Abstract
Acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) is the most common subtype of acute encephalopathy in Japan and is difficult to differentiate from prolonged febrile seizures (PFSs). This study aimed to explore the capability of machine learning to differentiate AESD from PFSs on the basis of early electroencephalogram (EEG) analyses. Sixty one children with AESD (n = 20) or PFS (n = 41) were included. Digital EEG data with bipolar montage collected within 48 h (1–48 h) after seizure onset were analyzed using absolute power spectrum (APS) and phase lag index (PLI) values in each EEG frequency band. The APS values in the theta, alpha, beta, and gamma bands were lower for AESD than those for PFS. By contrast, the mean PLI values for all frequency bands were higher for AESD than for PFS. Machine learning analysis revealed that the APS value in the beta bands provided the highest differentiation accuracy and positive predictive value for AESD (68.8%). The mean APS values across all electrodes in the beta band may be a useful tool for differentiating between early-phase AESD and PFS. This study demonstrates the potential for early automated diagnosis of AESD and PFS using EEG analysis.
Similar content being viewed by others
Introduction
Acute encephalopathy can occur related to any cause including metabolic, toxic or traumatic1. Acute encephalopathy with biphasic seizures and late reduced diffusion (AESD), a subtype of acute infection associated encephalopathy syndrome occurring in children, follows a biphasic clinical course and presents with both ‘early seizures’, typically presenting as convulsive status epilepticus during the febrile phase of a viral infection, and ‘late seizures’ that generally manifest as clusters of focal-onset seizures occurring 4–6 days after onset. The prognosis for survival in AESD is generally favorable, and fatalities are rare; however, > 60% of patients have neurological sequelae, including intellectual and motor disabilities1.
Differentiating AESD from prolonged febrile seizures (PFSs) with status epilepticus is difficult during the early phases of AESD2. In AESD, magnetic resonance imaging (MRI) of the brain has revealed no abnormalities at the onset of early seizures less than 2 days of onset, although subcortical high-signal patterns known as bright tree appearance (BTA) can be observed on diffusion-weighted MRI after the occurrence of late seizures more than 3 days after onset3. A biomarker that permits diagnosis of AESD within two days of onset could improve diagnostic accuracy and inform treatment decisions.
Scoring systems based on clinical symptoms and early biochemical data have recently been proposed for diagnostic purposes, although the positive predictive value of these systems for identifying AESD is < 30%3,4,5,6,7. Electroencephalography (EEG) is also used to differentiate between acute encephalopathy and febrile convulsions8. We performed a computed analysis of EEG data collected within 120 h after seizure onset and reported a significantly lower absolute power spectrum (APS) value of high-frequency components in patients with AESD than in patients with PFS9.
Furthermore, we postulated that the phase lag index (PLI) analysis could be applied to clarify the functional status of patients with AESD. PLI analysis enables objective quantitative assessment of neuronal synchronization, firing correlation, and phase coherence and has been used in various studies to help determine differential diagnoses and predict prognoses in conditions such as dementia10.
In this study, we performed APS and PLI analyses of EEG data recorded within 48 h of prolonged seizures in pediatric patients diagnosed with either AESD or PFS. This study aimed to explore whether these types of analyses and machine learning could serve as tools for differentiating between the early phases of AESD and PFS shortly after the onset of seizures. Additionally, we evaluated the brain function in patients in the early phases of AESD and used PLI analysis to compare these findings with comparable data from patients with PFS.
Results
Patient profiles
Sixty-one patients met the eligibility criteria for inclusion. Of these, 31 were male and 30 were female. The study population included 20 patients diagnosed with AESD and 41 with PFS. The clinical profile of the study population is summarized in Table 1, including sex ratio, mean age at seizure onset, time interval between seizure onset and EEG (hours), whether treatment for seizures was administered, seizure medications provided, and outcomes. Treatment for seizures was administered in nine of 20 patients with AESD and 22 of 41 patients with PFS, with no significant difference between groups in the type of treatment provided. The mean number of drugs used to treat seizures also did not differ between the AESD (0.75 drugs) and PFS (0.77 drugs) groups. Both groups included patients requiring two or more anticonvulsant drugs, four patients with AESD and four patients with PFS received two drugs, and one patient with AESD and four patients with PFS received three drugs. Regarding outcomes, 15 of 20 patients with AESD (75%) exhibited mild-to-severe sequelae, including 10 patients with mild-to-moderate sequelae and five patients with severe sequelae; five patients with AESD had no sequelae. In contrast, all patients with PFS had favorable outcomes without sequelae.
Differences in APS values between AESD and PFS
Figure 1 depicts the mean APS values in the AESD and PFS groups, both overall and by brain regions (frontal, centrotemporal, parieto-occipital). The mean overall APS values were significantly lower in the AESD group compared with the PFS group in the theta (Q < 0.001), alpha (Q < 0.01), beta (Q < 0.01), and gamma (Q < 0.01) frequency bands. Similarly, in both the frontal and parieto-occipital regions of the brain, the mean APS values were significantly lower in the AESD group compared with the PFS group in the theta (frontal, Q < 0.001; parieto-occipital, Q < 0.001), alpha (frontal, Q < 0.001; parieto-occipital, Q < 0.01), beta (frontal, Q < 0.001; parieto-occipital, Q < 0.01), and gamma (frontal, Q < 0.01; parieto-occipital, Q < 0.01) frequency bands. In the centrotemporal region of the brain, the mean APS value was significantly lower in AESD compared with PFS, only in the beta frequency band (Q < 0.01). In all the brain regions, the mean APS values in the delta frequency band did not significantly differ between the AESD and PFS groups. Particularly, in the frontal and centrotemporal regions, the mean APS values tended to be lower in the beta and gamma frequency bands among patients with AESD than among those with PFS (Supplementary Table S1).
Comparison of absolute power spectrum values between the AESD and PFS groups, overall and by brain region. The vertical axes represent the mean APS values, and the horizontal axes represent each frequency band. The graphs illustrate the mean APS values across all electrodes (overall) and in the frontal (average of the Fp1–F3 and Fp2–F4 electrodes), centrotemporal (average of C3 the T3 and C4–T4 electrodes), and parieto-occipital (average of P3 the O1 and P4–O2 electrodes) regions of the brain in all patients. The mean APS values in the theta, alpha, beta, and gamma frequency bands were significantly lower in the frontal regions of the patients with AESD than in those with PFS. AESD, acute encephalopathy with biphasic seizures and late reduced diffusion; APS, absolute power spectrum; PFS, prolonged febrile seizure.
Figure 2 presents the heat maps of the mean APS values across all patients for each electrode in both the AESD and PFS groups. This figure also highlights the brain regions where the electrode-specific mean APS values significantly differed between the AESD and PFS groups. In the beta and gamma frequency bands, the mean APS values were significantly lower for AESD than for PFS, particularly from the frontal to the centrotemporal regions.
Visual comparison of mean APS values for each electrode in the AESD group versus the PFS group. Heat maps were generated to visualize the mean APS values for each electrode in each frequency band in the AESD and PFS groups. Higher APS values are indicated in red. In the beta frequency band, the mean APS values in the frontal and temporal brain regions of the patients in the AESD group were significantly lower than those in the PFS group. AESD, acute encephalopathy with biphasic seizures and late reduced diffusion; APS, absolute power spectrum; PFS, prolonged febrile seizure.
Differences in PLI values between AESD and PFS
Functional connectivity between electrode pairs was evaluated using mean PLI values. Two-way analysis of variance was performed to compare the mean PLI values, both overall and for each electrode combination, between the AESD and PFS groups, with the patient group and electrode pairs as independent variables (Fig. 3).
Comparison of mean PLI values between the AESD and PFS groups. (a) Mean PLI values for all electrode pairs by frequency band and (b) mean PLI values for electrode pairs across different frequency bands, with coloring indicating electrode pairs for which mean PLI values significantly differed between the AESD and PFS groups. The AESD group exhibited significantly higher mean PLI values across all frequency bands. In the delta frequency band, the mean PLI values between the frontal pole and occipital regions were significantly higher in the AESD group compared with the PFS group. In the gamma frequency band, mean PLI values between the hemispheres were significantly higher in the AESD group than in the PFS group. AESD, acute encephalopathy with biphasic seizures and late reduced diffusion; PFS, prolonged febrile seizure; PLI, phase lag index.
In each frequency band, the overall mean PLI was significantly higher for AESD than for PFS (Q < 0.001 at all frequencies, Fig. 3a). For numerous electrode pairs in the delta and gamma frequency bands, there was a significantly higher functional connectivity (higher mean PLI values) in the AESD group than in the PFS group. In the delta frequency band, the pairs of frontal pole and occipital electrodes showed significantly higher mean PLI values in the AESD group than in the PFS group (Fig. 3b). Additionally, in the low-gamma frequency band, the mean PLI values between the left and right hemispheres were significantly higher in the AESD group than in the PFS group.
Subgroup analysis
To examine the effects of anticonvulsant drug use on EEG metrics, we performed within-group comparisons in AESD (n = 20; treated, n = 9; untreated, n = 11) and PFS (n = 41; treated, n = 22; untreated, n = 19) (Supplementary Table S2). In the AESD group, power spectral analysis showed no significant difference in power values between treated and untreated patients; however, gamma-band PLI tended to be higher in the treated than in the untreated subgroup (p = 0.05). In the PFS group, both gamma-band power and gamma-band PLI tended to be higher in the treated than in the untreated subgroup (p = 0.07 and p = 0.03, respectively).
To evaluate the effect of EEG recording timing, we conducted within-group comparisons stratified by < 24 h versus > 24 h from onset in AESD (n = 20; <24 h, n = 13; >24 h, n = 7) and PFS (n = 41; <24 h, n = 36; >24 h, n = 5) (Supplementary Table S3). In AESD, recordings obtained > 24 h after onset showed lower delta-band power and a trend toward lower theta-band PLI (p = 0.01 and p = 0.07, respectively). In PFS, beta-band power tended to be higher at > 24 h (p = 0.06), whereas PLI in the alpha, beta, and gamma bands tended to be lower at > 24 h than at < 24 h (p = 0.08, p < 0.001, and p = 0.09, respectively).
Prediction of outcomes in AESD
A multiple regression analysis was performed to identify the specific mean APS values that predicted the occurrence of sequelae in patients with AESD (Supplementary Table S4). Among the mean APS values evaluated for each frequency band, the APS values in the theta and gamma bands were significantly negatively correlated with poor outcomes, indicating that lower mean APS values were associated with unfavorable prognoses (theta, P < 0.01; gamma, P = 0.01). No significant correlations between the mean APS values and sequelae were observed in the other frequency bands.
A similar multiple regression analysis using mean PLI values as predictor variables revealed that higher mean PLI values in the delta band were significantly correlated with poorer outcomes (P < 0.01). Conversely, in the theta, alpha, and gamma bands, lower mean PLI values were significantly correlated with worse outcomes (P < 0.01 for all the bands). The beta band was the only frequency at which mean PLI values did not correlate with patient outcomes.
Distinguishing AESD from PFS using machine learning classification based on APS and PLI analyses
To evaluate the accuracy of the differentiation between AESD and PFS, machine learning with k-fold cross-validation was conducted using the mean APS and mean PLI values (Table 2). In classification based on the mean APS value, the beta frequency band demonstrated the highest sensitivity and specificity, with a sensitivity of 86.9% and a specificity of 80.1%. Additionally, for the APS-based classification in the beta frequency band, the positive predictive value (PPV) for AESD was 68.8%, and the negative predictive value (NPV) for PFS was 92.7%, representing the highest PPV and NPV among all frequency bands.
In the classification based on the mean PLI value, the theta frequency band also demonstrated the highest sensitivity and specificity, but with lower accuracy compared with the classification based on the mean APS value. In the theta band, the sensitivity of the PLI-based classification was 78.3%, the specificity was 74.4%, the PPV for AESD in the theta frequency band was 29.1%, and the NPV for PFS was 66.0%. All the data on the differentiation accuracy of the PLI-based classification in the theta band were lower than the corresponding results for classification based on the mean APS value in the beta band.
Discussion
Summary of results
Power spectrum and PLI analyses were performed using EEG data obtained within 48 h (1–48 h) of seizure onset in patients with AESD or PFS. The overall mean APS was lower in patients with AESD than in those with PFS across the theta, alpha, beta, and gamma frequency bands. Conversely, the overall mean PLI was higher in the AESD group than in the PFS group across all frequency bands. Among the mean APS and PLI values evaluated in the various frequency bands, the mean APS value in the beta frequency band demonstrated the highest potential accuracy for early differentiation between AESD and PFS.
Relationship between APS values and AESD
In this study, we analyzed EEG data obtained within 1–48 h before the abnormalities were visible on MRI in patients with AESD or PFS. The results revealed that the overall mean APS was significantly lower in patients with AESD than in those with PFS, particularly in the beta frequency band, where a lower APS was associated with a higher likelihood of AESD. In a previous study, delta wave power in the frontal region measured 6–10 h after onset was useful for the early differentiation of AESD from FS11. That study utilized APS values derived from a monopolar montage. However, EEGs recorded with a monopolar montage are susceptible to artifacts, particularly from the ears, which predominantly affect the beta frequency band. In contrast, bipolar montage EEGs tend to reduce such ear-related artifacts. The present study focused on EEG data recorded with a bipolar montage at an earlier stage of the disease, which may yield different results from those of the previous study.
The BTA phenomenon, as reported in AESD, predominantly occurs in the frontal regions of the brain, with relative sparing of the central, temporal, and occipital regions2,12,13. In our analysis of APS values by brain region, the most significant difference in the mean APS values between the AESD and PFS groups was observed in the frontal region, particularly in the beta and gamma bands, suggesting frontal-dominant impairment in AESD.
By contrast, within the AESD group, the overall mean APS values in the theta and gamma bands tended to be lower in patients with poorer outcomes, with the theta band showing a stronger correlation between APS values and outcomes. The generation of theta waves is associated with thalamocortical networks14. Thalamic lesions are often prominent in cases with poor outcomes15. Therefore, we hypothesized that the disruption of the thalamocortical network in severe AESD may be reflected in lower APS values.
Relationship between PLI values and AESD
We also examined the differences in PLI values between the AESD and PFS groups. The mean PLI values were higher in patients with AESD than in those with PFS across all frequency bands. Among the electrode pairs in the delta and gamma frequency bands, the mean PLI values were significantly higher in the AESD group than in the PFS group. These findings suggest that functional connectivity in different brain regions increases during AESD.
Histopathological analysis of the cerebrum in patients with AESD showed an increase in gemistocytic astrocytes at the corticomedullary junction, which may contribute to BTA16. It is hypothesized that axonal degeneration in structures, such as the arcuate fasciculus and uncinate fasciculus, may progress during AESD17, which could lead to the simplification of neural connections, a factor that may have contributed to the increased waveform synchronization observed in the PLI analysis in the present study.
Effects of anticonvulsant drug and recording time within groups on EEG analysis
Administration of benzodiazepines has been reported to increase slow-wave, decrease alpha-band, and augment fast-wave activities18. In particular, diazepam is thought to partially synchronize intracortical networks, and enhance EEG power in the beta and low-gamma (30–50 Hz) ranges by increasing neuronal firing rates. In our cohort, EEG metrics at frequencies > 20 Hz tended to increase in the presence of anticonvulsant drug; accordingly, gamma-band power was greater in the PFS group than in the AESD group in the current study, and this result may partly reflect the impact of anticonvulsant drugs. By contrast, within the AESD group, no significant difference in beta-band power was observed between treated and untreated subgroups, suggesting that intracortical network dysfunction present from the early phase of AESD may blunt the beta-power increase commonly seen after anticonvulsant administration.
Comparing EEGs recorded < 24 h versus 24–48 h after onset within each disease group revealed a time-dependent decrease in delta-band power in AESD, and in PFS an increase in beta-band power accompanied by a decrease in beta-band PLI. Consistent with these findings, Ogawa et al. reported that, in EEGs obtained within 24 h of onset, delta-band power declines over time with little change in beta power11. In exploratory subanalyses, changes observed from less than 24 h to 24–48 h consistently accentuated the AESD–PFS differences identified in the primary analysis. Because the number of patients was limited, we deemed a formal subanalysis underpowered and did not proceed. Nevertheless, analyses restricted to EEGs recorded at 24–48 h may provide more accurate discrimination.
Utility of APS and PLI values for early differentiation between AESD and PFS, and their potential clinical applications
This study aimed to explore the use of APS and PLI values in the diagnosis of AESD, which may be confused with PFS in its early phases. In our findings on the differentiation accuracy of the mean overall APS value in the beta frequency band, the PPV for AESD was 68.8% and the NPV for PFS was 92.7%. Compared with the mean overall APS values, the PLI values across all frequency bands showed poorer performance in accurately differentiating AESD from PFS. Collectively, these results suggest that the mean overall APS value in the beta band is the most promising candidate for accurate early differentiation between these conditions.
Several scoring systems have been developed to predict AESD based on clinical findings and laboratory test results3,4,5,6,7. Studies aimed at validating these scoring methods using clinical data have reported PPVs of 14–25% and NPVs of 95–100%, indicating high reliability in ruling out AESD17. In this study, the PPV for the mean overall APS value in the beta band was superior to the PPVs of previous scoring systems as reported in previous studies. Whether similar results can be achieved using other datasets remains a subject for future investigation.
The EEG analysis required for this study takes approximately 10 min, is semi-automated, and is therefore suitable for clinical application. Moreover, in patients suspected of having AESD during the early seizure phase, administration of a mitochondrial cocktail therapy comprising thiamine, vitamin C, vitamin E, biotin, coenzyme Q10, and L-carnitine after seizure onset may prevent progression to late seizures and the development of BTA on MRI19. This study suggests that proactively implementing such treatment in patients in whom AESD is suspected based on EEG findings may have the potential to improve prognosis.
Limitations
This study has some limitations. First, this retrospective analysis included only patients with AESD or PFS who met specific criteria. EEG recordings were performed at various times on selectively chosen patients, which may have introduced a selection bias. Second, future studies and prospective investigations are required to determine whether the present findings can be validated by replicating these findings or similar results in other patient groups. Third, the analyses in this study used a 12-channel electrode configuration with fewer than the number of electrodes in the standard international 10–20 system; it is unknown whether different results may be obtained using this standard method. Finally, because this study relied on data collected from other institutions, our analyses were based on the submitted case report forms. Consequently, we were unable to ascertain why treatment was not administered to patients with AESD or PFS during the acute phase. In future studies, we plan to implement a standardized data-collection form that records the rationale for treatment decisions, including reasons for withholding or deferring therapy.
Conclusions
In this study, the additional analyses using short-duration EEG data enabled semi-automated discrimination between AESD and PFS. The mean APS values were lower in the AESD group than in the PFS group, with this difference being most pronounced in the beta frequency band and in the frontal region of the brain. The mean PLI values were generally higher in the AESD group than in the PFS group. Among the metrics analyzed, the mean overall APS value in the beta band showed the greatest utility for distinguishing between AESD and PFS. These findings suggest that the APS value in the beta band can be useful for early differentiation between AESD and PFS during acute status epilepticus.
Materials and methods
Patients and methods
This study included patients who met the following criteria at Tottori University, Osaka City General Hospital, Saitama Children’s Medical Center, National Fukuoka Higashi Medical Center, Tokyo Women’s Medical University Yachiyo Medical Center, and Nagano Prefectural Hospital: (1) presentation of convulsive status epilepticus triggered by fever > 38.0 °C, defined as either a convulsive seizure lasting > 30 min or a series of recurrent seizures lasting > 30 min, occurring between 2003 and 2018; (2) no history of neurological disorders, such as Dravet syndrome or infantile epileptic spasms syndrome, before the onset of status epilepticus; (3) a final diagnosis of AESD or PFS; and (4) digital scalp EEG performed within 48 h (1–48 h) after seizure onset, during which no abnormal findings were detected on MRI.
The diagnosis of AESD was based on guidelines outlined by Mizuguchi et al., including the following: (1) presence of convulsive status epilepticus associated with fever; (2) presence of impaired consciousness lasting at least 24 h after the status epilepticus and with a severity score of either ≥ 20 on the Japan Coma Scale or < 11 on the Glasgow Coma Scale; (3) cerebrospinal fluid samples with a normal cell count and negative viral and bacterial cultures; (4) the presence of BTA observed on diffusion-weighted MRI, and (5) no known preexisting condition before the onset of seizures1. Patients diagnosed with hemiconvulsion-hemiplegia-epilepsy syndrome, who showed BTA on MRI in the acute phase of this condition, were excluded from this study. In this study, PFS was defined as meeting the following criteria: (1) convulsive status epilepticus associated with fever, (2) no consciousness impairment lasting > 24 h after status epilepticus, and (3) no neurological sequelae.
For both the AESD and PFS groups, we excluded patients whose guardians did not consent to participate in this study and those with any of the following conditions identified prior to the onset of seizures: neurological abnormalities, central nervous system inflammation, head trauma, cerebrovascular disorders, toxic encephalopathy, systemic diseases, or metabolic disorders.
This study was approved by the Institutional Review Boards of the Kagawa Prefectural University of Health Sciences and Tottori University Hospital (protocol code 295, approved on February 5, 2024).
Clinical profiles
Clinical information and EEG data were collected retrospectively by reviewing patients’ medical records. We reviewed the clinical data of each patient, including sex, age at onset of febrile status epilepticus, time interval between seizure onset and EEG, and outcomes quantified according to the Pediatric Cerebral Performance Category (PCPC) scale, which is commonly used to assess the extent of neurological sequelae in children20. We divided the patients into three outcome groups based on their PCPC scale scores, with PCPC scores of 1 indicating normal neurological performance, 2 or 3 mild-to-moderate disability, and 4 or 5 severe disability.
Electroencephalography (EEG) data acquisition and processing
Each patient underwent a scalp video-EEG with a duration of > 1 h using Nihon Kohden equipment for data acquisition. Scalp electrodes were positioned according to the international 10–20 system. EEG data were sampled at 200–500 Hz, with impedances < 5 kΩ. Low-cut and high-cut filters were set at 0.5 Hz and 60 Hz, respectively.
A reduced EEG montage involving fewer electrodes is often used for patients transported to the emergency department21. For the computed EEG analyses in this study, we used two montages as described in a previous study: bipolar montages (Fp1–F3, F3–C3, C3–P3, P3–O1, Fp2–F4, F4–C4, C4–P4, P4–O2, C3–T3, and C4–T4) were used for the power spectrum analysis, and monopolar montages with an average reference (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, T3, and T4) were used in the PLI analysis22. An EEG specialist certified by the College of Laboratory Medicine of Japan selected ten 30-s, non-artifact-free epochs. We used delta activity in sleep EEG to minimize electromyographic and cardiographic artifacts. All computational EEG analyses (i.e., APS analysis, PLI analysis, and machine learning classification) were performed using MATLAB version R2024a (MathWorks). APS analysis was included because it demonstrated the potential for differentiating between AESD and PFS in our previous pilot study involving a small number of patients9. Additionally, PLI analysis was used to measure functional connectivity of the brain during the acute phase of both diseases. For the power spectrum and PLI analyses, we analyzed all standard narrow frequency bands (delta band, 0.5–3.9 Hz; theta band, 4.0–7.9 Hz; alpha band, 8.0–12.9 Hz; beta band, 13.0–29.9 Hz; gamma band, 30.0–39.9 Hz).
EEG analyses
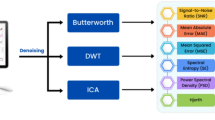
The two types of EEG analyses used in this study are shown in Fig. 4.
Workflow of EEG analysis. Digital EEG data were randomly divided into ten 30-s, non-artifact-free epochs. Absolute power spectra and phase lag index analyses were performed. The mean APS and PLI values for each electrode were calculated and recorded as the representative values for each patient. APS, absolute power spectrum; EEG, electroencephalography; PLI, phase lag index.
Power spectrum analysis
Power spectrum analysis was used in signal processing and data analysis to examine the power of each frequency band within a specific epoch of an EEG dataset. The power spectrum was calculated using fast Fourier transform on broadband bandpass-filtered data (0.5–60 Hz). The power spectrum of each electrode in each frequency band was calculated using a formula that defined the power spectrum for frequencies f = 0, 1, 2, …, N-1 as follows:
Power spectrum (f) = |X(f)|^2 = X(f) × X(f).
The spectral values are expressed as APS values. For each patient and frequency band, the APS value for each electrode was calculated for 10 epochs; these 10 APS values were then averaged to calculate a mean APS value. The mean APS values were calculated for three brain regions: the frontal region (mean of the values for Fp1–F3 and Fp2–F4), centrotemporal region (mean of the values for C3–T3 and C4–T4), and parieto-occipital region (mean of the values for P3–O1 and P4–O2). Additionally, the overall APS value, which represents the mean APS value for all electrodes, was calculated.
For both the AESD and PFS groups, a heat map was constructed using the mean APS values for each electrode across all patients in each group. The heat maps were generated using ATAMAP II for Windows (Kissei Comtec Co., Ltd.).
Phase lag index analysis
We applied a phase-based measure of functional connectivity, known as the PLI. This method involves calculating the level of synchronization between two electrodes by determining whether the phase of one signal consistently leads to or lags behind the other signal22,23. The PLI is defined as follows:
where the PLI value represents the mean signum of the phase difference \(\:\varDelta\:\phi\:\left({t}_{n}\right)\) between the two signals over a time period of length N. The instantaneous phase was extracted using the Hilbert transform of the narrow-bandpass-filtered EEG signal. We measured the PLI among all channel pairs for all 12 electrodes in five frequency bands (delta, theta, alpha, beta, and gamma). The PLI results were represented a 12-by-12 adjacency matrix for each data epoch. A PLI value close to zero indicates weak or inconsistent phase synchronization, whereas a PLI value close to one indicates strong phase synchronization. For each patient and frequency band, the PLI values between electrodes were calculated for each of the 10 epochs and averaged to determine the mean PLI value. Additionally, the overall PLI value, which represents the mean PLI value for all electrode combinations, was calculated.
Subgroup analysis
To investigate within-group effects of anticonvulsant drug use and recording time, we conducted the subgroup analyses within groups. The overall APS and PLI values were used as outcome measures. Recording time was dichotomized as < 24 h and > 24 h, and comparisons were performed separately within the AESD and PFS groups.
Statistical methods
Statistical analyses were performed using the IBM SPSS Statistics version 27 software (IBM Corp.). Clinical data presented in the patient profiles are reported as means and standard deviations. Welch’s t-test and chi-squared test were used to compare the clinical characteristics of the AESD and PFS groups.
The mean APS values were compared between the AESD and PFS groups using Welch’s t-test. A P value of < 0.05 was considered statistically significant. The Benjamini–Hochberg false discovery rate method was subsequently applied to account for multiple comparisons and adjust the expected proportion of false discoveries24. A false discovery rate threshold of Q = 0.01 (1%) was used, and results meeting this criterion were considered statistically significant.
Overall and the mean PLI values for each electrode combination were compared between the AESD and PFS groups. Welch’s t-test was used for the statistical analysis of intergroup comparisons, followed by application of the false discovery rate method to adjust for multiple comparisons. Differences with a Q value < 0.01 were considered statistically significant.
Two-way analysis of variance with Bonferroni correction was performed for detailed comparisons. Individual mean APS values were compared between the AESD and PFS groups, with patient group and electrodes as independent variables. Similarly, the individual mean PLI values in the AESD and PFS groups were compared using the patient group and electrode pairs as independent variables. Additionally, a multiple regression analysis was performed to examine the correlation between the outcome and mean APS or PLI values in the AESD group. In the subgroup analyses, the independent-samples t-test was performed separately within the AESD and PFS groups, to compare the overall APS and PFS values between the convulsant drug use or non-use, and between recording times < 24 h and > 24 h.
Classification architecture
To validate the accuracy of differentiation, k-fold cross-validation was performed using MATLAB. The data were divided into a training set (80%) and a test set (20%) for validation. The mean validation accuracy was calculated as the mean of five runs. The test dataset was used only for the final evaluation of the classifier performance. A support vector machine algorithm, which is among the most well-known machine learning models and is often used for classification tasks in supervised learning25, was used as a classifier in this study. The classifier evaluated the accuracy of using the mean APS and PLI values to discriminate between AESD and PFS.
Data availability
• The power spectrum analysis and phase lag index datasets generated and/or analyzed during the current study are available at Figshare ( [https://figshare.com/s/6ae66f4e982aeee05645](https://figshare.com/s/6ae66f4e982aeee05645) and [https://figshare.com/s/eadf57bc65b3d6af48d4](https://figshare.com/s/eadf57bc65b3d6af48d4).)・Custom code used for the analyses is available from the corresponding author upon reasonable request owing to patent considerations and ongoing commercialization plans. Editors and reviewers will be provided with access on request. Reader access will be granted under an institutional end-user license.
References
Mizuguchi, M. et al. Guidelines for the diagnosis and treatment of acute encephalopathy in childhood. Brain Dev. 43, 2–31. https://doi.org/10.1016/j.braindev.2020.08.001 (2021).
Tada, H. et al. Predictive score for early diagnosis of acute encephalopathy with biphasic seizures and late reduced diffusion (AESD). J. Neurol. Sci. 358, 62–65. https://doi.org/10.1016/j.jns.2015.08.016 (2015).
Takanashi, J. et al. Diffusion MRI abnormalities after prolonged febrile seizures with encephalopathy. Neurology 66, 1304–1309. https://doi.org/10.1212/01.wnl.0000210487.36667.a5 (2006). discussion 291.
Ichinose, F. et al. Incidence and risk factors of acute encephalopathy with biphasic seizures in febrile status epilepticus. Brain Dev. 44, 36–43. https://doi.org/10.1016/j.braindev.2021.07.004 (2022).
Maeda, M. et al. Predicting the onset of acute encephalopathy with biphasic seizures and late reduced diffusion by using early laboratory data. Front. Neurol. 12, 730535. https://doi.org/10.3389/fneur.2021.730535 (2021).
Uematsu, K., Matsumoto, H., Zaha, K., Mizuguchi, M. & Nonoyama, S. Prediction and assessment of acute encephalopathy syndromes immediately after febrile status epilepticus. Brain Dev. 45, 93–101. https://doi.org/10.1016/j.braindev.2022.10.004 (2023).
Yokochi, T. et al. Prediction of acute encephalopathy with biphasic seizures and late reduced diffusion in patients with febrile status epilepticus. Brain Dev. 38, 217–224. https://doi.org/10.1016/j.braindev.2015.07.007 (2016).
Ohno, A. et al. Acute encephalopathy with biphasic seizures and late reduced diffusion: predictive EEG findings. Brain Dev. 44, 221–228. https://doi.org/10.1016/j.braindev.2021.11.003 (2022).
Oguri, M. et al. Distinguishing acute encephalopathy with biphasic seizures and late reduced diffusion from prolonged febrile seizures by acute phase EEG spectrum analysis. Yonago Acta Med. 59, 1–14 (2016).
van Straaten, E. C. et al. Disturbed phase relations in white matter hyperintensity based vascular dementia: an EEG directed connectivity study. Clin. Neurophysiol. 126, 497–504. https://doi.org/10.1016/j.clinph.2014.05.018 (2015).
Ogawa, E., Sakaguchi, Y., Kaneko, T., Saito, O. & Miyama, S. Early-phase EEG power spectrum analysis May differentiate acute encephalopathy with biphasic seizures and late reduced diffusion from prolonged febrile seizures. Epilepsy Res. 210, 107515. https://doi.org/10.1016/j.eplepsyres.2025.107515 (2025).
Maegaki, Y. et al. Clinical characteristics of acute encephalopathy of obscure origin: a biphasic clinical course is a common feature. Neuropediatrics 37, 269–277. https://doi.org/10.1055/s-2006-955928 (2006).
Takanashi, J. Two newly proposed infectious encephalitis/encephalopathy syndromes. Brain Dev. 31, 521–528. https://doi.org/10.1016/j.braindev.2009.02.012 (2009).
Saadatmehr, B. et al. Evolution of cross-frequency coupling between endogenous oscillations over the Temporal cortex in very premature neonates. Cereb. Cortex. 33, 278–289. https://doi.org/10.1093/cercor/bhac067 (2022).
Kurahashi, N. et al. Thalamic lesions in acute encephalopathy with biphasic seizures and late reduced diffusion. Pediatr. Neurol. 51, 701–705. https://doi.org/10.1016/j.pediatrneurol.2014.07.013 (2014).
Takanashi, J. I. et al. Loss of myelinated axons and astrocytosis in an autopsy case of acute encephalopathy with biphasic seizures and late reduced diffusion. Brain Dev. 40, 947–951. https://doi.org/10.1016/j.braindev.2018.06.004 (2018).
Nishiyama, M. et al. Prediction of AESD and neurological sequelae in febrile status epilepticus. Brain Dev. 43, 616–625. https://doi.org/10.1016/j.braindev.2021.01.004 (2021).
Matejcek, M. Pharmaco-encephalgraphy: the value of quantified EEG in psychopharmacology. Pharmacopsychiatry 12, 126–136. https://doi.org/10.1055/s-0028-1094603 (1979).
Omata, T. et al. Efficacy of a mitochondrial drug cocktail in preventing acute encephalopathy with biphasic seizures and late reduced diffusion. J. Neurol. Sci. 15, 123245. https://doi.org/10.1016/j.jns.2024.123245 (2024). Epub 2024 Sep 17.
Imataka, G., Kuwashima, S. & Yoshihara, S. A comprehensive review of pediatric acute encephalopathy. J. Clin. Med. 11, 5921. https://doi.org/10.3390/jcm11195921 (2022).
Kanamori, K., Sakaguchi, Y. & Miyama, S. The utility of limited-montage electroencephalography for seizure detection in children. Pediatr. Neurol. 137, 1–5. https://doi.org/10.1016/j.pediatrneurol.2022.08.011 (2022).
Smith, R. J. et al. Computational characteristics of interictal EEG as objective markers of epileptic spasms. Epilepsy Res. 176, 106704. https://doi.org/10.1016/j.eplepsyres.2021.106704 (2021).
Stam, C. J., Nolte, G. & Daffertshofer, A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 28, 1178–1193. https://doi.org/10.1002/hbm.20346 (2007).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R Stat. Soc. Ser. B Methodol. 57, 289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x (1995).
Yassin, W. et al. Machine-learning classification using neuroimaging data in schizophrenia, autism, ultra-high risk and first-episode psychosis. Transl Psychiatry. 10, 278. https://doi.org/10.1038/s41398-020-00965-5 (2020).
Acknowledgements
We are sincerely grateful to Professor Masashi Nishiyama for his valuable advice on software development. We extend our appreciation to the collaborating doctors who provided us with the EEG data. This research was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Early-Career Scientists (Grant No. 22K15904). The funders had no role in the design of the study; collection, analyses, or interpretation of the data; writing of the manuscript; or decision to publish the results.
Funding
This research was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Early-Career Scientists (Grant No. 22K15904). The funders had no role in the design of the study; collection, analyses, or interpretation of the data; writing of the manuscript; or decision to publish the results.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.O., T.O., and Y.M. Methodology: M.O. Software: M.O. and M.Y. Validation: M.O. and T.O. Formal analysis: T.O., I.K., S, H., I.U., Y.N., Y.I., and S.L. Investigation: O.T., Y.M., and I.K. Resources: T.O. and M.O. Data curation: T.O., A.O., and M.O. Writing—original draft preparation: M.O. Writing—review and editing: T.O. and Y.M. Visualization: M.O. Supervision: Y.M. Project administration: Y.M. Funding acquisition: M.O. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Tottori University Hospital (protocol code 295, approved on February 5, 2024). Prior to enrollment in the study, the parents or guardians of the participants were provided with an opt-out document, and consent was obtained regarding the purpose, methods, safety considerations, and potential risks of their participation in the research. We confirm that we have read the journal’s position on issues involved in ethical publication and that this report is consistent with those guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Oguri, M., Okanishi, T., Kuki, I. et al. Automated differentiation of acute encephalopathy with biphasic seizures and late reduced diffusion and prolonged febrile seizures in acute phase. Sci Rep 15, 32948 (2025). https://doi.org/10.1038/s41598-025-17828-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17828-y