Abstract
Renal cell carcinoma accounts for a significant number of kidney malignancy-related fatalities globally. Perirenal Fat Thickness (PRFT) may indicate a state of nutritional excess in patients, which is potentially directly linked to both the incidence and prognosis of kidney cancer. This study investigated the association between perirenal fat thickness (PRFT) and overall survival (OS), as well as the predictive value of PRFT for postoperative estimated glomerular filtration rate (eGFR) in patients with renal cell carcinoma (RCC). A retrospective cohort of 1647 RCC patients from 2014 to 2021 was analyzed, divided into radical nephrectomy (RN) and partial nephrectomy (PN) groups. Preoperative measurements included visceral fat area, PRFT, and subcutaneous fat area. Kaplan-Meier curves compared OS between high and low PRFT groups, while Cox regression analyses identified prognostic factors for OS, and linear regression analyses assessed predictors of postoperative eGFR. PRFT significantly influenced OS in RN patients in univariate analysis (HR: 0.32; 95% CI: 0.19–0.52; P < 0.001), but not in PN patients. After adjusting for covariates such as age, sex, sarcomatoid features, necrosis, T stage, Fuhrman grade, smoking status, subcutaneous adipose tissue, and BMI, PRFT remained an independent risk factor for OS (HR: 0.56; 95% CI: 0.33–0.96; P < 0.001). Kaplan-Meier analysis showed that higher PRFT was associated with improved OS in RN patients. Univariate linear regression revealed that high PRFT correlated with reduced postoperative eGFR in both RN (β = -0.2, P = 0.002) and PN (β = -0.34, P < 0.001) groups; however, this correlation was not significant after multivariate adjustment. In conclusion, Low PRFT is independently associated with higher mortality in RCC patients undergoing RN. High PRFT is associated with reduced postoperative eGFR in initial analyses, but this association was not significant in multivariate analysis.
Similar content being viewed by others
Introduction
Renal cell carcinoma (RCC) represents the most prevalent solid tumor of the kidney and accounts for 90% of all kidney malignancies1. As the third deadliest tumor of the urinary system2RCC affects nearly 430,000 individuals globally each year, with around 150,000 fatalities attributed to the disease annually. Surgical resection, which includes both radical nephrectomy (RN) and partial nephrectomy (PN), remains the cornerstone treatment for RCC, boasting a 5-year cancer-specific survival rate exceeding 90%3. Despite these favorable oncological outcomes, the preservation of kidney function and management of chronic kidney disease (CKD) have become increasingly important. CKD affects at least 25% of RCC patients, particularly those who undergo RN4. Given that CKD is not only a risk factor for RCC5 but also significantly increases the risk of cardiovascular morbidity and mortality, it substantially worsens the prognosis and diminishes the quality of life for RCC patients. Therefore, it is crucial to identify RCC patients at risk of developing CKD to better tailor clinical interventions.
Visceral adipose tissue (VAT) surrounds the abdominal organs, primarily located in the omentum, mesentery, and retroperitoneum. Perirenal fat tissue (PRFT), a subset of VAT adjacent to the kidneys6can be distinctly identified from other VAT regions using computed tomography (CT)7. PRFT is composed of both adipocyte precursor cells and mature adipocytes, contributing to its developmental and functional heterogeneity compared to other visceral fat components. Chen et al. highlighted that the unique anatomical position and developmental heterogeneity of PRFT might render it particularly responsive to the development of CKD. They demonstrated that PRFT has a higher predictive value for CKD than total, subcutaneous, or other visceral fats in patients with type 2 diabetes mellitus (T2DM)8. While these findings have primarily been observed in patients with diabetes or obesity, we hypothesize that PRFT may similarly serve as a valuable predictive marker for CKD incidence in patients with RCC.
Adiposity significantly influences the prognosis of RCC patients. Body mass index (BMI), a conventional measure of general obesity, is paradoxically associated with both a higher risk of developing RCC and improved overall survival (OS) and progression-free survival (PFS), a phenomenon known as the “obesity paradox”. The impact of VAT on survival in RCC patients remains a subject of debate in current research9. Limited studies have explored the relationship between PRFT and RCC prognosis10. According to research by Jui-Peng et al., increased perinephric fat is predictive of malignant pathology in renal tumors11. Additionally, high PRFT has been associated with poorer PFS in patients with localized clear cell RCC. However, evidence linking PRFT to the prognosis in other histological subtypes of RCC is absent. Building on the hypothesis that PRFT is closely associated with CKD, our study further investigates the impact of post-CKD conditions on the prognosis of RCC patients. We also examined cardiovascular events—a reflection of life quality and a known consequence of CKD.
Hence, our study comprised both cross-sectional and retrospective cohort analyses. Initially, we compared PRFT between pre-CKD and non-pre-CKD groups. We then assessed the impact of PRFT on the incidence of CKD among Chinese RCC patients and compared these effects with total body fat (TBF), subcutaneous adipose tissue (SAT), and VAT. Additionally, the influence of various risk factors on OS and PFS was evaluated, including the effects of CKD and their association with cardiovascular events. These analyses were conducted for each histological type of RCC separately.
Methods
Patient selection and study design
Patient selection and study design
This retrospective study was conducted at Sun Yat-sen University Cancer Center, and included participants enrolled from 2000 to 2022. Eligible participants were histologically confirmed RCC patients who underwent PN or RN. Only patients with available preoperative abdominal CT or CT urography imaging data were included. The exclusion criteria were: (1) diagnosis of bilateral RCC; (2) presence of a solitary kidney; (3) prior renal surgery, ablative therapies, targeted therapies, or immunotherapies; (4) follow-up duration less than one year; (5) preexisting CKD conditions such as renal artery stenosis, ischemic nephropathy, hypertensive nephropathy, chronic pyelonephritis, hydronephrosis due to kidney stones, kidney atrophy, or kidney loss. This study received approval from the institutional review board and the ethical committee of Sun Yat-sen University Cancer Center. All procedures were conducted in accordance with the ethical standards of the Declaration of Helsinki and reported according to the STROCSS criteria12.
Measurement of SAT, VAT and PRFT
Abdominal CT scans were obtained using a CT scanner with settings that included a field of view of 350 mm, a matrix of 512 × 512, slice collimation of 128, gantry rotation time of 0.5–0.6 s, a pitch of 1.0, scan time of 0.4 s, tube voltage of 120 kV, and auto-adjusted tube current (Auto mA). The scans extended from the diaphragm to the S1 vertebra. The measurements of VAT and SAT were conducted in accordance with established protocols7. Briefly, both VAT and SAT areas were measured at the level of the umbilicus using Mimics Researcher 21 software. These areas were manually delineated by outlining the abdominal muscular wall, and fat-containing pixels were automatically identified within a window width of −195 to −45 Hounsfield units. TAT area was equivalent to the sum area of SAT and VAT.
PRFT was measured following the methodology of a previous study13. Measurements were taken only from the healthy side of the body, at the central slice of the renal hilum, defined as the central slice of the pelvis. PRFT was calculated as the sum of PRFT from three directions: (1) anteriorly, the vertical distance from the anterior renal fascia or the closest visceral organ to the top of the anterior border of the kidney; (2) laterally, the vertical distance from the lateral perirenal fascia or the closest visceral organ to the top of the lateral border of the kidney; and (3) posteriorly, the vertical distance from the posterior renal fascia to the top of the posterior border of the kidney. All imaging segmentation and area measurements were performed by a trained researcher.
Consistent with numerous previous studies, the cohorts were dichotomized using sex-specific median values to differentiate the high-value group from the low-value group. In this study, for patients undergoing RN, the sex-specific cutoff values for VAT, SAT, and PRFT were 197.57 cm2233.65 mm2and 20.10 mm for males, and 168.88 cm2308.06 mm2and 14.04 mm for females. Conversely, for patients undergoing PN, the sex-specific cutoff values for VAT, SAT, and PRFT were 232.35 cm2270.91 cm2and 21.84 mm for males, and 150.95 cm2310.08 cm2and 10.96 mm for females.
Clinical data collection
The following clinical data were collected for analysis: (1) patient baseline characteristics before nephrectomy including sex, age and history of smoking, diabetes, coronary heart disease or hypertension; (2) anthropometric data, including height, weight, systolic blood pressure (SBP), and diastolic blood pressure (DBP); (3) renal function indicators, including serum creatinine (SCr) and cystatin C (Cys C) measured within 7 days before surgery and within 7 days after surgery; (4) tumor features including tumor histological max diameter, location, histological type, histological necrosis, sarcomatoid component presence, embolus presence, T-stage, and Fuhrman grade; (5) blood biochemical measurements before surgery including total cholesterol, high-density lipoprotein-C, and blood glucose; and (6) surgery information including surgical methods, warm ischemia usage, and ischemia time. The estimated glomerular filtration rate (eGFR) was calculated using the MDRD formula. Chronic kidney disease (CKD) was defined as an eGFR of < 60 mL/min/1.73 m² according to KDIGO guidelines. Follow-up assessments were conducted from the date of nephrectomy until the last available follow-up or death. OS was defined as the time from the date of surgery to the date of death from any cause or the last follow-up.
Statistical analysis
Continuous variables were expressed as mean ± SD for normally distributed data and median (interquartile range [IQR]) for non-normally distributed data. Categorical variables were presented as frequencies (percentages). Univariable and multivariable Cox proportional hazards regression analyses were performed using the Surv function in the survival package to identify potential predictive factors associated with prognosis. Body components were modeled continuously using restricted cubic splines (RCS) within Cox proportional hazard models’ predict function in the rms package. OS and its associated risk factors, including VAT, SAT, and PRFT, were assessed using Kaplan-Meier survival analysis, with log-rank tests for comparison. The Kaplan-Meier curves were plotted using the ggsurvplot function in the survminer package. Subgroup analyses were visualized using the forestplot function in the forestplot package. To explore predictors of post-operative eGFR, univariate and multivariate linear regression analyses were conducted using the lm function in the stats package. Scatter plots and fitting curves were generated using the ggplot function in the ggplot2 package. Variables significant in univariate analyses were included in the multivariate models. A p-value of < 0.05 was summarized as statistically significant in all tests.
Results
Characteristics of RCC patients and cross-section analysis
A total of 1,647 patients with RCC who had available survival data were included in the cross-sectional analysis, comprising 501 patients who underwent RN and 1,146 who underwent PN(Fig. 1). The baseline characteristics of these participants are detailed in Table 1. Clear cell carcinoma was the most common diagnosis, present in 374 (74.7%) of the RN group and 666 (58.1%) of the PN group. The median values for VAT, SAT, PRFT, and BMI were as follows: in the RN group, VAT was 185.37 cm2 (IQR: 119.52–250.70), SAT was 261.98 cm2 (IQR: 179.88-354.89), PRFT was 17.00 mm (IQR: 8.53–27.97), and BMI was 22.80 kg/m2 (IQR: 20.76–24.84); in the PN group, VAT was 206.19 cm2 (IQR: 129.35-276.24), SAT was 291.66 cm2 (IQR: 221.85-363.82), PRFT was 16.90 mm (IQR: 8.58–28.59), and BMI was 23.82 kg/m2 (IQR: 21.48–25.79). Baseline characteristics of the participants with postoperative creatinine data are presented in Supplementary Table 1.
Flowchart of study population in the study and measurement of PRFT, VAT and SAT. As shown in B, axial CT images at the level of umbilicus used for evaluating the VAT and SAT, using automated mask segmentation of Mimic Research Software with the fat density ranging from 150 to 50 hU. The green mask represented the SAT and the yellow mask manefist the VAT. As shown in C, PRFT was measured in the central slice of renal hilium. As shown I the D and E, PRFT was measured in three directions : (1) anterior, the vertical distance from top of anterior border of kidney to anterior renal fascia or the closest visceral organ; (2) lateral, the vertical distance from top of lateral border of kidney to lateral perirenal fascia or the closest visceral organ; and (3) posterior, the vertical distance from top of posterior border of kidney to posterior renal fascia.
Analysis for prediction of OS
In univariate analysis, the presence of histological necrosis (HR = 2.30, 95%CI: 1.48–3.6, and HR = 4.08, 95%CI: 1.58–10.5, respectively) and higher Fuhrman grades (HR = 4.16, 95%CI: 2.39–7.22, and HR = 3.63, 95%CI: 1.49–8.28, respectively) were significantly associated with poorer OS in both RN and PN patients. Additional factors significantly associated with worse prognosis in RN patients included being male (HR = 2.20, 95% CI: 1.32–3.69), aged over 60 years (HR = 2.08, 95% CI: 1.34–3.23), presence of a sarcomatoid component (HR = 3.83, 95% CI: 1.83–8.05), higher T stage (HR = 2.68, 95% CI: 1.70–4.21), and smoking (HR = 1.73, 95% CI: 1.11–2.69). These factors were not significant in PN patients. Subgroup analyses further demonstrated the role of PRFT in overall survival (Supplementary Fig. 1).
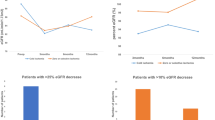
Concerning body composition, a BMI > 24 (HR = 0.89, 95% CI: 0.83–0.96), higher SAT (HR = 0.54, 95% CI: 0.34–0.86), and higher PRFT (HR = 0.32, 95% CI: 0.19–0.52) were associated with better prognosis in RN patients but had no significant impact in PN patients (Fig. 2). The predicted hazard ratios for each body composition parameter, including PRFT, VAT, and SAT, are illustrated using spline models (Supplementary Fig. 2). In the multifactorial analysis, PRFT was identified as an independent predictor of OS in RN patients across various body composition metrics (Table 2).
Analysis for prediction of post-eGFR
To investigate the effects of SAT, VAT, and PRFT on postoperative eGFR, we analyzed data from 356 RN patients and 492 PN patients.
Risk factors significantly associated with a decrease in postoperative eGFR in both RN and PN patients included being male (RN: β = −3.96, P = 0.046; PN: β = −13.67, P < 0.001), aged over 60 years (RN: β = −5.335, P = 0.009; PN: β = −18.12, P < 0.001), hypertension (RN: β = −9.44, P < 0.001; PN: β = −12.79, P < 0.001), and diabetes mellitus (RN: β = −10.34, P = 0.003; PN: β = −9.17, P = 0.014). Additionally, higher preoperative eGFR was predictive of better postoperative eGFR in both groups (RN: β = 0.19, P < 0.001; PN: β = 0.59, P < 0.001). Preoperative CHO levels were associated with higher postoperative eGFR in RN patients only (β = 2.19, P = 0.036 and β = −0.89, P < 0.42, respectively). High PRFT (RN: β = −0.2, P = 0.002; PN: β = −0.34, P < 0.001) and VAT (RN: β = −0.27, P = 0.006; PN: β = −0.56, P < 0.001) were identified as risk factors for greater eGFR decline in both patient groups. In contrast, BMI showed a significant association with eGFR decline only in PN patients (β = −0.64, P < 0.049), with no significant impact observed in RN patients (β = −0.36, P = 0.224). SAT did not show any significant association with eGFR in either group (β = 0.03, P = 0.66 and β = −0.12, P < 0.244, respectively) (Fig. 3).
The scatter plot with fitting linear regression model of patients with RCC undergoing RN and PN. The y axis represented eGFR after surgery, and the x axis represented PRFT(A, B), VAT(C, D) and SAT(E, F) measured before surgery. Linear regression model was also visualized (black line) with corresponding 95% confidence intervals (the shaded area).
After adjusting for factors such as sex, age, hypertension, and diabetes, somatic components like PRFT and VAT ceased to be independent predictors of eGFR decline (RN: PRFT β = −0.10, P = 0.225; VAT β = −0.04, P = 0.754. PN: PRFT β = −0.006, P = 0.938; VAT β = 0.001, P = 0.989). Conversely, preoperative eGFR continued to be an independent predictor of postoperative eGFR in both RN and PN patients (Table 3).
Validation of PRFT correlation with OS and post-eGFR in XXX hospital data
Data from our center identified PRFT as a significant predictor of overall survival (OS) following radical nephrectomy (RN), with higher PRFT levels associated with better OS. Preliminary analyses indicated that elevated PRFT was linked to a reduction in postoperative estimated glomerular filtration rate (eGFR) in the RN group. To validate these findings in a larger and more diverse population, we collaborated with another center and collected data from 214 RN patients. The results confirmed that higher PRFT was correlated with improved OS (HR = 0.50, 95% CI: 0.29–0.89, p = 0.017) and was significantly associated with a decrease in postoperative eGFR (R2 = 0.017, p = 0.033) (Supplementary Fig. 3), further supporting our initial observations.
Discussion
In our study, no significant association was observed between BMI and OS in patients undergoing either RN or PN. Traditionally used as a categorical measure of obesity, BMI has been extensively studied and is known to predispose individuals to RCC14. Epidemiological studies have consistently shown that while a higher BMI is linked to an increased risk of RCC, it paradoxically appears to confer a better prognosis on these patients15,16,17. However, variations in findings across retrospective studies may be influenced by differences in ethnicity and BMI categories18,19. To more accurately evaluate the impact of obesity on the development and progression of RCC, it is essential to consider not just overall obesity but the distribution of body fat, particularly the role of specific adipose compartments. Perirenal fat, a crucial component of visceral fat20,21is directly involved in cytokine secretion, influencing renal function22,23,24,25 and thus playing a notable role in RCC pathology. This detailed consideration of body fat distribution has opened new avenues in RCC research and is pivotal in developing future therapeutic strategies.
Recent investigations have underscored the significance of perirenal fat thickness and volume in clinical outcomes, with substantial correlations confirmed by a p-value of less than 0.0222. Despite this, the role of perirenal fat as a predictor of survival in RCC patients continues to be debated. For instance, Du, Yiqing et al. found that a higher proportion of perirenal adipose tissue independently correlates with increased mortality risk in patients undergoing surgical treatment for RCC, based on a study involving 421 patients26. In contrast, our prior research demonstrated that high PRFT is associated with a better prognosis in patients with metastatic RCC (mRCC) who were treated surgically and received anti-VEGF therapies within a cohort of 358 Chinese patients27. Further analysis within our larger Chinese RCC cohort indicated that PRFT significantly correlates with better OS in patients undergoing RN, as assessed through CT body composition analysis. Considering the retrospective nature of these findings, it is essential to conduct additional prospective studies to confirm and extend these observations.
The differential impact of PRFT on OS between patients undergoing RN and PN may be influenced by several factors. Observational data show 5-year OS rates of 95% for PN patients and 83% for RN patients28. Although PN patients exhibit comparable OS rates, suggesting minimal influence of PRFT on their survival, PRFT significantly correlates with improved OS in RN patients. RN is typically indicated for larger or locally advanced tumors, which are associated with greater tumor aggressiveness and poorer prognosis29. Consequently, the role of perirenal fat may be more pronounced in radical resections due to its potential relationship with tumor biology and overall patient health. The surgical removal of the entire kidney and surrounding structures, including perirenal fat, in RN patients could significantly alter physiological and metabolic statuses. Perirenal fat is known not only as an energy reserve but also as a source of various cytokines and hormones that regulate metabolism and inflammation30. Thus, the excision of the kidney and adjacent fat could disrupt the balance of these critical factors, influencing survival outcomes. Furthermore, variations in clinical and biomarker profiles at baseline between patients choosing RN versus PN might also explain the distinct impacts of perirenal fat on 5-year OS across these surgical groups.
This study is the first to assess PRFT and its association with risk factors for CKD following RN and PN. Our findings indicate that high PRFT is significantly associated with a decline in postoperative eGFR in patients with RCC who underwent both RN and PN. This relationship highlights the potential of PRFT as a predictive marker for the development of CKD post-RCC.
Previous investigations into the connection between perirenal fat and CKD have generally involved relatively small sample sizes. Chen et al. demonstrated that perirenal fat serves as a more accurate predictor of CKD than total, subcutaneous, or visceral fat in individuals with T2DM8. Similarly, a cross-sectional study by Fang et al., which included 171 patients with T2DM and employed ultrasound to measure PRFT, identified a negative correlation between perirenal fat thickness and eGFR31. These studies support the hypothesis that PRFT could be a valuable predictive marker for CKD development following RCC, although additional validation through prospective studies is necessary.
The mechanisms through which perirenal fat contributes to the increased risk of CKD are multifaceted and not yet fully understood. Elevated levels of perirenal fat are associated with enhanced local inflammation and oxidative stress, which may damage renal tissues and facilitate the progression of CKD32. Perirenal adipose tissue serves not only as an energy reserve but also as a source of various adipokines, including adiponectin, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). These adipokines are critical in regulating energy balance, inflammatory responses, and metabolic functions33. Abnormal secretion of adipokines by perirenal fat in the context of CKD may lead to metabolic disorders and inflammatory conditions, thereby exacerbating kidney damage34. Moreover, elevated levels of perirenal fat are correlated with insulin resistance, a common feature in patients with diabetes mellitus and metabolic syndrome, which are established risk factors for CKD35. This adipose tissue may promote insulin resistance through the secretion of inflammatory mediators and adipokines, further complicating renal health. Additionally, high levels of perirenal fat are linked to increased blood pressure, potentially due to direct effects on renal vascular function and increased vascular tension from adipose-derived substances such as angiotensin36. Significant accumulation of perirenal fat may also exert direct physical pressure on the kidneys, thereby impacting renal blood flow and function37. Collectively, local inflammation, oxidative stress, adipokine secretion, and physical compression associated with perirenal fat may contribute significantly to the onset of chronic renal failure in patients undergoing RN and PN.
This study has several limitations. First, its retrospective design is prone to recall and selection biases, which could impact the generalizability and accuracy of our findings. Due to the retrospective nature of data collection, detailed demographic and imaging information for the validation cohort was not fully accessible38. Nevertheless, we applied the same PRFT measurement protocol and cut-off value as used in the training cohort. While this approach supports initial external validation, the absence of comprehensive validation cohort dataa limits further subgroup comparisons and warrants caution in interpreting generalizability39. Although higher PRFT was observed more frequently in patients with higher T-stage tumors, the association did not remain significant after adjusting for confounders. Moreover, due to incomplete recurrence and metastasis data, PFS and DFS could not be reliably assessed in this retrospective cohort. Future prospective studies are needed to further elucidate the relationship between PRFT and tumor aggressiveness or cancer progression. Despite this, our cohort is notably the largest examining perirenal fat’s association with survival and renal function following radical nephrectomy (RN) and partial nephrectomy (PN). Moreover, while the study was primarily conducted at one center, it was validated with data from another center. Nonetheless, this may limit the external validity of our findings. All patients in this study were of Han Chinese ethnicity and shared a relatively homogeneous regional background. Although detailed lifestyle information (e.g., dietary patterns or physical activity) was not available, the limited ethnic and cultural variability may have reduced confounding from population heterogeneity. However, this also restricts the generalizability of our results, as body composition and disease progression may differ across populations and healthcare settings. Second, the median follow-up period of 39.2 months, while sufficient for assessing short- and medium-term outcomes, may not fully capture late complications or distant recurrences, particularly in chronic conditions such as chronic kidney disease or long-term survival analyses. Although we observed an association between high perirenal fat thickness (PRFT) and reduced postoperative eGFR in both RN and PN groups, this association did not reach statistical significance after adjusting for multiple covariates. The p-value was below 0.05, but the low R² value suggests a limited strength of the association. Thus, we approached these results with caution. While PRFT may be a potential predictor, its predictive ability was not strongly validated in this study. Furthermore, we acknowledge that the absence of subgroup analysis stratified by baseline renal function (e.g., preoperative eGFR) is a limitation of our study. This may have prevented us from identifying whether PRFT impacts outcomes differently in patients with varying renal reserves. Additionally, we did not perform stratified analysis based on histological subtypes of RCC due to the predominance of clear cell RCC and the relatively small number of patients with non-clear cell histologies. As different RCC subtypes may exhibit distinct biological behaviors and metabolic profiles, future studies with more histologically diverse cohorts are needed to evaluate whether PRFT holds differential prognostic value across subtypes. Larger sample sizes or longer follow-up periods may be necessary in future research to further explore the relationship between PRFT and postoperative renal function. Additionally, while genomic analyses of PRFT offer great scientific potential, limitations in available datasets and resources constrained this study. Future research could delve deeper into the interactions between PRFT, overall survival (OS), and postoperative renal function. Despite these limitations, we believe our study’s findings regarding the predictive value of PRFT for postoperative eGFR in patients undergoing RN and PN will provide valuable insights for clinical practice and decision-making.
Conclusions
This study investigated the prognostic significance of PRFT in patients with RCC. Our findings revealed that PRFT was a significant predictor of OS following RN, with higher PRFT associated with improved OS (HR: 0.32, P < 0.001). This prognostic relevance was not observed in patients undergoing PN. Furthermore, multivariate analyses confirmed that low PRFT was an independent risk factor for increased mortality in patients undergoing RN (HR: 0.56, P < 0.001). Although initial analyses indicated that high PRFT correlated with a decrease in postoperative eGFR for both RN and PN groups, these associations were not statistically significant after adjustment for covariates. These findings highlight the critical role of PRFT as a prognostic marker in RCC, particularly for patients undergoing RN, underscoring its potential in enhancing patient risk stratification and informing management strategies.
Data availability
The study data underlying the findings of current work were deposited at the Research Data Deposit platform (Available at http://www.researchdata.org.cn/). The datasets used and analysed during the current study available from the corresponding author(vonbasten@163.com) on reasonable request.
Change history
25 November 2025
Department of Anesthesiology, Affiliated Hospital of Hebei University, Baoding, China.
References
Huang, H., Chen, S., Li, W., Wu, X. & Xing, J. High perirenal fat thickness predicts a poor progression-free survival in patients with localized clear cell renal cell carcinoma. Urol. Oncol. 36(4), 157.e151-157.e156 (2018).
Global Burden of Disease et al. Cancer incidence, mortality, years of life lost, years lived with disability, and Disability-Adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol. 8 (3), 420–444 (2022).
Li, L. et al. Risk of chronic kidney disease after cancer nephrectomy. Nat. Rev. Nephrol. 10 (3), 135–145 (2014).
Chang, A., Finelli, A., Berns, J. S. & Rosner, M. Chronic kidney disease in patients with renal cell carcinoma. Adv. Chronic Kidney Dis. 21 (1), 91–95 (2014).
Saly, D. L., Eswarappa, M. S., Street, S. E. & Deshpande, P. Renal cell cancer and chronic kidney disease. Adv. Chronic Kidney Dis. 28(5), 460-468.e461 (2021).
Liu, B. X., Sun, W. & Kong, X. Q. Perirenal fat: A unique fat pad and potential target for cardiovascular disease. Angiology 70 (7), 584–593 (2019).
Caan, B. J. et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 4 (6), 798–804 (2018).
Chen, X. et al. Perirenal fat thickness is significantly associated with the risk for development of chronic kidney disease in patients with diabetes. Diabetes 70 (10), 2322–2332 (2021).
Vrieling, A. et al. Body composition in relation to clinical outcomes in renal cell cancer: A systematic review and Meta-analysis. Eur. Urol. Focus. 4 (3), 420–434 (2018).
Sanchez, A. et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol. 21 (2), 283–293 (2020).
Tsai, J. P., Lin, D. C., Huang, W. M., Chen, M. & Chen, Y. H. Comparison of perinephric fat measurements between malignant and benign renal tumours. J. Int. Med. Res. 50 (9), 3000605221125086 (2022).
Mathew, G. et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int. J. Surg. 96, 106165 (2021).
Davidiuk, A. J. et al. Mayo adhesive probability score: an accurate image-based scoring system to predict adherent perinephric fat in partial nephrectomy. Eur. Urol. 66 (6), 1165–1171 (2014).
Elmaleh-Sachs, A. et al. Obesity management in adults: A review. JAMA 330 (20), 2000–2015 (2023).
Choi, Y. et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int. J. Cancer. 132 (3), 625–634 (2013).
Ahmedov, V., Kizilay, F. & Cureklibatir, I. Prognostic significance of body mass index and other tumor and patient characteristics in non-metastatic renal cell carcinoma. Urol. J. 15 (3), 96–103 (2018).
Ji, J., Yao, Y., Guan, F., Luo, L. & Zhang, G. Impact of BMI on the survival of renal cell carcinoma patients treated with targeted therapy: A systematic review and Meta-Analysis. Nutr. Cancer. 75 (9), 1768–1782 (2023).
Otunctemur, A., Dursun, M., Ozer, K., Horsanali, O. & Ozbek, E. Renal cell carcinoma and visceral adipose index: a new risk parameter. Int. Braz J. Urol. 42 (5), 955–959 (2016).
Maurits, J. S. F., Sedelaar, J. P. M., Aben, K. K. H., Kiemeney, L. & Vrieling, A. Association of visceral and subcutaneous adiposity with tumor stage and Fuhrman grade in renal cell carcinoma. Sci. Rep. 12 (1), 16718 (2022).
Chau, Y. Y. et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell. Biol. 16 (4), 367–375 (2014).
Zhang, H., Li, Y., Ibanez, C. F. & Xie, M. Perirenal adipose tissue contains a subpopulation of cold-inducible adipocytes derived from brown-to-white conversion. Elife 13 (2024).
Favre, G. et al. Perirenal fat thickness measured with computed tomography is a reliable estimate of perirenal fat mass. PLoS One. 12 (4), e0175561 (2017).
D’Marco, L. et al. Perirenal fat thickness is associated with metabolic risk factors in patients with chronic kidney disease. Kidney Res. Clin. Pract. 38 (3), 365–372 (2019).
Shen, F. C., Cheng, B. C. & Chen, J. F. Peri-renal fat thickness is positively associated with the urine albumin excretion rate in patients with type 2 diabetes. Obes. Res. Clin. Pract. 14 (4), 345–349 (2020).
Preza-Fernandes, J. et al. A hint for the obesity paradox and the link between obesity, perirenal adipose tissue and renal cell carcinoma progression. Sci. Rep. 12 (1), 19956 (2022).
Du, Y. et al. Perirenal fat as a new independent prognostic factor in patients with surgically treated clear cell renal cell carcinoma. Clin. Genitourin. Cancer. 20 (1), e75–e80 (2022).
Ning, K. et al. Perirenal fat thickness significantly associated with prognosis of metastatic renal cell cancer patients receiving Anti-VEGF therapy. Nutrients 14, 16 (2022).
Weight, C. J. et al. Partial nephrectomy is associated with improved overall survival compared to radical nephrectomy in patients with unanticipated benign renal tumours. Eur. Urol. 58 (2), 293–298 (2010).
Mir, M. C. et al. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: A systematic review and Meta-analysis of comparative studies. Eur. Urol. 71 (4), 606–617 (2017).
Hammoud, S. H., AlZaim, I., Al-Dhaheri, Y., Eid, A. H. & El-Yazbi, A. F. Perirenal adipose tissue inflammation: novel insights linking metabolic dysfunction to renal diseases. Front. Endocrinol. (Lausanne). 12, 707126 (2021).
Xu, S. et al. Para-perirenal fat thickness is associated with reduced glomerular filtration rate regardless of other obesity-related indicators in patients with type 2 diabetes mellitus. PLoS One. 18 (10), e0293464 (2023).
Grigoras, A. et al. Perirenal adipose tissue-current knowledge and future opportunities. J Clin. Med 10(6). (2021).
Lin, L., Li, Z., Chen, K., Shao, Y. & Li, X. Uncovering somatic genetic drivers in prostate cancer through comprehensive genome-wide analysis. Geroscience 47 (3), 5039–5056. https://doi.org/10.1007/s11357-025-01623-8 (2025).
Lin, L., Wang, W., Shao, Y., Li, X. & Zhou, L. National prevalence and incidence of benign prostatic hyperplasia/lower urinary tract symptoms and validated risk factors pattern. Aging Male. 28 (1), 2478875. https://doi.org/10.1080/13685538.2025.2478875 (2025).
Tsay, K., Safari, S., Abu-Samra, A., Kremers, J. & Tzekov, R. Pre-stimulus bioelectrical activity in light-adapted ERG under blue versus white background. Vis. Neurosci. 40, E004 (2023). PMID: 38087845; PMCID: PMC11016353.
O’Hare, L. & Hibbard, P. B. Support for the efficient coding account of visual discomfort. Vis Neurosci. 41, E008. https://doi.org/10.1017/S0952523824000051 (2024) (PMID: 39721939; PMCID: PMC11730975).
Manno, C. et al. Relationship of para- and perirenal fat and epicardial fat with metabolic parameters in overweight and obese subjects. Eat. Weight Disord. 24 (1), 67–72 (2019).
Chughtai, H. L. et al. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension 56 (5), 901–906 (2010).
Ricci, M. A. et al. Morbid obesity and hypertension: the role of perirenal fat. J. Clin. Hypertens. (Greenwich). 20 (10), 1430–1437 (2018).
Funding
This study was supported by the National Natural Science Foundation of China (No. 82203288, 82203320, 82273031, 82273311, 82303922 and 82373225), China National Postdoctoral Program for Innovative Talents (NO. BX20220363), China Postdoctoral Science Foundation (NO. 2023M734038) and the Natural Science Foundation for Distinguished Young Scholars of Guangdong Province (No. 2021B1515020077), Wuhan Natural Science Foundation Exploration Project(2024040801020372).
Author information
Authors and Affiliations
Contributions
W.Z.: Conceptualization, Supervision, Project administration, Writing—Review & Editing; Y. P.: Methodology, Formal analysis, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization; H.T.: Writing—Original Draft, Software, Validation, Methodology, Formal analysis; Y.X.: Validation, Data Curation, Resources; L.Y.: Writing—Review & Editing; L.Z.:Investigation, Software, Methodology; Q.F.: Software, Validation, Methodology; Y.H.: Data Curation; T.H.: Data Curation; D.W.: Data Curation; B.Y.: Data Curation; X.Y.: Data Curation; Z.Z.: Supervision, Funding acquisition; F.Z.: Supervision, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved and reviewed by “The Ethics Review Board of Sun Yat-sen University Cancer Center (SYSUCC; Guangzhou, China)”. Approved number: B2024-228-01. Given the retrospective nature of the study and the use of anonymized data, the requirement for informed consent was waived by “The Ethics Review Board of Sun Yat-sen University Cancer Center”. Relevant statements, including the Ethics Approval Statement and Waived Informed Consent Statement, have been uploaded for reference.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, H., Xie, Y., Yang, L. et al. Perirenal fat thickness may be a significant predictor of prognosis and postoperative renal function in renal cancer surgery patients. Sci Rep 15, 32341 (2025). https://doi.org/10.1038/s41598-025-17865-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17865-7