusions
Overall, this study indicates a close association between lipid mediators, including oxidative lipids, and both SARS-COV-2 and thrombi. More importantly, oxidative lipids metabolites, in particular, 20-hydroxy and 14(15)-EpETE, could potentially serve as predictive indicators of thrombosis risk. And these differential metabolites in patients with SARS-COV-2 with or without thrombus were predominantly clustered within the ARA and serotonergic synapses metabolism signaling pathway, which maybe valuable in revealing the mechanism of thrombosis caused by SARS-COV-2. Healthcare providers should regularly assess the lipid profiles of SARS-COV-2-positive individuals in the clinical setting. Nonetheless, it is crucial to conduct extensive long-term prospective cohort studies to better elucidate the diagnostic significance of these innovative markers for the early identification of SARS-COV-2-associated thrombosis. In the post-COVID-19 era, our research group aims to enhance precise cardiovascular prevention and treatment by investigating the mechanisms that connect oxidized lipids to cardiovascular diseases.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).
Gu, S. X. et al. Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 18, 194–209 (2021).
The Lancet, H. COVID-19 coagulopathy: An evolving story. Lancet Haematol. 7, e425 (2020).
Nopp, S. et al. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res. Pract. Thromb. Haemost. 4, 1178–1191 (2020).
Deitelzweig, S. et al. Thrombotic and bleeding events, mortality, and anticoagulant use among 546,656 hospitalized patients with COVID-19 in the united states: A retrospective cohort study. J. Thromb. Thrombolysis. 53, 766–776 (2022).
Zhang, L. et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: Prevalence, risk factors, and outcome. Circulation 142, 114–128 (2020).
Chen, S. et al. DVT incidence and risk factors in critically ill patients with COVID-19. J. Thromb. Thrombolysis. 51, 33–39 (2021).
Fox, S. E. et al. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from new Orleans. Lancet Respir Med. 8, 681–686 (2020).
Ackermann, M. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 383, 120–128 (2020).
Carsana, L. et al. Pulmonary post-mortem findings in a series of COVID-19 cases from Northern Italy: A two-centre descriptive study. Lancet Infect. Dis. 20, 1135–1140 (2020).
Connors, J. M. & Levy, J. H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 135, 2033–2040 (2020).
Mehra, M. R. et al. Cardiovascular disease, drug therapy, and mortality in Covid-19. N. Engl. J. Med. 382, e102 (2020).
Guo, T. et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5, 811–818 (2020).
Inciardi, R. M. et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5, 819–824 (2020).
Rapkiewicz, A. V. et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 24, 100434 (2020).
Ding, W. Y. et al. Relationship between lipoproteins, thrombosis, and atrial fibrillation. Cardiovasc. Res. 118, 716–731 (2022).
Nayeem, M. A. Role of Oxylipins in cardiovascular diseases. Acta Pharmacol. Sin. 39, 1142–1154 (2018).
Yeung, J. et al. Omega-6 DPA and its 12-lipoxygenase-oxidized lipids regulate platelet reactivity in a nongenomic PPARalpha-dependent manner. Blood Adv. 4, 4522–4537 (2020).
Cummings, H. E. et al. Cutting edge: leukotriene C4 activates mouse platelets in plasma exclusively through the type 2 Cysteinyl leukotriene receptor. J. Immunol. 191, 5807–5810 (2013).
Arehart, E. et al. Acceleration of cardiovascular disease by a dysfunctional Prostacyclin receptor mutation: Potential implications for cyclooxygenase-2 Inhibition. Circ. Res. 102, 986–993 (2008).
Veyradier, A. et al. Improvement of von Willebrand factor proteolysis after prostacyclin infusion in severe pulmonary arterial hypertension. Circulation 102, 2460–2462 (2000).
Stanger, L. et al. The oxylipin analog CS585 prevents platelet activation and thrombosis through activation of the prostacyclin receptor. Blood 142, 1556–1569 (2023).
Wu, Z. & McGoogan, J. M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323, 1239–1242 (2020).
The Novel Coronavirus Pneumonia Emergency Response Epidemiology T. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly. 2, 113–122 (2020).
Kanehisa, M. et al. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D77 (2025).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Malas, M. B. et al. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine 29, 100639 (2020).
Abu-Farha, M. et al. The role of lipid metabolism in COVID-19 virus infection and as a drug target. Int. J. Mol. Sci. 21, 3544 (2020).
Casari, I. et al. Dissecting lipid metabolism alterations in SARS-CoV-2. Prog. Lipid Res. 82, 101092 (2021).
Grootemaat, A. E. et al. Lipid and nucleocapsid N-Protein accumulation in COVID-19 patient lung and infected cells. Microbiol. Spectr. 10, e0127121 (2022).
Nardacci, R. et al. Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis. Cell. Death Dis. 12, 263 (2021).
Toelzer, C. et al. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science 370, 725–730 (2020).
Vangaveti, V., Baune, B. T. & Kennedy, R. L. Hydroxyoctadecadienoic acids: Novel regulators of macrophage differentiation and atherogenesis. Ther. Adv. Endocrinol. Metab. 1, 51–60 (2010).
Szczuko, M. et al. Lipoxins, RevD1 and 9, 13 HODE as the most important derivatives after an early incident of ischemic stroke. Sci. Rep. 10, 12849 (2020).
Dennis, E. A. & Norris, P. C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 15, 511–523 (2015).
Regidor, P. A. et al. Acute severe SARS COVID-19 patients produce pro-resolving lipids mediators and eicosanoids. Eur. Rev. Med. Pharmacol. Sci. 25, 6782–6796 (2021).
Vargaftig, B. B. & Zirinis, P. Platelet aggregation induced by arachidonic acid is accompanied by release of potential inflammatory mediators distinct from PGE2 and PGF2. Nat. New. Biol. 244, 114–116 (1973).
Song, J. W. et al. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell. Metab. 32, 188–202e5 (2020).
Wu, D. et al. Plasma metabolomic and lipidomic alterations associated with COVID-19. Natl. Sci. Rev. 7, 1157–1168 (2020).
Hirata, T. & Narumiya, S. Prostanoid receptors. Chem. Rev. 111, 6209–6230 (2011).
Basu, S. Novel cyclooxygenase-catalyzed bioactive prostaglandin F2α from physiology to new principles in inflammation. Med. Res. Rev. 27, 435–468 (2007).
Yalcin Kehribar, D. et al. The assessment of the serum levels of TWEAK and prostaglandin F2alpha in COVID-19. Turk. J. Med. Sci. 50, 1786–1791 (2020).
Kida, M. et al. Urinary lipid profile of patients with coronavirus diseases 2019. Front. Med. (Lausanne). 9, 941563 (2022).
Yang, C. C. et al. Induction of heme oxygenase-1 by 15d-prostaglandin J(2) mediated via a ROS-dependent Sp1 and AP-1 cascade suppresses lipopolysaccharide-triggered interleukin-6 expression in mouse brain microvascular endothelial cells. Antioxid. (Basel) 11, 719 (2022).
Matsumoto, T. et al. Constrictor prostanoids and uridine adenosine tetraphosphate: Vascular mediators and therapeutic targets in hypertension and diabetes. Br. J. Pharmacol. 172, 3980–4001 (2015).
Yao, L. et al. Regulation of YAP by mammalian target of rapamycin complex 1 in endothelial cells controls blood pressure through COX-2/mPGES-1/PGE(2) cascade. Hypertension 74, 936–946 (2019).
Lee, E. J. et al. 15-Keto prostaglandin E(2) suppresses STAT3 signaling and inhibits breast cancer cell growth and progression. Redox Biol. 23, 101175 (2019).
Lagarde, M., Guichardant, M. & Bernoud-Hubac, N. Anti-inflammatory and anti-virus potential of poxytrins, especially protectin DX. Biochimie 179, 281–284 (2020).
Serhan, C. N., Chiang, N. & Van Dyke, T. E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 (2008).
Gorog, D. A. et al. Current and novel biomarkers of thrombotic risk in COVID-19: A consensus statement from the international COVID-19 thrombosis biomarkers colloquium. Nat. Rev. Cardiol. 19, 475–495 (2022).
Kvasnicka, A. et al. Long-chain polyunsaturated fatty acid-containing phosphatidylcholines predict survival rate in patients after heart failure. Heliyon 10, e39979 (2024).
Helmersson, J. et al. Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation 109, 1729–1734 (2004).
Seo, K. W. et al. Participation of 5-lipoxygenase-derived LTB(4) in 4-hydroxynonenal-enhanced MMP-2 production in vascular smooth muscle cells. Atherosclerosis 208, 56–61 (2010).
Ruscica, M. et al. Omega-3 and cardiovascular prevention—Is this still a choice? Pharmacol. Res. 182, 106342 (2022).
Reiner, M. F. et al. Omega-3 fatty acids and markers of thrombosis in patients with atrial fibrillation. Nutrients 16, 178 (2024).
Shibabaw, T. Omega-3 polyunsaturated fatty acids: Anti-inflammatory and anti-hypertriglyceridemia mechanisms in cardiovascular disease. Mol. Cell. Biochem. 476, 993–1003 (2021).
Mason, R. P., Libby, P. & Bhatt, D. L. Emerging mechanisms of cardiovascular protection for the Omega-3 fatty acid eicosapentaenoic acid. Arterioscler. Thromb. Vasc. Biol. 40, 1135–1147 (2020).
Liu, Y. et al. Metabolic profiling of murine plasma reveals eicosapentaenoic acid metabolites protecting against endothelial activation and atherosclerosis. Br. J. Pharmacol. 175, 1190–1204 (2018).
Wang, C. et al. Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. Br. J. Pharmacol. 174, 2358–2372 (2017).
Nagatake, T. et al. 12-Hydroxyeicosapentaenoic acid inhibits foam cell formation and ameliorates high-fat diet-induced pathology of atherosclerosis in mice. Sci. Rep. 11, 10426 (2021).
Dyall, S. C. et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: recent advances in the understanding of their biosynthesis, structures, and functions. Prog Lipid Res. 86, 101165 (2022).
Kim, A. S. & Conte, M. S. Specialized pro-resolving lipid mediators in cardiovascular disease, diagnosis, and therapy. Adv. Drug Deliv. Rev. 159, 170–179 (2020).
Alnouri, M. W. et al. SPMs exert anti-inflammatory and pro-resolving effects through positive allosteric modulation of the prostaglandin EP4 receptor. Proc. Natl. Acad. Sci. U S A. 121, e2407130121 (2024).
Diaz Del Campo, L. S. et al. Resolvin D2 attenuates cardiovascular damage in angiotensin II-induced hypertension. Hypertension 80, 84–96 (2023).
Manson, J. E. et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 380, 23–32 (2019).
Mourikis, P. et al. Icosapent ethyl reduces arterial thrombosis by inhibition of cyclooxygenase-1-induced platelet reactivity. Sci. Transl. Med. 17, eado0610 (2025).
Laguzzi, F. et al. Role of polyunsaturated fat in modifying cardiovascular risk associated with family history of cardiovascular disease: Pooled de Novo results from 15 observational studies. Circulation 149, 305–316 (2024).
Johnston, S. C. et al. Clopidogrel and aspirin in acute ischemic stroke and High-Risk TIA. N. Engl. J. Med. 379, 215–225 (2018).
Knight, R. et al. Association of COVID-19 with major arterial and venous thrombotic diseases: A Population-Wide cohort study of 48 million adults in England and Wales. Circulation 146, 892–906 (2022).
Whiteley, W. & Wood, A. Risk of arterial and venous thromboses after COVID-19. Lancet Infect. Dis. 22, 1093–1094 (2022).
Funding
This study was supported by National Natural Science Foundation of China (82073367), Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (2022-LCYJ-PY-20), National Key Research and Development Program of China (2023YFC2415000).
Author information
Authors and Affiliations
Contributions
D.B.P. and M.X. contributed to the conception and design of the study. M.X. and F.C. performed the experiments. D.B.P. collected the study data and wrote the paper. H.X.S. helped with the experiments and statistical analysis of the study. All authors read and approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Nanjing Drum Tower Hospital affiliated with Nanjing University Medical School (Project Number: 2022-360-03) and all experiments were performed in accordance with approved clinical trial protocols and regulations. All the patients have been informed and signed informed consent before the experiments.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Peng, D., Chen, F., Sun, H. et al. Profiling of lipid mediators and oxylipins in SARS-CoV-2 infection associated thrombosis. Sci Rep 15, 31904 (2025). https://doi.org/10.1038/s41598-025-17722-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17722-7