Abstract
As the phytochromes play a key role in plant light perception, they also modulate stress responses. The tomato mutant c.v. Moneymaker lacking PHYTOCHROME A (PHYA) exhibits tolerance to heat stress during the vegetative growth phase; however, this response does not continue into the reproductive stage. In this study, the response of phyA at the reproductive stage was improved through the exogenous application of 4-chlorophenoxy acetic acid (4-CPA) and ethanol under heat stress, either at 37 °C in controlled culture room or fluctuating high temperatures (approximately 50 °C and 30 °C in midday and night, respectively) during the summer in greenhouse. 4-CPA, a synthesized plant growth regulator with functions similar to that of auxins, induced growth and flower formation at the flowering stage when sprayed on phyA compared to that in the non-treated plants. Similarly, 4-CPA application improved fruit setting and fruit characteristics, and the quantity or quality of the phyA mutant. The expression of numerous heat-related genes, such as heat shock factors (HSFs), heat shock proteins (HSPs), and reactive oxygen species (ROS) scavengers, was upregulated in phyA as a result of 4-CPA application. Ethanol application showed better growth when sprayed on phyA than non-treated plants, and HSFA1a and HSP70 expression was significantly stimulated by this treatment. On the other hand, applying either 4-CPA or ethanol-induced auxin and gibberellin production by stimulating the expression of genes involved in hormone production. Finally, electrolyte leakage (EL) and malondialdehyde (MDA) levels were inhibited after both treatments. In contrast, proline production increased. Therefore, applying 4-CPA or ethanol improved phyA mutant tolerance, allowing the mutant to tolerate heat stress during all growth stages. Furthermore, the quality of tomato fruits is improved by the application of these chemicals.
Similar content being viewed by others
Introduction
Among plant photoreceptors, PHYTOCHROMEs (PHYs) are the well-characterized and are essential for regulating key developmental processes, including germination, de-etiolation, and flowering1,2. The number and types of PHYs vary across plant species. For instance, both the tomato and Arabidopsis genomes contain five PHY genes; however the types differ with PHYA, B1, B2, E, and F found in tomatoes3 and PHYA, B, C, D, and E in Arabidopsis4. Rice contains only three PHYs (PHYA, B, and C)5.
The tomato phytochrome A (phyA) mutant exhibited thermotolerance, particularly during the vegetative growth stage, characterized by enhanced membrane stability, reduced electrolyte leakage (EL) and malondialdehyde (MDA) accumulation, and increased proline levels, contributing to the osmotic balance and stress adaptation. These physiological traits were supported by the upregulation of stress-responsive genes, such as HSFA2, HSFB1, and GRP, which are associated with improved heat resistance. However, the phyA mutant displays defects during the fruiting stage and produces smaller parthenocarpic fruits. Unlike the WT, the phyA mutant failed to stimulate the expression of heat shock factors (HSFs) and heat shock proteins (HSPs), which are important for stress adaptation6. Improving the growth and productivity of the phyA mutant during the reproductive stage is a promising area for future research.
4-Chlorophenoxy acetic acid (4-CPA) is an auxin that influences fruit setting in tomatoes. In particular, applying 4-CPA after anthesis increased the percentage of fruit set, number of fruits per plant, weight, fruit diameter, number of fruits per cluster, and yield per cluster at lower temperatures7. 4-CPA is a plant growth regulator that enhances fruit set during summer8, and can also induce parthenocarpy and fruit development without fertilization9. When the synthetic auxin 4-CPA was applied to flowers, it successfully induced parthenocarpic ovarian growth9.
Chemical priming is a useful strategy for improving plant stress resistance10. Various chemical compounds can activate the molecular processes that regulate tolerance to environmental stress. Recent studies have demonstrated that applying ethanol to plants can improve their tolerance to environmental stresses. Ethanol is widely available and is considered environmentally- and human-friendly11. It has emerged as a promising agent for enhancing plant stress tolerance as an alternative to transgenic approaches and traditional breeding methods. It mitigates drought stress in Arabidopsis and improves drought tolerance in wheat and rice12. Application of 20 mM ethanol improved growth in drought-stressed soybean plants by increasing biomass, leaf area per trifoliate, gas exchange features, water-use efficiency, photosynthetic pigment content, and leaf relative water content13. Additionally, ethanol enhances tolerance to high-salinity stress by detoxifying reactive oxygen species (ROS) in both Arabidopsis and rice14. It also increases heat stress tolerance in Arabidopsis by activating unfolded protein response signaling, which is facilitated by putrescine accumulation15. Ethanol treatment alleviates salt stress in soybean plants by boosting ROS detoxification mechanisms, enhancing antioxidant enzyme activity, and promoting osmotic adjustment by increasing proline and amino acid levels16. Moreover, ethanol reduces oxidative damage caused by stress in Arabidopsis by suppressing ROS accumulation17. Ethanol-induced heat stress tolerance in tomatoes is mainly the result of increased expression of stress-related genes encoding late embryogenesis-abundant (LEA) proteins, ROS elimination enzymes, and activated gluconeogenesis11. Ethanol stimulates the unfolded protein response, making plants more heat tolerant15.
The aim of this study was to identify the phyA response to heat stress during the reproductive stage after exogenous application of 4-CPA and ethanol. Because the phyA mutant exhibited heat tolerance in vegetative growth stage6, if application of 4-CPA and ethanol enhance heat tolerance in reproductive growth stage, combination of the phyA mutation and chemical treatments enhances heat tolerance in both stages. 4-CPA and ethanol improved plant growth of the phyA mutant under heat stress, either at 37 °C or fluctuating high temperatures during the summer in greenhouse conditions. Furthermore, both chemical treatments improved fruit quality, such as increase in sugar content and ascorbic acid. These results indicate that tolerance to heat stress in both vegetative and reproductive growth stages is improved by combination of the phyA mutation and chemical treatments.
Results
Enhancement of heat tolerance by applying 4-CPA and ethanol
Under HS conditions at 37°C in controlled cultivation room and in greenhouse environments (approximately 50 °C and 30 °C in midday and night, respectively), applying 4-CPA and ethanol significantly affected plant growth. Plants treated with 4-CPA and ethanol exhibited improved phenotypic traits under stress conditions, including enhanced height and stem thickness, compared to that of the control plants (Fig. 1a–c, Supplementary Fig. S1a). These results indicated that 4-CPA and ethanol application improved plant tolerance to HS.
Morphological response under heat stress treated with 4-CPA or ethanol (EtOH). (a) Plant phenotype under 37 °C. Several parameters, such as plant height (b), plant thickness (c), number of flower/cluster (d), and rate of fruit set (e) were measured. Statistical analysis was performed to determine significant differences compared to the control at p < 0.05 (*) and at p < 0.01 (**) (n ≥ 4).
Tomato flowers are highly susceptible to HS, which can cause morphological changes in flower structure18. Observations under stable high-temperature conditions (37 °C) revealed that flowers treated with 4-CPA developed faster than those in the control and ethanol-treated groups (Supplementary Fig. S1a).
Applying 4-CPA has been reported to increase fruit set, yield, and economic benefits during summer tomato production. In the present study, 4-CPA application significantly increased the number of flowers / clusters, and these flowers successfully developed into fruits under stressful conditions (Fig. 1d). This indicated that 4-CPA application improved fruit set (Fig. 1e). Although ethanol application also enhanced flower development and fruit set, the differences observed were not statistically significant compared with the control (Fig. 1e). Fruit characteristics, including weight, length, and diameter, were measured under HS conditions. Applying 4-CPA increased fruit weight, length, and diameter significantly compared to those of the control. Ethanol application also improved these fruit characteristics; however, the differences were not statistically significant compared to the control treatment (Fig. 2a–d).
Fruit quality under heat stress. (a) Fruit phenotype and color. Application of 4-CPA exhibited orange-colored fruits, but others did red-colored fruits. Morphological parameters, such as fruit weight (b), fruit length (c), and fruit diameter (d) were investigated. The contents of lycopene (e) or β-carotene (f) in 4-CPA-treated tomato fruits was decreased or increased, respectively, leading to the orange-colored fruits. Brix (g) and ascorbic acid (h) levels were increased in both 4-CPA and EtOH. Statistical analysis was performed to determine significant differences compared to the control at p < 0.05 (*) and at p < 0.01 (**) (n ≥ 3).
Fruit quality after 4-CPA and ethanol application
Fruit quality was evaluated by measuring key parameters, including total soluble solids (Brix %), ascorbic acid content, lycopene, and β-carotene levels, for both applications. Brix increased significantly with the 4-CPA and ethanol treatments (Fig. 2g). To confirm increase in Brix by ethanol application, another cultivar (Sicilian Rouge) was used. Application of ethanol also increased Brix value although other characteristics, such as fruit weight, diameter, and length, were unchanged (Supplementary Fig. S2).
Additionally, the ascorbic acid content increased with both applications (Fig. 2h). Differences in the tomato color were observed after 4-CPA application compared to other treatments, prompting an analysis of lycopene and β-carotene content in the fruits. The results showed that application with 4-CPA inhibited lycopene accumulation while significantly increasing β-carotene levels compared to the control and ethanol-treated fruits. This explained the orange coloration observed in the tomato fruits treated with 4-CPA.
Physiological responses to chemical treatments under HS
The physiological responses of the plant were evaluated under three conditions, i.e., normal conditions (25 °C), 37 °C, and greenhouse conditions. The plants were subjected to these conditions for 3 weeks for the HS treatment.
Membrane thermostability is a reliable parameter for assessing plant tolerance to HS. The membrane stability of the phyA mutant following 4-CPA and ethanol treatment was investigated by measuring the EL of tomato leaves. At 37 °C and under greenhouse environments, plants treated with 4-CPA and ethanol exhibited the lowest EL values, which were significantly lower than those of the control. However, there was no significant difference in the EL between the chemical treatments and the control under normal conditions (Fig. 3a).
Physiological analysis under three conditions, at 25 °C, at 37 °C, and under greenhouse conditions. Electrolyte leakage (EL) (a), malondialdehyde (MDA) accumulation (b), and proline level (c) were investigated. Statistical analysis was performed to determine significant differences compared to the control at p < 0.05 (*) and at p < 0.01 (**) (n ≥ 4).
MDA is an end product of polyunsaturated fatty acid peroxidation in cells and serves as a marker of oxidative stress. There were no significant differences in the MDA levels among the control, 4-CPA, and ethanol treatments under normal conditions. However, both applications significantly reduced MDA accumulation at 37°C and under greenhouse conditions compared to the control (Fig. 3b). These findings indicated that 4-CPA and ethanol application enhanced the membrane thermostability of green organs and inhibited membrane lipid peroxidation under HS during the reproductive stage in the phyA mutant.
Proline is a key abiotic stress indicator in plants and contributes to stabilizing the subcellular structure and scavenging of free radicals. There were no significant differences in proline levels between the control and the two treatments under normal conditions. However, the 4-CPA and ethanol applications resulted in the highest proline accumulation at 37°C and under greenhouse conditions (Fig. 3c).
Changes in stomata features under HS conditions with 4-CPA and ethanol applications
Stomata are small pores in the aboveground organs of plants that facilitate gas exchange and water regulation between plants and their surrounding environment. Their development is highly sensitive to environmental fluctuations, including temperature stress19. Stomata number, stomatal pore length, diameter, and area were measured in plants grown at 37°C. The stomatal number of 4-CPA- and ethanol-treated plants was significantly higher than that of the control plants (Fig. 4b, Supplementary Fig. S3). Additionally, stomatal pore length and diameter were significantly larger in the treated plants, leading to an increase in stomatal pore area relative to that of the control (Fig. 4).
Stomata response under heat stress. (a) Stomata under heat stress treated with 4-CPA or EtOH were observed with microscope. Bars indicate 5 μm length. Stomatal phenotypes, such as stomata number (b), stomatal length (c), stomatal aperture (d), and stomatal pore area were investigated. Statistical analysis was performed to determine significant differences compared to the control at p < 0.01 (**) (n ≥ 16).
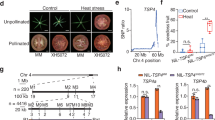
Fertile pollen directly contributes to crop productivity and is essential for the survival, fitness, and dispersal of flowering plants20. Pollen fertility was assessed in flowers from 4-CPA and ethanol-treated plants compared to the control plants, 2 weeks after exposure to HS at 37°C. There were no significant differences in pollen viability among the treatments, and high viability was observed in all groups (Supplementary Fig. S4a), suggesting that pollen viability was not significantly affected by HS.
The pollen tube growth was investigated in vivo. Temperatures above 30°C impair tomato pollen germination and pollen tube growth21. Pollen tube germination was observed 24 h after hand-pollination of the stigma at 25 °C. Although all pollen showed high viability under stress, the pollen tubes from the control and ethanol-treated plants did not grow, whereas only pollen from the 4-CPA-treated plants grew under HS (Supplementary Fig. S4b), indicating that 4-CPA application promoted pollen tube growth under HS.
4-CPA and ethanol treatment enhanced expression of HSFs, HSPs, and several hormone-related genes under HS during the fruiting stage
HS reduces the efficiency of plant physiological and biochemical processes by influencing molecular mechanisms. The ability of plants to tolerate various abiotic stresses, including HS, is considerably influenced by HSFs and HSPs22. The expression levels of heat-responsive genes, including HSFs and HSPs, were examined in control plants and in plants treated with 4-CPA or ethanol under greenhouse conditions. The expression levels of several HSF genes, such as HSFA1a, were upregulated in both 4-CPA- and ethanol-treated plants compared to those in the control, while HSFA1b, HSFA2, and HSFB1 were upregulated after 4-CPA treatment. The expression levels of HSFA4a and HSFA5 did not differ significantly across the treatments (Fig. 5a). Additionally, the expression of HSP70 was upregulated in both 4-CPA- and ethanol-treated plants compared to in the control, whereas the expression of HSP90 significantly increased only in response to 4-CPA treatment (Fig. 5b). These results suggested the involvement of HSFs and HSPs in heat response mechanisms activated by 4-CPA or ethanol.
Expression level of heat shock transcription factors (HSFs) genes and heat shock protein (HSPs) genes under heat stress treated with 4-CPA or EtOH. The expression levels of HSFs (HSFA1a, HSFA1b, HSFA2, HSFB1, HSFA4a, and HSFA5) (a) and of HSPs (HPS70 and HSP90) (b) were examined. Statistical analysis was performed to determine significant differences compared to the control at p < 0.01 (**) (n ≥ 3).
Ascorbate peroxidase (APX), catalase (CAT), and superoxide dismutase (SOD) are enzymatic antioxidant defense systems in plants against environmental stress factors that are essential for scavenging ROS and reducing oxidative stress23. In this study, the expression levels of APX1, APX2, CAT1, CAT2, and SOD were measured after exposure to HS. The expression of APX1 did not show a significant increase in 4-CPA- or ethanol-treated phyA plants compared to non-treated plants (Fig. 6). APX2 expression was significantly stimulated by both chemical treatments compared to that in the non-treated plants (Fig. 6). A similar result was observed for the expression of CAT1 and CAT2, in which 4-CPA-treated phyA showed significant upregulation compared to the ethanol or non-treated group (Fig. 6). SOD expression was similar to the CAT genes expression results, and 4-CPA application significantly stimulated SOD expression compared to the other treatments (Fig. 6). These results indicated that phyA plants treated with 4-CPA stimulated expression of APX2, CAT1, CAT2, and SOD for ROS scavenging, whereas ethanol stimulated APX2.
Expression level of enzymes for antioxidant defense systems, such as ascorbate peroxidase (APX), catalase (CAT), and superoxide dismutase (SOD) under heat stress treated with 4-CPA or EtOH. Statistical analysis was performed to determine significant differences compared to the control at p < 0.05 (*) and at p < 0.01 (**) (n ≥ 3).
Tomato contains a single euAP3 lineage gene, Tomato APETALA3 (TAP3), and Tomato MADS-box gene 6 lineage (TM6), both of which play distinct roles in floral development. TAP3 is essential for specifying petal and stamen identities, whereas TM6 is crucial for stamen differentiation24. The expression level of TM6 significantly increased in both treatments (Supplementary Fig. S5), indicating that both treatments enhanced floral organ development.
The tryptophan aminotransferase A (TAA) family produces tryptophan-derived indole-3-pyruvic acid (IPA), while the YUC family functions in converting IPA to indole-3-acetic acid (IAA) in Arabidopsis through a quantification process of IPA. Two gene families encoding key enzymes in the TAA/YUC biosynthesis pathway have been identified in tomatoes, providing new insights into the regulatory mechanisms of auxins during tomato development25. The expression level of TAR2a, which is categorized in this family, increased significantly following 4-CPA application, and TAR2b exhibited significant upregulation under both 4-CPA and ethanol treatments compared to the control plants (Fig. 7a1, a2). Auxin response factor 5 (ARF5) is highly expressed in the leaves, flowers, and early immature green fruit in tomatoes, suggesting its role in the development of these tissues and organs. Overexpression of SlARF5 increases plant height26. SlARF7 exhibits a bidirectional regulatory effect on tomato fruit development by modulating fruit growth-related genes (e.g., EXPANSIN5) and mediating crosstalk between auxins and gibberellins (GAs)27. The expression levels of both SlARF5 and SlARF7 increased significantly with 4-CPA and ethanol application compared to those in the control plants (Fig. 7a3, a4). The increased expression of TAR2a and TAR2b in tomatoes may enhance auxin biosynthesis, which is associated with growth regulation, developmental changes, and stress responses. Similarly, the upregulation of ARF5 and ARF7 suggested improved auxin signaling (Fig. 7a1–a4), significantly affecting fruit development, fruit set, and the potential production of parthenocarpic fruits.
Expression level of auxin signaling-related (a) or gibberellin signaling-related genes (b) under heat stress treated with 4-CPA or EtOH. The expression level of auxin signaling-related genes, such as TAR2a, TAR2b, ARF5, and ARF7, were measured. The expression level of gibberellin signaling-related genes, such as GA20OX1, GA3OX2, GA2OX1, GA2OX5, and GID1, were also investigated. Statistical analysis was performed to determine significant differences compared to the control at p < 0.05 (*) and at p < 0.01 (**) (n ≥ 3).
GAs are plant hormones that regulate various aspects of plant growth, including flowering, fruit setting, hypocotyl and stem elongation, root expansion, seed germination, and fruit development28. Bioactive GAs are synthesized in the cytosol from GA12 and GA53 through the activity of two 2-oxoglutarate-dependent dioxygenases (2-ODDs) families, GA 20-oxidases (GA20ox), and GA 3-oxidases (GA3ox) (Shohat et al., 2021). In this study, the expression levels of two genes related to GA biosynthesis, GA20OX1 and GA3OX2, were analyzed. The expression of GA20OX1 increased significantly after ethanol application, whereas GA3OX2 expression increased significantly after 4-CPA treatment. These findings suggested that gibberellin activation occurs in both applications (Fig. 7b1, b2). GA deactivation, primarily catalyzed by GA 2-oxidases (GA2ox), reduces the levels of bioactive GAs29. The expression levels of the two GA2ox genes, GA2OX1 and GA2OX5, were evaluated. The expression of GA2OX1 decreased following ethanol application, while GA2OX5 expression decreased under both 4-CPA and ethanol treatments. GA deactivation may be reduced in both applications (Fig. 7b3, b4). GIBBERELLIN INSENSITIVE DWARF1 (GID1) is a receptor and nucleocytoplasmic protein that plays a critical role in GA signaling. In the presence of GA, GID1 undergoes a conformational change that enhances its ability to bind to DELLA proteins. DELLA proteins typically suppress GA signaling by inhibiting transcription factors involved in GA-regulated genetic programs30. In the present study, the expression level of GID1 increased significantly with both 4-CPA and ethanol treatments (Fig. 7b5). These results suggested that 4-CPA and ethanol treatment stimulate GA activity by enhancing biosynthesis, reducing deactivation, and promoting GA signaling.
The physiology and abundance of stomata are critical for plant adaptation and acclimatization to diverse habitats under specific environmental conditions. The ability of plants to adjust the number, size, and distribution of stomata on their leaves contributes to their fitness under changing environments. MUTE and FAMA genes, which encode bHLH-type transcription factors, regulate the initiation and progression of stomatal lineages. These genes promote cell division and fate transitions during stomatal development31. In the present study, 4-CPA significantly increased the expression of MUTE and FAMA under HS conditions (Supplementary Fig. S6). STOMATAL DENSITY AND DISTRIBUTION 1 (AtSDD1) acts as a negative regulator of stomatal density in Arabidopsis, optimizing transpiration and water-use efficiency. In the present study, the expression of SDD1 decreased significantly following ethanol application, suggesting enhanced stomatal development under stressful conditions (Supplementary Fig. S6). Overexpression of SDD1-like gene reduces stomatal density and prevents dehydration in both Arabidopsis and cultivated tomatoes31. However, the expression level of SDD1-like did not differ between the plants treated with 4-CPA and ethanol and the control plants (Supplementary Fig. S6). Applying 4-CPA and ethanol may enhance stomatal development and function under stressful conditions, potentially contributing to improved stress tolerance in plants.
Fruit quality is determined by several characteristics, including visual characteristics such as fruit size and color, taste characteristics such as sweetness32, and nutritional value characteristics such as vitamin C content, which has an antioxidant effect33.
FRUIT WEIGHT 2.2 (FW2.2) is a member of the CELL NUMBER REGULATOR gene family that negatively regulates cell division and affects tomato fruit size and weight in tomato34. The fascinated (FAS) gene is essential for controlling the number of fruit locules, directly affecting fruit size and shape35. The expression of FW2.2 decreased significantly after both chemical treatments compared with that in non-treated phyA plants. Only the expression of the FAS gene was significantly downregulated in 4-CPA-treated plants compared to that in ethanol-treated and non-treated plants (Fig. 8a), indicating fruit size stimulation after 4-CPA application.
Regarding fruit color, the phytoene synthase (PSY1) gene regulates the biosynthesis of carotenoids during tomato fruit development and ripening36. The carotenoid isomerase (CRTISO) gene is responsible for the isomerization of prolycopene to all-trans lycopene in the carotenoid synthesis pathway37. The expression levels of both PSY1 and CRTISO genes did not differ markedly between the treated and non-treated phyA plants (Fig. 8b). On the other hand, lycopene β-cyclase (LYCB) is a crucial enzyme for producing β-carotene38. The 4-CPA-treated plants showed a significantly higher expression level of LYCB compared to ethanol-treated and non-treated phyA plants (Fig. 8b), indicating the reason for the high β-carotene in tomato fruit treated with 4-CPA (Fig. 2f).
For sweetness, the sucrose synthase (SUS) gene is crucial for plant growth and sucrose metabolism39. In this study, the expression of the SUS gene increased significantly with both 4-CPA and ethanol application compared to that in untreated plants (Fig. 8c), indicating higher sugar accumulation in their fruits.
Regarding the vitamin C content, GDP-Mannose 3′,5′-epimerase (GME) in the D-mannose/L-galactose pathway is a crucial enzyme in the biosynthesis of vitamin C40. In the last stage of this pathway, L-galactono-1, 4-lactone is transformed into ascorbate by L-galactono-1,4-lactone dehydrogenase (GLDH)41. In this study, the expression level of GME increased significantly in ethanol-treated phyA plants compared to that in 4-CPA-treated and untreated plants. GLDH expression was significantly higher in both 4-CPA- and ethanol-treated plants than in the non-treated phyA plants (Fig. 8d), indicating the induction of ascorbic acid after both treatments.
Discussion
PHYA is a key gene involved in sensing far-red light signals and plays a critical role in various developmental stages of plant growth, including flowering and seed development42. Additionally, the PHYA gene has been linked to stress responses, particularly HS, for which the phyA mutant exhibits a tolerance mechanism compared to the Moneymaker WT plants. This tolerance was more pronounced during the vegetative growth stage. However, during the transition to the reproductive stage, tolerance was observed at flowering, but this diminished during the fruiting stage6. Application of 4-CPA and ethanol-induced phyA tolerance during the flowering and fruiting stages (Fig. 1a). These induced tolerance responses begin by protecting the cell membrane from heat damage. Plants treated with these chemicals exhibited lower MDA and EL accumulation (Fig. 3a, b), which are key indicators of membrane lipid peroxidation and membrane permeability43. Furthermore, proline accumulation induction supported phyA tolerance activation following the chemical treatment (Fig. 3c). Proline is an important osmoprotectant that stabilizes cellular structures and mitigates heat damage44.
The phyA plants treated with 4-CPA and ethanol exhibited an increase in stomata number and stomatal pore area compared with non-treated phyA plants (Fig. 4c, d). This response suggested an enhanced level of water transpiration, which may facilitate transpiration cooling, a mechanism that helps to mitigate the effects of high temperatures45. An increased stomata response in 4-CPA-treated plants was linked to the upregulation of MUTE and FAMA genes (Supplementary Fig. S6), which are essential for stomata development46. Overexpression of either MUTE or FAMA increases stomatal density and index in tomatoes, with the MUTE gene having a particularly significant effect46. Conversely, the increase in stomata number and pore areas may also be attributed to the downregulation of the SDD1 gene (Supplementary Fig. S6). This gene negatively regulates stomatal density and plays a role in optimizing transpiration and water-use efficiently31.
The phyA-treated plants treated with 4-CPA showed an increased expression of HSFs and HSPs under HS conditions (Fig. 5). Generally, HS causes growth cessation. Arabidopsis HSFA1 is considered a master regulator of heat tolerance and its function in tomato plants is similar to that of HSA1a47. When HSFs, particularly HSFA1, HSFA2, and HSFB1 are stimulated, they induce the expression of HSP, including HSP70 and HSP90. These chaperones are critical in preventing protein denaturation and reducing ROS accumulation48. This mechanism was evident in the 4-CPA-treated plants, in which the expressions of HSFA1a, HSFA1b, HSFA2, and HSFB1 were upregulated. Consequently, the expressions of HSP70 and HSP90 were induced (Fig. 5), leading to reduced lipid membrane peroxidation as indicated by lower MDA accumulation (Fig. 3b). Similarly, ethanol-treated phyA plants exhibited higher expression of the tomato master HSFA1a than untreated plants, along with a significant upregulation of HSP70 (Fig. 5). This likely contributed to enhanced cellular protection against membrane lipid peroxidation (Fig. 3b).
Plant growth was also stimulated during the upregulation of HSFs and HSPs and the protection of cell membranes in the chemically treated plants (Fig. 5). The chemically treated plants exhibited healthier phenotypes, with significant increases in plant height and stem thickness compared to the non-treated phyA plants during the reproductive stage (Fig. 1a–c). Generally, plant defense responses to HS are mediated by endogenous phytohormones that stimulate defense mechanisms. Auxins play an important role in HS protection by promoting stem elongation and leaf hyponasty49. In the present study, the expression of auxin signaling genes, including TAR2 and ARF, was significantly upregulated in 4-CPA- and ethanol-treated plants (Fig. 7a), indicating increased auxin production. Moreover, GA is involved in plant height, leaf expansion, dry matter accumulation, tissue differentiation, and cell division, and plays a role in tolerance to HS in tomatoes50. In this study, the expression of GA biosynthesis genes (GA20OX1 and GA3OX2) was upregulated, whereas that of GA inhibitor genes (GA2OXs) was downregulated in 4-CPA- and ethanol-treated plants compared to that in untreated plants. GA20OX1 expression was markedly increased by ethanol application, and GA3OX2 expression was significantly upregulated by 4-CPA treatment. Similarly, GA2OX1 and GA2OX5 were inhibited by ethanol, with a similar inhibitory effect on GA2OX5 observed in the 4-CPA-treated plants (Fig. 7b1–4). Additionally, GID1, which encodes the gibberellin receptor, was significantly upregulated in response to both chemical treatments (Fig. 7b5). These results suggested that both the auxin and GA pathways were activated following chemical application, enhancing plant growth. The induction of auxin by 4-CPA application was consistent with previous findings that growth regulators such as 4-CPA increase internal IAA levels during the early stages of tomato fruit development51. Moreover, the stimulation of HSP90 in 4-CPA-treated plants (Fig. 5b2) supported the activation of ARFs and repression of Aux/IAAs transcriptional repressors52. The mechanism underlying auxin induction by ethanol treatment remains unclear. However, the induction of HSP70 may elicit a response similar to HSP90, facilitating auxin stimulation. GA induction by both 4-CPA and ethanol treatments appears to result from the crosstalk between the auxin and GA biosynthesis pathways. Specifically, ARF genes can stimulate GA3OXs expression while repressing GA2OXs, thereby enhancing GA production53. This interplay was clearly observed in the chemically treated plants (Fig. 7b).
For reproductive organs, it has been reported that the setting of tomato fruit is controlled by successful pollination and fertilization, which trigger fruit development through the auxin and GA signaling pathways54. Tomato parthenocarpy can be promoted by GA3 or 2,4-D application, resulting in fruits similar to those formed after pollination55. In this study, increased auxin- and GA-related gene expression (Fig. 7) may have enhanced flower and fruit development in 4-CPA-treated phyA plants compared to untreated plants. The flower number, fruit set, and fruit weight were significantly improved, and parthenocarpic fruits were observed (Figs. 1d, e, 2b, and Supplementary Fig. S1c). In addition to the roles of auxins and GA in promoting reproductive organ development, the FA gene was upregulated in 4-CPA-treated plants, contributing to flowering induction. This was accompanied by the upregulation of the TM6 gene (Supplementary Fig. S5), regulating normal flower development56. Conversely, the ethanol treatment significantly increased TM6 expression (Supplementary Fig. S5) and partially improved fruit development (Fig. 1e). However, these improvements were not statistically significant compared to those in the non-treated plants.
Although fruit size differed among the treated plants, fruit quality was consistent between the 4-CPA- and ethanol-treated plants when compared to the non-treated plants. Fruit quality was improved by both treatments, as evidenced by increased Brix levels and ascorbic acid content (Fig. 2). The increase in Brix levels can be attributed to GA stimulation following chemical treatment, consistent with previous findings that GA production can enhance sugar content in seedless tomatoes57. In addition to the upregulation of the SUS gene in phyA mutant after being treated with 4-CPA or ethanol (Fig. 8c), this gene catalyzes the reversible reaction of sucrose + UDP ⇄ UDP-glucose + fructose58. The increase in the ascorbic acid content was due to the induction of GLDH gene expression (Fig. 8d2), which is involved in the transformation of l-galactono-1,4-lactone into ascorbate41. This stimulation might be linked to hormone induction (Fig. 7) or the induction of parthenocarpy because parthenocarpic tomato fruits have a higher vitamin C content than seeded fruits59.
Along with Brix values and vitamin C content, fruit color is a critical factor in determining fruit quality. Lycopene and β-carotene are the key pigments responsible for tomato fruit color60. Fruits from 4-CPA-treated plants exhibited high β-carotene and low lycopene content (Fig. 2e, f), resulting in an orange-colored tomato fruit (Fig. 2a). This effect may be attributed to 4-CPA’s role as a synthetic auxin, as previous studies have shown that exogenous auxin application in tomatoes can increase β-carotene content and decrease lycopene levels at the later stages of development compared to non-treated plants60. Induction of LYC-B gene expression after 4-CPA application (Fig. 8b3) is involved in the β-carotene stimulation because of its function as a crucial enzyme for β-carotene production38.
Ethanol can both increase and decrease reactive oxygen species (ROS) in plants, depending on the context. Ethanol can initially increase ROS production61, but under stress conditions, it can also decrease ROS levels14,17. Increase in ROS enhances stomatal closure62. Stomatal aperture under heat stress conditions was observed in this study, ROS level may decrease, and stomata were opened as shown in previous report, in which ethanol suppressed ROS accumulation in high light condition17. Furthermore, auxin generally works as a positive regulator for stomatal opening63. These effects probably promote stomatal opening in this study.
In summary, this study reveals a strategy for improving heat stress tolerance in tomato by utilizing exogenous application of 4-CPA and ethanol to activate key hormonal and stress-responsive genes in the phyA mutant tomato plants. These findings provide an insight into the molecular and physiological mechanisms underlying heat tolerance and offer tools for developing resilient tomato cultivars through chemical treatment-based approaches. Furthermore, this study demonstrated that applying 4-CPA to flower clusters induces various heat tolerance mechanisms, enhancing the development and fruit formation in phyA mutant plants. Additionally, ethanol application enhanced plant tolerance to HS, as evidenced by physiological responses, while improving fruit quality (Fig. 9).
Materials and methods
Plant materials and growth conditions
The phyA mutant seeds (Solanum lycopersicum cv. Moneymaker) were grown in soil or Rockwool cubes and incubated at 25 °C under a long-day photoperiod (16 h light/8 h dark) until the flowering stage. Plants were exposed to heat stress (HS) under two different conditions after flowering: a constant high temperature of 37 °C and fluctuating high temperatures under greenhouse conditions (from July to October). The temperatures were approximately 50 °C and 30 °C in sunny midday and night, respectively, from July to mid September.
Chemical treatment
Three different groups were designed for the chemical treatment. The first group consisted of phyA mutant without any treatment, the second group was sprayed with 4-CPA at 20 ppm, and the third group was sprayed with 20 mM ethanol. These concentrations of 4-CPA and ethanol were based on the result of tomatoes; application of 4-CPA at 20 ppm increased numbers of fruits and plant yield8 and application of 20 mM ethanol enhanced heat tolerance of tomato11. The chemicals were dissolved into water. For the 4-CPA application, flower clusters were targeted and sprayed once per week. From 10 to 20 mL of 4-CPA was sprayed for one flower cluster. In the third group, ethanol was sprayed onto the whole plant once per week for one month until the first fruit was set. From 50 to 100 mL of ethanol was sprayed for each whole plant. All the groups were subjected to HS conditions until the plants reached the flowering stage.
Morphological phenotype
The morphological characteristics of the tomato plants’ vegetative organs, including plant stem height and stem thickness, were measured. Plant height was measured using a ruler, and stem thickness was determined using a digital caliper. The number of flowers and clusters and the percentage of developed flowers and clusters under HS conditions were recorded as the floral characteristics. The average fruit fresh weight (FW), length, diameter, fruit set percentage, and parthenocarpy occurrence were recorded as the fruiting characteristics to evaluate the treatment responses under HS conditions.
EL measurement
EL in tomato leaves was analyzed as previously described6. Briefly, the leaf surface was washed with Milli-Q water (MQ) to remove ions from the surface and the leaves were submerged in a tube filled with MQ to cover all parts of the leaf. The tube was incubated in a water bath at 43 ± 1 °C for 1 h. The initial ionic conductivity (IC1) was measured after cooling to room temperature (23 ± 2 °C). The samples were then autoclaved at 121 °C for 10 min, cooled to room temperature, and the second ionic conductivity (IC2) was measured. A conductivity meter (Lutron Electronics Co., Inc., Coopersburg, PA, USA) was used to measure the ionic conductivity. The percentage of EL was calculated using the following formula: EL (%) = IC1 / IC2 × 100.
Measuring proline and MDA levels
Proline content in the leaf samples was determined as previously described64. The absorbance at 520 nm (A520) was measured using a DU-800 spectrophotometer (Beckman Coulter, Inc., Brea, CA, USA). Proline concentration was calculated using the following equation:
The MDA levels were measured as described43 and calculated using the following formula:
Microscopic analysis of stomata
Fresh leaflets were collected to prepare the leaf samples for stomatal analysis. A piece of thick tape was applied to the upper surface of the leaflet and gently removed to peel off the epidermis, thereby exposing a thin transparent layer of surface cells. The epidermal layer was then placed on a microscope slide, and the leaflet was carefully trimmed using a sharp scalpel. One drop of water was added to the sample, and a coverslip was placed over it. The stomatal number, pore length, and aperture were examined using an Olympus BX50 microscope (Olympus, Tokyo, Japan). As described previously65, the stomatal number was determined under 40 × magnification with a counting area of 92.7 mm2, whereas the pore length and aperture were measured under 100 × magnification. ImageJ software was used for measuring.
RNA isolation and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from leaves after 4 weeks of exposure to HS to investigate heat- and stress-responsive genes during the flowering stage. Total RNA was isolated from the three groups of plant samples using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. A total of 2 µg of RNA was used to synthesize cDNA using a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific). The primers used for the real-time PCR are listed in Table S1. RT-PCR amplification and detection were performed using THUNDERBIRD SYBR qPCR Mix (Toyobo, Osaka, Japan) on a 7900HT real-time PCR system (Applied Biosystems/Thermo Fisher Scientific). The relative transcript abundance was calculated using the comparative CT method. The ΔCT of WT under HS as a subthreshold factor in the ΔΔCT subtraction formula for comparison with the phy treatment was calculated as follows: ΔΔCT (ΔCT − ΔCT, WT (stress)). The tomato EXPRESSED gene was used as an endogenous control for the gene expression analysis66.
Pollen fertility and pollen tube growth test
Newly blooming flowers were collected daily from plants grown under HS conditions to extract pollen grains. Anther cones were isolated from the flowers and left to dry for 3–4 h. Each anther cone was divided into 2–3 parts, and pollen grains were extracted by gently tapping the cones. Pollen was collected in a tube for subsequent microscopic analysis of pollen fertility and pollen tube growth.
Pollen fertility was analyzed by staining pollen grains with potassium iodide, as described previously67. The stained pollen was observed under an Olympus BX50 microscope. The fertile pollen grains were manually counted across at least four microscopic fields.
The flowers were emasculated 1 day before flower opening. Manual cross-pollination using pollen extracted after the stress treatment was performed the following day. After 24 h, the pistils were collected and immersed in a fixing solution (3:1 ethanol:acetic acid) for 12 h, followed by immersion in 75% ethanol for 6–8 h, and transfer to a softening solution (5 M NaOH) for 12–16 h. An aniline blue working solution was prepared 1 day in advance by diluting 0.01% (v/v) aniline blue stock solution with 0.1 M K2HPO4 (pH 10) at a 1:10 ratio and storing it at 4 °C in the dark overnight. The pistils were then transferred to the aniline blue working solution for 24 h, mounted on a glass slide with glycerol as the mounting agent, and flattened by firmly pressing the cover glass. The sections were observed under an Olympus BX50 UV microscope.
Lycopene and β-carotene contents
Fresh tomatoes (1 g) were mixed with 1 mL of acetone: hexane (4:6) and placed on ice for 10 min to measure lycopene content. The mixture was centrifuged at 1,370 × g for 10 min and the absorbance was measured at 663, 645, 505, and 453 nm68 using a DU-800 spectrophotometer (Beckman Coulter). The following formula was used:
Fresh tomatoes (1 g) were mixed with 1 mL of acetone and placed on ice with shaking for 15 min. To measure β-carotene content. The mixture was vigorously mixed for 10 min, centrifuged at 1370 × g for 10 min, and the absorbance was measured at 449 nm using a DU-800 spectrophotometer (Beckman Coulter, Inc). Using the following formula, Y = aX + b, where Y is the response (Absorbance), X is the concentration of β-carotene, a is the slope, and b is the intercept of the standard curve69.
Ascorbic acid content
Ascorbic acid content was measured as described previously70. Tomato fruits (1 g) were homogenized and mixed with 2 mL of 5% metaphosphoric acid (w/v). After centrifugation at 12,000 × g for 3 min, the supernatant was collected as the crude extract. Total ascorbic acid content was measured using a reflectometer (RQflex Plus 10).
Statistical analyses
Analysis of variance was performed using the ASTATA database to analyze the quantitative data, with means compared using Duncan’s multiple range test (p < 0.05) or Tukey HSD post-hoc test.
Data availability
Data available under request to the corresponding author.
References
Gavassi, M. A., Monteiro, C. C., Campos, M. L., Melo, H. C. & Carvalho, R. F. Phytochromes are key regulators of abiotic stress responses in tomato. Sci. Hortic. 222, 126–135. https://doi.org/10.1016/j.scienta.2017.04.035 (2017).
Song, C. et al. 3D structures of plant phytochrome A as Pr and Pfr from solid-state NMR: implications for molecular function. Front. Plant Sci. 9, 2018. https://doi.org/10.3389/fpls.2018.00498 (2018).
Hauser, B. A., Cordonnier-Pratt, M.-M., Daniel-Vedele, F. & Pratt, L. H. The phytochrome gene family in tomato includes a novel subfamily. Plant Mol. Biol. 29, 1143–1155. https://doi.org/10.1007/BF00020458 (1995).
Sharrock, R. A. & Clack, T. Patterns of expression and normalized levels of the five Arabidopsis phytochromes. Plant Physiol. 130, 442–456. https://doi.org/10.1104/pp.005389 (2002).
Sun, W. et al. The rice phytochrome genes, PHYA and PHYB, have synergistic effects on anther development and pollen viability. Sci. Rep. 7, 6439. https://doi.org/10.1038/s41598-017-06909-2 (2017).
Abdellatif, I. M. Y. et al. Functional characterization of tomato phytochrome A and B1B2 mutants in response to heat stress. Int. J. Mol. Sci. 23, 452. https://doi.org/10.3390/ijms23031681 (2022).
Poonia, S., Raiger, P. R., Ram, M. & Kuri, R. Optimal use of plant growth regulators for improved growth, yield, and economic returns of winter tomato (Solanum lycopersicum) in arid regions. Ann. Arid Zone 63, 107–112 (2024).
Sasaki, H., Yano, T. & Yamasaki, A. Reduction of high temperature inhibition in tomato fruit set by plant growth regulators. Japan Agric. Res. Q. 39, 135–138 (2005).
Mariotti, L., Picciarelli, P., Lombardi, L. & Ceccarelli, N. Fruit-set and early fruit growth in tomato are associated with increases in indoleacetic acid, cytokinin, and bioactive gibberellin contents. J. Plant Growth Regul. 30, 405–415. https://doi.org/10.1007/s00344-011-9204-1 (2011).
Bashir, K. et al. Chemical application improves stress resilience in plants. Plant Mol. Biol. 115, 47. https://doi.org/10.1007/s11103-025-01566-w (2025).
Todaka, D. et al. Application of ethanol alleviates heat damage to leaf growth and yield in tomato. Front. Plant Sci. 15, 2024. https://doi.org/10.3389/fpls.2024.1325365 (2024).
Bashir, K. et al. Ethanol-mediated novel survival strategy against drought stress in plants. Plant Cell Physiol. 63, 1181–1192. https://doi.org/10.1093/pcp/pcac114 (2022).
Rahman, M. M. et al. Ethanol positively modulates photosynthetic traits, antioxidant defense and osmoprotectant levels to enhance drought acclimatization in soybean. Antioxid. Basel 11, 452. https://doi.org/10.3390/antiox11030516 (2022).
Nguyen, H. M. et al. Ethanol enhances high-salinity stress tolerance by detoxifying reactive oxygen species in Arabidopsis thaliana and rice. Front. Plant Sci. 8, 2017. https://doi.org/10.3389/fpls.2017.01001 (2017).
Matsui, A. et al. Ethanol induces heat tolerance in plants by stimulating unfolded protein response. Plant Mol. Biol. 110, 131–145. https://doi.org/10.1007/s11103-022-01291-8 (2022).
Das, A. K. et al. Ethanol treatment enhances physiological and biochemical responses to mitigate saline toxicity in soybean. Plants 11, 452. https://doi.org/10.3390/plants11030272 (2022).
Sako, K., Nagashima, R., Tamoi, M. & Seki, M. Exogenous ethanol treatment alleviates oxidative damage of Arabidopsis thaliana under conditions of high-light stress. Plant Biotechnol. (Tokyo) 38, 339–344. https://doi.org/10.5511/plantbiotechnology.21.0715a (2021).
Alsamir, M., Mahmood, T., Trethowan, R. & Ahmad, N. An overview of heat stress in tomato (Solanum lycopersicum L.). Saudi J. Biol. Sci. 28, 1654–1663. https://doi.org/10.1016/j.sjbs.2020.11.088 (2021).
Samakovli, D., Tichá, T. & Šamaj, J. HSP90 chaperones regulate stomatal differentiation under normal and heat stress conditions. Plant Signal. Behav. 15, 1789817. https://doi.org/10.1080/15592324.2020.1789817 (2020).
Jiang, L. et al. MSH7 confers quantitative variation in pollen fertility and boosts grain yield in maize. Plant Biotechnol. J. 22, 1372–1386. https://doi.org/10.1111/pbi.14272 (2024).
Karapanos, I. C., Akoumianakis, K. A., Olympios, C. M. & Passam, H. C. The effect of substrate, ADP and uncoupler on the respiration of tomato pollen during incubation in vitro at moderately high temperature. Sex Plant Reprod. 22, 133–140. https://doi.org/10.1007/s00497-009-0098-z (2009).
Ul Haq, S. et al. Heat shock proteins: dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 20, 785. https://doi.org/10.3390/ijms20215321 (2019).
Sharma, P., Jha, A. B., Dubey, R. S. & Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 217037. https://doi.org/10.1155/2012/217037 (2012).
de Martino, G., Pan, I., Emmanuel, E., Levy, A. & Irish, V. F. Functional analyses of two tomato APETALA3 genes demonstrate diversification in their roles in regulating floral development. Plant Cell 18, 1833–1845. https://doi.org/10.1105/tpc.106.042978 (2006).
Meng, S. et al. Analysis of YUC and TAA/TAR gene families in tomato. Horticulturae 9, 665 (2023).
Lin, Q. et al. Tomato SlARF5 participate in the flower organ initiation process and control plant height. BMC Plant Biol. 24, 993. https://doi.org/10.1186/s12870-024-05707-z (2024).
Li, B. J., Bao, R. X., Shi, Y. N., Grierson, D. & Chen, K. S. Auxin response factors: important keys for understanding regulatory mechanisms of fleshy fruit development and ripening. Hortic. Res. 11, uhae209. https://doi.org/10.1093/hr/uhae209 (2024).
Wu, M. et al. Gibberellins involved in fruit ripening and softening by mediating multiple hormonal signals in tomato. Hortic. Res. 11, uhad275. https://doi.org/10.1093/hr/uhad275 (2024).
Shohat, H., Eliaz, N. I. & Weiss, D. Gibberellin in tomato: metabolism, signaling and role in drought responses. Mol. Hortic. 1, 15. https://doi.org/10.1186/s43897-021-00019-4 (2021).
Gazara, R. K., Moharana, K. C., Bellieny-Rabelo, D. & Venancio, T. M. Expansion and diversification of the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1 (GID1) family in land plants. Plant Mol. Biol. 97, 435–449. https://doi.org/10.1007/s11103-018-0750-9 (2018).
Morales-Navarro, S. et al. Overexpression of a SDD1-like gene from wild tomato decreases stomatal density and enhances dehydration avoidance in arabidopsis and cultivated tomato. Front. Plant Sci. 9, 2018. https://doi.org/10.3389/fpls.2018.00940 (2018).
Flores, F. B. et al. The effectiveness of grafting to improve tomato fruit quality. Sci. Hortic. 125, 211–217. https://doi.org/10.1016/j.scienta.2010.03.026 (2010).
Zhang, J. et al. A comprehensive evaluation of tomato fruit quality and identification of volatile compounds. Plants 12, 2947 (2023).
Beauchet, A. et al. The CELL NUMBER REGULATOR FW2.2 protein regulates cell-to-cell communication in tomato by modulating callose deposition at plasmodesmata. Plant Physiol. 196, 883–901. https://doi.org/10.1093/plphys/kiae198 (2024).
Cong, B., Barrero, L. S. & Tanksley, S. D. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nat. Genet. 40, 800–804. https://doi.org/10.1038/ng.144 (2008).
Enfissi, E. M. et al. Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotechnol. J. 3, 17–27. https://doi.org/10.1111/j.1467-7652.2004.00091.x (2005).
Park, H., Kreunen, S. S., Cuttriss, A. J., DellaPenna, D. & Pogson, B. J. Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14, 321–332. https://doi.org/10.1105/tpc.010302 (2002).
Guo, F., Zhou, W., Zhang, J., Xu, Q. & Deng, X. Effect of the citrus lycopene β-cyclase transgene on carotenoid metabolism in transgenic tomato fruits. PLoS ONE 7, e32221. https://doi.org/10.1371/journal.pone.0032221 (2012).
Li, M. et al. Sucrose synthase gene family in Brassica juncea: genomic organization, evolutionary comparisons, and expression regulation. PeerJ 9, e10878. https://doi.org/10.7717/peerj.10878 (2021).
Zhang, C. et al. Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 30, 389–398. https://doi.org/10.1007/s00299-010-0939-0 (2011).
Schertl, P. et al. L-galactono-1,4-lactone dehydrogenase (GLDH) forms part of three subcomplexes of mitochondrial complex I in Arabidopsis thaliana. J. Biol. Chem. 287, 14412–14419. https://doi.org/10.1074/jbc.M111.305144 (2012).
Lei, Y. et al. Functional dissection of phytochrome A in plants. Front. Plant Sci. 15, 2024. https://doi.org/10.3389/fpls.2024.1340260 (2024).
Shi, X., Jiang, F., Wen, J. & Wu, Z. Overexpression of Solanum habrochaites microRNA319d (sha-miR319d) confers chilling and heat stress tolerance in tomato (S. lycopersicum). BMC Plant Biol. 19, 214. https://doi.org/10.1186/s12870-019-1823-x (2019).
Tiwari, Y. K. Proline as a key player in heat stress tolerance: insights from maize. Discover Agric. 2, 121. https://doi.org/10.1007/s44279-024-00084-5 (2024).
Sadok, W., Lopez, J. R. & Smith, K. P. Transpiration increases under high-temperature stress: potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 44, 2102–2116. https://doi.org/10.1111/pce.13970 (2021).
Ortega, A., de Marcos, A., Illescas-Miranda, J., Mena, M. & Fenoll, C. The tomato genome encodes SPCH, MUTE, and FAMA candidates that can replace the endogenous functions of their arabidopsis orthologs. Front. Plant Sci. 10, 2019. https://doi.org/10.3389/fpls.2019.01300 (2019).
Hoshikawa, K., Pham, D., Ezura, H., Schafleitner, R. & Nakashima, K. Genetic and molecular mechanisms conferring heat stress tolerance in tomato plants. Front. Plant Sci. 12, 2021. https://doi.org/10.3389/fpls.2021.786688 (2021).
Hahn, A., Bublak, D., Schleiff, E. & Scharf, K. D. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell 23, 741–755. https://doi.org/10.1105/tpc.110.076018 (2011).
Küpers, J. J., Oskam, L. & Pierik, R. Photoreceptors regulate plant developmental plasticity through auxin. Plants 9, 1452. https://doi.org/10.3390/plants9080940 (2020).
Guo, T. et al. Heat stress mitigation in tomato (Solanum lycopersicum L.) through foliar application of gibberellic acid. Sci. Rep. 12, 11324. https://doi.org/10.1038/s41598-022-15590-z (2022).
Gemici, M., Turkyilmaz, B. & Tan, K. Effects of 2,4-D and 4-CPA on yield and quality of the tomato, Lycopersicon esculentum Miller. J. Faculty Sci. 29, 24–32 (2006).
Li, N. et al. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 11, 2020. https://doi.org/10.3389/fpls.2020.627969 (2021).
Weiss, D. & Ori, N. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol. 144, 1240–1246. https://doi.org/10.1104/pp.107.100370 (2007).
de Jong, M., Mariani, C. & Vriezen, W. H. The role of auxin and gibberellin in tomato fruit set. J. Exp. Bot. 60, 1523–1532. https://doi.org/10.1093/jxb/erp094 (2009).
Serrani, J. C., Fos, M., Atarés, A. & García-Martínez, J. L. Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv micro-tom of tomato. J. Plant Growth Regul. 26, 211–221. https://doi.org/10.1007/s00344-007-9014-7 (2007).
Fonseca, R. et al. Insights into the functional role of tomato TM6 as a transcriptional regulator of flower development. Hortic. Res. 11, uhae019. https://doi.org/10.1093/hr/uhae019 (2024).
Setiawan, A., Multi, R. & Purwantoro, A. Seedlessness and fruit quality traits of gibberellin induced parthenocarpic fruit in seven tomato genotypes (Solanum lycopersicum L.). J. Agric. Sci. 8, 84–91 (2016).
Kawaguchi, K. et al. Functional disruption of cell wall invertase inhibitor by genome editing increases sugar content of tomato fruit without decrease fruit weight. Sci. Rep. 11, 21534. https://doi.org/10.1038/s41598-021-00966-4 (2021).
Dominic, S. et al. Phenolic profile, nutritional composition, functional properties, and antioxidant activity of newly grown parthenocarpic and normal seeded tomato. J. Chem. 2021, 8826325. https://doi.org/10.1155/2021/8826325 (2021).
Li, J. et al. Effects of exogenous auxin on pigments and primary metabolite profile of postharvest tomato fruit during ripening. Sci. Hortic. 219, 90–97. https://doi.org/10.1016/j.scienta.2017.03.011 (2017).
Bailey, S. M., Pietsch, E. C. & Cunningham, C. C. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic. Biol. Med. 27, 891–900. https://doi.org/10.1016/s0891-5849(99)00138-0 (1999).
Sierla, M., Waszczak, C., Vahisalu, T. & Kangasjärvi, J. Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 171, 1569–1580. https://doi.org/10.1104/pp.16.00328 (2016).
Daszkowska-Golec, A. & Szarejko, I. Open or close the gate – stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 4(235), 2013. https://doi.org/10.3389/fpls.2013.00138 (2013).
Khan, S. H. et al. Effect of drought stress on Tomato cv. Bombino. J. Food Process. Technol. 6, 7 (2015).
Abdellatif, I. M. Y. et al. Stimulation of tomato drought tolerance by PHYTOCHROME A and B1B2 mutations. Int. J. Mol. Sci. 24, 1560 (2023).
Choi, S. W. et al. Evaluation of internal control genes for quantitative realtime PCR analyses for studying fruit development of dwarf tomato cultivar “Micro-Tom”. Plant Biotechnol. (Tokyo) 35, 225–235. https://doi.org/10.5511/plantbiotechnology.18.0525a (2018).
Sulusoglu, M. & Cavusoglu, A. In vitro pollen viability and pollen germination in cherry laurel (Prunus laurocerasus L.). Sci. World J. 2014, 657123. https://doi.org/10.1155/2014/657123 (2014).
Soytong, M., Guevarra, P., Mateo, J. M. & Galvez, H. Evaluation of tomatoes fruits flesh colour, beta-carotene and lycopene content. Int. J. Agric. Technol. 17, 727–736 (2021).
Hagos, M., Redi-Abshiro, M., Chandravanshi, B. S. & Yaya, E. E. Development of analytical methods for determination of β-Carotene in pumpkin (Cucurbita maxima) flesh, peel, and seed powder samples. Int. J. Anal. Chem. 2022, 9363692. https://doi.org/10.1155/2022/9363692 (2022).
Miura, K. et al. Accumulation of antioxidants and antioxidant activity in tomato, Solanum lycopersicum, are enhanced by the transcription factor SlICE1. Plant Biotechnol. 29, 261–269. https://doi.org/10.5511/plantbiotechnology.12.0303b (2012).
Acknowledgements
We would like to thank Ms. Yuriko Nagai, Ms. Junko Hayashi, Ms. Kazuko Ito, and Ms. Ayako Kobayashi at T-PIRC from University of Tsukuba, Japan, for their technical support.
Funding
This work supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid (22H02295), Program on Open Innovation Platform with Enterprise, Research Institute and Academia, Japan Science and Technology Agency (JST-OPERA, JPMJOP1851), and Adopting Sustainable Partnerships for Innovative Research Ecosystem, Japan Science and Technology Agency (JST-ASPIRE, JPMJAP24A3).
Author information
Authors and Affiliations
Contributions
R.A.H.A: investigation, methodology, visualization, analysis, and writing-original draft; I.M.Y.A.: validation, investigation, and writing-review and editing; N.O.: investigation; M.K.: investigation; M.B.F.A.: investigation; D.T.: data curation and writing-review and editing; M.S.: writing-review and editing; K.M.: funding acquisition, resources, project administration, and writing-review and editing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, R.A.H., Abdellatif, I.M.Y., Oka, N. et al. Application of 4-CPA or ethanol enhances plant growth and fruit quality of phyA mutant under heat stress. Sci Rep 15, 32388 (2025). https://doi.org/10.1038/s41598-025-17929-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17929-8