Abstract
As graphene and related materials increasingly integrate into various industries, producing high-quality graphene sheets on a large scale becomes crucial. Here, we present a unique approach to the large-scale production of graphene through laser-assisted graphite expansion followed by ultrasonic exfoliation. The present method utilizes laser technology to significantly expand graphite, achieving an expansion rate of 800 mL g−1 with a deficient energy consumption of just two watts laser. The graphene produced via ultrasonication of expanded graphite samples exhibited high quality (ID/IG) ~ 0.13 and a few layers with a (I2D/IG) ~ 0.52. Free-standing films with different thicknesses (11–69 µm) were successfully prepared through filtration, reaching significant electrical conductivity up to (~ 1707 S cm−1). The as-prepared graphene films are further explored for electromagnetic interference (EMI) shielding. The highest shielding effectiveness (SE) was recorded for the graphene film with a thickness of 69 µm, reaching a value of ~ 72 dB. Meanwhile, the graphene film with an 11 µm thickness achieves the highest absolute effectiveness (SSE/t) of ~ 58,666 dB cm2 g−1, surpassing most current graphene and MXene films, which typically present values in the range of 10,000 to 40,000 dB cm2 g−1. This present laser-assisted expansion incorporating a sonication exfoliation strategy paves a new way to produce graphene on a large scale.
Similar content being viewed by others
Introduction
Graphene, a single-layer carbon allotrope arranged in a hexagonal lattice, has garnered significant attention from academia 1 and industry 2 in recent years. The isolation of graphene by Novoselov et al. 3 through micromechanical cleavage of graphite with scotch tape marked a pivotal moment. Their groundbreaking work led to the Nobel Prize in 2010. Graphene’s 2D hexagonal carbon lattice structure gives its distinct properties, including a remarkable tensile strength of 130 GPa and a Young’s modulus of 1.0 TPa 4, exceptional thermal conductivity at 5 × 103 W mK−1 5, electrical conductivity up to 80 MS m−1 6, a high surface area of 2630 m2 g−1 7, outstanding electron charge transport properties (~ 200,000 cm2 V−1 s−1) 8, and excellent optical characteristics with an absorption rate of about 2.3% over a wide wavelength range 9. These exceptional properties have made graphene a potent candidate for a variety of applications, including electronic devices 10, optical devices 11, electrochemical sensing 12, biosensing 13, energy conversion 14, energy storage, including supercapacitors and batteries 15,16, water treatment 17, desalination 18, medicine 19, military 20, coating 21, printing 22, catalysis 23, photocatalysis 24, advanced composite material 25. There are two main types of graphene preparation methods: bottom-up and top-down. The bottom-up approach is growing graphene continuously by breaking the chemical bonds of carbon-containing compounds and depositing carbon atoms on the substrate by certain methods, such as chemical vapor deposition 26, epitaxial growth 27, and plasma synthesis 28. Top-down methods obtain graphene from bulk synthetic or natural graphite by breaking the van der Waals forces between graphite layers with the assistance of external forces 29.
The top-down methods mainly include exfoliation via ultrasonic waves 30, ball milling 31, high shear delamination 32, high-pressure homogenization 33, microfluidics 34, and electrochemical intercalation 35. The intercalation-assisted exfoliation of graphite to graphene offers several significant advantages, such as the versatility of the method by using different intercalants, including anions such as SO42− 36, BF4− 37, ClO4− 38 and C2O42− 39 and alkali earth metals such as Li 40, K 41, and Na 42, the properties of graphene can be tailored, offering the possibility to modify its electronic structure 43 or enhance its reactivity 44. Additionally, it enables the scalable production of graphene 45, making it an attractive method for large-scale applications 46.
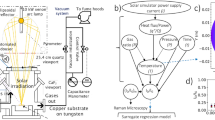
The typical procedure of intercalation-assisted exfoliation involves the intercalation of a guest (such as ClO4- 47 or SO4− 48) and the subsequent exfoliation of the host (graphite). Guest intercalation is achieved via chemical routes, which form the graphite intercalated compound (GIC), while host exfoliation refers to isolating atomic layers from the intercalation compound (host + guest) 49. This exfoliation process begins with thermal treatment of the GIC to yield thermally expanded graphite (TEG), followed by sonication or any similar form of external forces to exfoliate TEG, which creates a suspension of the exfoliated nanosheets, allowing for their individualization and dispersion 50. Expanded graphite (EG) refers to graphite expanded along the c-axis up to several hundred times 51. EG can be produced through various methods utilizing different intercalation and oxidation agents. For instance, intercalation agents such as H2SO4 52, HNO3 53, or HClO4 54 can be combined with oxidation agents like potassium permanganate 55, ammonium persulphate 56, or hydrogen peroxide 57 to facilitate the production process. The subsequent treatment can involve conventional heating 58, microwave heating 59, or room temperature processes 60 to achieve the desired EG structure. These methods come with several disadvantages. These processes necessitate substantial time consumption, particularly in the washing and drying stages. The intercalated graphite is washed for several hours to reach a neutral pH 61 and then oven-dried for approximately 12 to 24 h 62. The process of thermally expanding the intercalated graphite varies and requires temperatures ranging from 600 to 1200 degrees Celsius for heat expansion and power inputs between 350 to 800W for microwave expansion, as shown in Fig. 1c. To the best of our knowledge, this study is the first to achieve unprecedented intercalated graphite expansion using a laser source. This novel approach enhances the characteristics of the expanded graphite produced, significantly simplifying the exfoliation process and making it more efficient and scalable for various applications. In the present work, we report a unique approach for synthesizing graphene from natural graphite flakes under laser irradiation using perchloric acid intercalation, achieving an expansion volume of 800 mL g−1. Subsequently, the resultant expanded graphite is exfoliated into graphene sheets using probe sonication with a frequency of 24 kHz and power of 750 watts for 1 h. The graphene samples exhibited high quality (ID/IG) ~ 0.13 with few layers characteristic with a (I2D/IG) ~ 0.52. Free-standing graphene films with varying thicknesses from 11 µm to 69 µm have been fabricated and tested for their EMI shielding properties. They showed significant electrical conductivity reaching up to 1700 S cm−1 for the 11 µm thick film. SE for the films ranged between 20 dB for 11 µm thick film and 72 dB for 69 µm thick film. Compared to state-of-the-art graphene and MXenes that fall in the category of free-standing films, they showed significant absolute shielding effectiveness ranging from (~ 37,714 to ~ 58,666) dB cm2 g−1.
Results and discussion
The expansion volume of expanded graphite is a crucial parameter that drives its exfoliation efficiency 63. The expansion process is pivotal for evaluating performance, influenced by variables such as the type of intercalant used, the degree of intercalation, and the expansion temperature 64. Figure 1c illustrates the expansion volume of graphite, measured in milliliters per gram (mL g−1) on the x-axis, and its correlation with three different metrics on the y-axis: conventional heat in degrees Celsius (°C), microwave irradiation in Watts (W), and laser output, also in Watts (W). It compiles results from previous literature, primarily focusing on perchloric acid as the intercalating agent. Conventional heating methods demonstrate an increase in expansion volume with temperature, starting slightly above 150 mL g−1 and just over 550 mL g−1 at the highest temperature recorded at 900 °C 65. In contrast, the microwave irradiation method demonstrates a relatively higher and more variable expansion volume, ranging from 150 to 750 mL g−1, which correlates proportionally to the increasing microwave power applied between 200 and 800W. This pattern suggests that microwave irradiation may be more effective than conventional heating for expanding graphite. The present research introduces an advanced laser technique to expand the intercalated graphite. This innovative method has achieved the highest expansion volume recorded for expanded graphite at 800 mL g−1 while utilizing the lowest amount of power (2 W) compared to other methods. These findings emphasize the superior efficacy of laser-induced expansion, highlighting its potential as the optimal technique for graphite expansion for subsequent exfoliation to graphene due to its unparalleled volume increase and energy efficiency. Figure 1b shows the expansion ratio of graphite into expanded graphite with the same mass calculated using Eq. (1).
The three methods—laser, microwave, and conventional heating—exhibit significant differences in expansion volume, cost, and energy efficiency. Comparative performance analysis of synthesis methods across six criteria was done in a diagram Figure S5, using ranges derived from literature values compiled in Tables S4 and S5. Figure S4 is a photograph of the laser setup. The continuous-wave blue laser (CW-blue laser) is directed at the intercalated graphite; when the laser is on, the localized heat from the laser heats the GIC to high temperatures, causing perchloric acid to vaporize from within the graphite layers, as shown in Fig. 1a. A suction fan swiftly removes these vapors, ensuring the operator’s safety and preserving the experiment’s integrity. The ceramic tube, being highly resistant to heat and not prone to damage from the laser, serves as a barrier to protect the surrounding area, including the work surface or floor, from accidental laser exposure. Surrounding the ceramic tube, a container collects the lightweight laser-expanded graphite produced. Figure 2a,b show the starting material, intercalated graphite, before and during laser irradiation, respectively. Figure 2c–e is a closer demonstration of the expansion process. Figure 2c shows a single flake of intercalated graphite before laser irradiation, suspended in the air with the help of an acoustic levitation device, with a full image in Figure S3 (Camera setting and setup mentioned in the Measurements section). Figure 2d shows the flake while laser irradiates, while Fig. 2e shows the expanded flake after laser irradiation. Figure 2f presents the laser power threshold required for the expansion of intercalated graphite. “No expansion” refers to the condition where laser irradiation does not lead to any noticeable material expansion to the naked eye. “Slow expansion” (Supplementary video 2) indicates a gradual expansion of the graphite particles relative to a high-power output, whereas “Instant expansion” (Supplementary video 3) denotes the rapid evaporation of gas particles, causing the layers of graphite to expand quickly. It can be observed that approximately 2 watts of laser output is required to expand the intercalated graphite successfully. For a more detailed analysis, Fig. 2g focuses on the expansion in the current range of 0.4 to 0.6 amperes, confirming that the expansion of graphite particles can be accomplished with a laser output as low as 2 watts. To demonstrate the scalability of the present process, we utilized a commercial laser engraver, as depicted in Figure S4. As illustrated in Supplementary Video 1, the intercalated graphite undergoes expansion through the laser engraver, achieving laser-expanded graphite (LEG) with reduced time and minimal effort. Figure 3a shows that expansion volume and temperature rise as the laser output increases. Expansion remains minimal at low laser powers (0.2 W to 0.8 W). However, a sharp increase in expansion volume is observed between 1 and 2 W, suggesting that the laser has reached a localized temperature high enough to decompose perchloric acid particles. Beyond 2 W, expansion volume plateaus at around 800 mL g−1, indicating a saturation point where additional energy input does not significantly enhance expansion. The temperature, however, continues increasing linearly with power, exceeding 700 °C at the highest tested power (Figure S6). This suggests that while thermal effects continue, the expansion reaches a limit. Figure 3b shows Thermogravimetric analysis (TGA) revealed that LEG remains stable up to nearly 900 °C, meaning that the temperature increase in the experiment does not induce full decomposition or degradation.
(a) Intercalated graphite before laser irradiation. (b) Intercalated graphite during laser irradiation. (c) A single flake of intercalated graphite before laser irradiation. (d) Flake is being irradiated with a laser and started to expand. (e) Expanded graphite flake after laser irradiation. (f) Laser optimization analysis as a function of Ampere vs Watt with a constant voltage of 3.5 Volt. (g) Detailed analysis of the optimization in a smaller range.
The absorption of laser energy by graphite follows the photothermal effect 84, where incident photons interact with the electronic structure of the material, leading to rapid thermal energy conversion 85. When a laser beam strikes the graphite surface, its energy is absorbed by free electrons in the delocalized π-electron system of graphite, exciting them to higher energy states 86,87,88,89. These high-energy electrons then undergo electron–phonon coupling, transferring their excess energy to the atomic lattice as vibrational energy 90,91,92. This process leads to a localized and rapid temperature rise, which propagates through the material due to graphite’s high thermal conductivity 90. Upon laser irradiation, the intercalated perchloric acid undergoes thermal decomposition, releasing gases that expand the graphite structure 91:
The rapid formation of O2, Cl2, and H2O vapor creates a high internal pressure between graphene layers, forcing expansion.
The continuous-wave laser irradiates the intercalated graphite from the top surface, and while a thermal gradient does form, the high thermal conductivity of graphite allows rapid heat dissipation. Additionally, the intercalation lowers the expansion temperature, enabling relatively uniform expansion through the thickness.
X-ray diffraction (XRD) analysis was carried out on both the graphene and graphite to assess the crystallization degree of the synthesized graphene. In Fig. 4b, two characteristic XRD peaks at 2θ values of approximately 26.99° and 55.13° can be ascribed to the (002) and (004) crystallographic planes of graphite, respectively 92. The graphene XRD peaks are broader and exhibit reduced intensity compared to graphite ones. According to the calculations from Table S1, the Crystallite size (Dp) of graphite is 36.48 nm, whereas it is 7.53 for graphene. This reduction in crystallite size suggests a transformation in the material’s crystalline domains from larger graphite to smaller graphene sheets 93. XPS analysis was performed to evaluate the composition of graphene further. Figure S2b shows the survey spectra of graphene, in which only C and O can be observed. Figure 4c shows the C 1s spectra of graphene. Oxygen in graphene is evidenced by the O 1s peak observed in the XPS survey spectra. The intensity of this peak, relative to the C 1s peak, indicates the oxygen concentration on the graphene surface. The higher the O 1s peak intensity, the greater the oxygen content. The sharp and large carbon 1s (C1s) peak of graphene represented the graphitic carbon sp2 (C–C) and consisted of peaks corresponding to carbon bound (C–C) at ~ 284.4 eV and (C-O) at around 286 eV, respectively 94. However, for graphite, the carbon 1s (C1s) peak also prominently represents graphitic carbon sp2 (C–C) bonding but typically shows a slightly broader and less symmetric peak centered at approximately 284.5 eV, reflecting the multi-layered structure and varying electronic environments 95. Additionally, oxygen-containing functional groups are less pronounced, with any observable (C-O) bonding peaks around 286 eV; however, if they appear, they have generally lower intensity than graphene due to graphite’s bulk nature 96. Raman spectroscopy was done to identify distinctive peaks in graphite, intercalated graphite, expanded graphite, and graphene. The D Peak centered on ~ 1350 cm-1 manifests the structural defects in sheets arising due to disruption in regular aromatic sp2 networks that are so-called non-sp2 or sp3 defects 97. The G peak, located around 1580 cm⁻1, corresponds to the E2g phonon at the Brillouin zone center of graphene 98. The 2D peak, observed at approximately 2700 cm⁻1, results from second-order Raman scattering by in-plane transverse optical phonons near the boundary of the Brillouin zone of graphene 99. Figure 4a shows the Raman spectra of graphite, intercalated graphite, LEG, and graphene. The Raman spectra of LEG flakes show peaks at 1348, 1586, and 2689 cm-1, corresponding to the D, G, and 2D bands, respectively. These bands are the most characteristic bands of a graphitic material 100. The effect of laser treatment appears in the significant decrease of the D peak, with an intensity ratio of the D and G bands (ID/IG) of NG 0.27, compared with LEG 0.11, the laser treatment is performed on intercalated graphite, leading to rapid thermal expansion and delamination rather than direct laser ablation of graphite basal planes. This process avoids excessive bond breaking and defect formation, preserving the sp2 carbon network. As a result, the D peak is negligible. Figure S2a illustrates the Raman spectrum of the intercalated graphite. It exhibits a peak at 1627 cm⁻1 (G peak), which, according to Liu, Daozhi, et al. 101, indicates that the intercalation stage is between stages 1 and 2. This peak serves as an indication of the successful intercalation process. As shown in Fig. 4a, the decreased intensity of the D band in graphene and the increased intensity of the 2D band compared to graphite indicates that it has few layer characteristics and fewer defects 99. The I2D/IG ratio depends on the number of graphene layers 102. Typically, the I2D/IG ratio is ~ 2–3 for monolayer graphene, 2 > I2D/IG > 1 for bilayer graphene, and I2D/IG < 1 for multilayer graphene 103. An I2D/IG of ~ 0.5 is reported to correspond to a few-layered graphene (2–8 layers) 104. As shown in Fig. 4a, the I2D/IG for graphite is ~ 0.33, while in Fig. 4f, I2D/IG of graphene ranges from ~ 0.46 to ~ 0.53, averaging ~ 0.49 which indicates the presence of few to few-layer graphene and the success of the exfoliation process 105. It is essential to assess the quality of the obtained exfoliated graphene samples. As per the findings by AC Ferrari et al., 106 defect can be monitored by estimating the Full Width at Half Maximum (FWHM) of the G band, indicating that a broader G band is associated with a higher level of disorder. Additionally, the defect ratio, defined as the ratio of the intensity of the D band to the G band, is a crucial quantitative indicator for assessing the degree of disorder in graphene 107. To gain a deeper insight, it is essential to investigate the nature of defects, whether basal plane or edge defects. However, edge defects are inevitable since the ultrasonication process reduces flake sizes by causing fragmentation from kink bands 106. As shown in Fig. 4e, the statistical histograms for graphene samples’ ID/IG ratios vary between approximately 0.13 and 0.21, with an average value of around 0.16. For further clarity, the correlation between the defect ratio (ID/IG) and FWHM of the G band has been plotted in Fig. 4d. The graph showed that values of FWHM of the G band are predominantly clustered in the range of 23–28 cm-1 for graphene. In other words, the range of defect ratios and the narrowed broadening of the G band in the graphene samples strongly suggest that the defects present are mainly due to edge defects, as opposed to defects in the basal plane.
(a) Raman spectra of graphite, intercalated graphite, LEG, and graphene. (b) XRD patterns of graphite and graphene. (c) Convoluted fitting of XPS spectrum of C 1 s of graphene. (d) ID/IG ratio as a function of FWHM(G) in graphene. (e) Statistical Histogram of ID/IG ratio of graphene. (f) Statistical Histogram of I2D/IG ratio of graphene. All are mapped across a 20 µm × 20 µm region.
The scanning electron microscope (SEM) examined natural graphite (NG), GIC, LEG, and graphene morphological features. Figure S7a-c showed that graphite possessed a smooth surface, displaying no apparent porous characteristics. After undergoing intercalation treatment with perchloric acid, the layer spacing of GIC increased noticeably, resulting in an accordion-like structure with more expansive spaces (Fig. 5a). Furthermore, when GICs were subjected to laser irradiation, the intercalating species between the layers evaporated quickly, critical to the effective expansion of the graphite layers. Consequently, this process yields a worm-like structure, as shown in Fig. 5b. Figure 5c shows a highly dispersed array of graphene flakes with various shapes and sizes, suggesting a successful exfoliation process. EDX was used to analyze the elemental composition of the samples (Figure S1e-h). As shown in Figure S1f., the chlorine content increased significantly compared to graphite after the intercalation process, confirming the successful intercalation of the graphite sample. Figure S1g confirms the successful evaporation of the intercalation species, as the laser-expanded graphite contains no chlorine content and consists only of carbon. Figure S1h illustrates the known elemental composition of graphene, which consists of solely carbon atoms. Figure S1a-d shows the cross-sectional SEM of the free-standing graphene films. The cross-sectional SEM image displays the free-standing graphene films. As the added graphene volume to vacuum filtration increases, the thickness of the films also increases, ranging from approximately 11 to 69 µm. TEM and high-resolution TEM (HRTEM) analyses were conducted further to investigate the morphology and orientation of graphene samples. Figure 5d shows TEM images of LEG, showing multiple flakes stacked atop one another, consistent with the selected area electron diffraction (SAED) patterns that show relatively higher crystalline than graphene samples. Figure 5e shows a representative low-magnification TEM image of the graphene flakes. The lateral sizes of the flakes are relatively large, mainly in a few micrometers. Folded and wrinkled regions are observed in most of the flakes, and the SAED patterns of the sample consist of diffraction spots attributable to graphene 108. Figure 5f shows an HRTEM image, revealing a highly crystalline structure of the graphene with some defects due to exfoliation by ultrasonication. The Fast Fourier Transform (FFT) of the graphene shows a set of hexagonal patterns in the diffraction spots. Furthermore, Figure S2c shows the Electron Energy Loss Spectroscopy (EELS) for graphene. The C K-edge spectra of the graphene sample show an energy loss of around 284 eV. A clear feature of the peak at 284 eV is attributed to the C = C π* resonance, and the peak at 292 eV is attributed to the carbon sp2 bond (C–C σ* resonance) 109, which is consistent with the XPS spectrum in Fig. 4c. Figure 5g–i presents an analysis of graphene flakes using a high-resolution image with atomic force microscopy (AFM). Figure 5g shows a topographic image of graphene flakes on a silicon substrate, with two regions of interest, Region 1 and Region 2, marked for detailed analysis. Figure 5h,i illustrate the height profiles for the marked regions and provide insights into the layer thickness of graphene flakes. Graphene flake thickness ranges from 2 to 4 nm, indicating the presence of a few-layer graphene consistent with the Raman analysis.
SEM at different magnifications of intercalated graphite (a), expanded graphite (b), and graphene (c). TEM of laser expanded graphite along with its SAED, LEG (d) Graphene (e) HRTEM of graphene (f) along with FFT. (g) AFM image of the graphene flakes. (h,i) Analysis of the thickness of the flakes in two different regions.
Electro Magnetic Interference shielding performance of graphene films was investigated in the X-band frequency range (8.2–12.4 GHz). Electromagnetic waves (EMWs) can be shielded by reflection, absorption, and multiple internal reflections 110. It is worth noting that the reflection of EMWs depends on the electrical conductivity of the shielding materials. At the same time, the absorption of EMWs is based on the electrical and magnetic dipoles of materials. In addition, multiple internal reflections are related to the abundant scattering centers and multiple interfaces in the structure of shielding materials 111. The graphene film with a thickness of 11 μm demonstrates sheet resistance of 1.9473 Ω/square, resistivity of 5.8418 µΩ m, and an excellent electrical conductivity of 1706.5 S cm−1. The thickness, density, and electrical conductivity of the films are mentioned in Table S2. Considering their optimal electrical properties, EMI shielding was measured in the X-band range for the graphene films. The total EMI (SET), absorption (SEA), and reflection (SER) of the Graphene film with a thickness of 69 μm are shown in Fig. 6a, delivering shielding efficiency values of approximately 72 dB, 51 dB, and 21 dB corresponding to SET, SEA, and SER respectively. Notably, SER of the graphene film-69 μm presents a high value of nearly 21 dB, implying that the graphene film reflects more than 99% of EMWs. In this case, EMW reflection is dominant in the EMI shielding of graphene films. The remaining EMWs are further absorbed inside the film, contributing greatly to a super high value of SET. Film thickness is believed to be crucial in EMI shielding performance 112. Therefore, graphene films with different thicknesses are successfully fabricated by changing the mass of the graphene. The EMI SET significantly increases with an increase in the thickness of the graphene films (Fig. 6b). The highest EMI SET is recorded at 72 dB for graphene film-69 μm. The thicker films provide a lengthened path for EMWs to travel, leading to plenty of interfaces and internal absorption and reflection, thus achieving more EMW consumption. Figure 5g,h are cross-sectional SEM images of the films with the lowest and highest thickness. Furthermore, the shielding behaviors of SEA and SER for graphene films with different thicknesses are plotted in Fig. 6c, revealing that SEA plays an important role in the shielding effectiveness for all graphene films. To further elucidate the EMI shielding mechanism, the power coefficients of reflection(R), absorption (A), and transmission (T) are calculated and summarized in Fig. 6d. Noticeably, the values of R (> 0.94) are much higher than those of A (< 0.06) for all graphene films, demonstrating that most incident EMWs are reflected at the interfaces of graphene films due to impedance mismatch and the reflection of EMWs dominates the shielding mechanism. A small quantity of remaining EMWs can enter the graphene film to be consumed by electromagnetic energy conversion. To sum up, the shielding process of the EMWs in graphene films is subject to a reflection-dominant shielding mechanism. The outstanding EMI shielding performance of the graphene films is derived from their high electrical conductivity and stacked porous architecture. The proposed mechanism, leading to the high EMI SE of graphene films, is shown in Fig. 6e. Specifically, most of the incident EM waves (> 90%) can be reflected when initially encountering the graphene films. This is owing to the interface impedance mismatch caused by the abundant free electrons on the surface of the conductive graphene films 112. After that, the remaining EMWs penetrate graphene films and interact with the high-density electron carriers (electrons and holes) in conduction paths for further dissipation 113. Meanwhile, the penetrated EMWs suffer from multiple internal reflections in the pores and the cavities of the graphene films, leading to further attenuation of EMWs. After the effective reflection and absorption, only a tiny amount of EMWs can pass through graphene films. High electrical conductivity and porous structure synergistically endow the graphene films with high-performance EMI SE. Specific shielding effectiveness and absolute effectiveness are other criteria used to estimate the potential application of EMI shielding materials, which can be calculated using Eqs. (7,8). All the graphene films (11 µm to 69 µm) present low densities of 0.310, 0.257, 0.273, and 0.236 g cm−3, respectively. Benefiting from the high electrical conductivity and low density, Graphene film -11 μm delivers the highest SSE/t of 58,666.7 dB cm2 g−1. With the increase of the thickness of the graphene film, the SSE/t diminishes to a lower value, which is probably owing to the enhanced EMI SE stemming from the longer EMW traveling path cannot compromise the increased thickness and weight of thicker graphene films, leading to lower SSE/t performance. The values of SSE/t for all the graphene films, along with Graphene and MXene films from recent literature, are calculated and presented in Fig. 6f and Table S3. MXene has emerged as a material of significant interest due to its potential for EMI shielding applications 102,103. While MXenes exhibit higher electrical conductivity, which is a favorable attribute for EMI shielding, they tend to have a lower absolute EMI shielding efficiency than the present work. This lower efficiency can be attributed to the higher weight of MXene composites compared to graphene films 114,115.
(a) SET, SER, and SEA for GF-69 μm Films. (b) EMI SE is used for graphene films with various thicknesses. (c) SET, SER, and SEA for graphene films with various thicknesses. (d) Power coefficients of reflection (R), transmission (T), and absorption (A) of graphene films as a function of thickness. (e) The possible EMI shielding mechanism of graphene films. (f) SSE/t of graphene films with different thicknesses compared with Graphene and MXene films from literature.
Conclusion
In this work, an innovative approach was developed to synthesize graphene utilizing a laser through the expansion of graphite with a simple preparation process for effective EMI shielding efficiency. Under optimally tuned laser conditions, LEG is efficiently produced using a mere two Watts of output power, completing expansion up to 800 mL g−1 in seconds. The present findings reveal that free-standing graphene produced from LEG exhibits exceptional electrical conductivity of 1706 S cm−1, EMI shielding efficiency of 72 dB for 69 µm thick free standing graphene film, and a remarkable absolute shielding effectiveness of 58,666 dB cm2 g−2 for 11 µm thickness film. A comprehensive suite of characterization techniques was employed to investigate the morphology and structure of the samples systematically. Raman spectroscopy analysis confirmed the high quality of the graphene produced, as evidenced by an ID/IG ratio of approximately 0.13 and an I2D/IG ratio of around 0.52. Further analyses of AFM and HRTEM revealed that the graphene sheets predominantly exhibit a folded morphology, with few-layer graphene sheets entangling each other, consistent with the Raman data.
Experimental section
Chemicals and reagents
Graphite flakes (average size of 100 μm), perchloric acid (HClO4, 70 wt.%), deionized water, and isopropyl alcohol (C3H8O, 99%) were purchased from Sigma-Aldrich. All the chemicals used in the experiments were used without further purification.
Synthesis of expanded graphite
Typically, 1 g of graphite flakes and 2.5 mL of perchloric acid were mixed in a weight ratio 1:4 for 30 min at 150 °C to form a graphite intercalated compound. GIC was taken into a porcelain dish and placed in the laser setup mentioned in Fig. 1a, with a 2 cm distance from the CW-blue laser source. After being irradiated under the laser for 30 s, LEG was collected.
Synthesis of graphene and graphene films
LEG was then exfoliated in isopropyl alcohol by sonication using a probe sonicator for 1 h. The supernatant with exfoliated graphene was collected after centrifuging at 900 RPM for 45 min.
Desired amounts of the exfoliated graphene suspension were filtrated under a vacuum to form free-standing graphene films with different thicknesses.
Characterization and measurements
The crystallinity of the samples was measured using X-ray diffraction (XRD Bruker D2 phaser). Raman analysis of the starting materials and the exfoliated samples were performed by Renishaw spectrometer using 532 nm laser (2.33 eV) excitation and 50 × objective lens. The laser power was kept below 1 mW to prevent sample damage. 20 spectra were recorded (each one at a different location) for each sample to create statistical data for the samples. Morphology of the samples was done by scanning electron microscopy ((SEM) Nova NanoSEM 650, beam resolution 0.8 nm). AFM analysis was done using the Park system FX50 module. XPS spectra were recorded using a Thermo Scientific K-Alpha spectrometer. Intercalated graphite samples were irradiated using a CW-blue laser with a wavelength of 455 nm, focus length of 40 mm, spot size of 0.16–0.18 mm, and output optical power of 20 W. Digital images were taken using a Sony Alpha A6400 camera with an 18 Mm lens, 24.2 megapixels, and iso was adjusted to 100. Graphite samples were suspended in the air using a DC 12Volts ultrasonic levitation device. The graphite’s expansion volume (EV) was calculated by equation 72. EG was moved into a measuring cylinder for volume testing, and the average value was calculated after removing the maximum and minimum values, considering the accuracy of the testing process.
where EV is the graphite expansion volume, mL g−1; V is the volume after expansion, mL; m is the mass of the specimen, g.
EMI shielding performance of graphene films was measured using a 2-port network analyzer (ENA5071C, Agilent Technologies, USA) in the X-band frequency range (8.2–12.4 GHz). All the graphene film samples were cut into a rectangular shape, slightly larger than the sample holder, to avoid any leakage paths from the edges. Electromagnetic radiation includes reflection (R), absorption (A), and transmission (T) (A + R + T = 1). The total EMI shielding effectiveness (SET), reflection effectiveness (SER), and absorption effectiveness (SEA) were calculated following equations below:
Taking the thickness and density of films into account, the Specific shielding effectiveness (SSE) and Absolute effectiveness (SSE/t) can be calculated using the following equation:
Electrical characterization of the samples was performed on thin films prepared from the dispersions. Sheet resistance measurements were carried out by four-point probe technique using Ossila Four-Point Probe system. The conductivity values of the thin films were calculated from the measured sheet resistances using Eq. (9).
where σel is the electrical conductivity; Rs is the sheet resistance and t is the thickness of the film.
Raman fitting analysis was done using Lorentzian equation which is shown below.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Tiwari, S. K., Sahoo, S., Wang, N. & Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 5(1), 10–29. https://doi.org/10.1016/j.jsamd.2020.01.006 (2020).
Zhu, Y., Ji, H., Cheng, H.-M. & Ruoff, R. S. Mass production and industrial applications of graphene materials. Natl. Sci. Rev. 5(1), 90–101. https://doi.org/10.1093/nsr/nwx055 (2018).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306(5696), 666–669. https://doi.org/10.1126/science.1102896 (2004).
Lee, C., Wei, X., Kysar, J. W. & Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321(5887), 385–388. https://doi.org/10.1126/science.1157996 (2008).
Balandin, A. A. et al. Superior thermal conductivity of single-layer graphene. Nano Lett. 8(3), 902–907. https://doi.org/10.1021/nl0731872 (2008).
Rizzi, L. et al. Quantifying the influence of graphene film nanostructure on the macroscopic electrical conductivity. Nano Express 1(2), 020035. https://doi.org/10.1088/2632-959X/abb37a (2020).
Zhang, S., Wang, H., Liu, J. & Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 261, 127098. https://doi.org/10.1016/j.matlet.2019.127098 (2020).
Bolotin, K. I. et al. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 146(9–10), 351–355. https://doi.org/10.1016/j.ssc.2008.02.024 (2008).
Nair, R. R. et al. Fine structure constant defines visual transparency of graphene. Science 320(5881), 1308–1308. https://doi.org/10.1126/science.1156965 (2008).
Schwierz, F. Graphene transistors. Nat. Nanotechnol. 5(7), 487–496. https://doi.org/10.1038/nnano.2010.89 (2010).
Jo, G. et al. The application of graphene as electrodes in electrical and optical devices. Nanotechnology 23(11), 112001. https://doi.org/10.1088/0957-4484/23/11/112001 (2012).
Pumera, M., Ambrosi, A., Bonanni, A., Chng, E. L. K. & Poh, H. L. Graphene for electrochemical sensing and biosensing. TrAC Trends Anal. Chem. 29(9), 954–965. https://doi.org/10.1016/j.trac.2010.05.011 (2010).
Szunerits, S. & Boukherroub, R. Graphene-based biosensors. Interface Focus 8(3), 20160132. https://doi.org/10.1098/rsfs.2016.0132 (2018).
Sahoo, N. G., Pan, Y., Li, L. & Chan, S. H. Graphene-based materials for energy conversion. Adv. Mater. 24(30), 4203–4210. https://doi.org/10.1002/adma.201104971 (2012).
Raccichini, R., Varzi, A., Passerini, S. & Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 14(3), 271–279. https://doi.org/10.1038/nmat4170 (2015).
Zhu, J., Yang, D., Yin, Z., Yan, Q. & Zhang, H. Graphene and graphene-based materials for energy storage applications. Small 10(17), 3480–3498. https://doi.org/10.1002/smll.201303202 (2014).
Ali, I. et al. Water treatment by new-generation graphene materials: Hope for bright future. Environ. Sci. Pollut. Res. 25(8), 7315–7329. https://doi.org/10.1007/s11356-018-1315-9 (2018).
Cohen-Tanugi, D. & Grossman, J. C. Water desalination across nanoporous graphene. Nano Lett. 12(7), 3602–3608. https://doi.org/10.1021/nl3012853 (2012).
Priyadarsini, S., Mohanty, S., Mukherjee, S., Basu, S. & Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 8(2), 123–137. https://doi.org/10.1007/s40097-018-0265-6 (2018).
Abreu, I., Ferreira, D. P. &Fangueiro, R. Versatile Graphene-Based Fibrous Systems for Military Applications.
Ollik, K. & Lieder, M. Review of the application of graphene-based coatings as anticorrosion layers. Coatings 10(9), 883. https://doi.org/10.3390/coatings10090883 (2020).
Tran, T. S., Dutta, N. K. & Choudhury, N. R. Graphene inks for printed flexible electronics: Graphene dispersions, ink formulations, printing techniques and applications. Adv. Colloid Interface Sci. 261, 41–61. https://doi.org/10.1016/j.cis.2018.09.003 (2018).
Yan, Y. et al. A recent trend: Application of graphene in catalysis. Carbon Lett. 31(2), 177–199. https://doi.org/10.1007/s42823-020-00200-7 (2021).
Li, X., Yu, J., Wageh, S., Al-Ghamdi, A. A. & Xie, J. Graphene in photocatalysis: A review. Small 12(48), 6640–6696. https://doi.org/10.1002/smll.201600382 (2016).
Stankovich, S. et al. Graphene-based composite materials. Nature 442(7100), 282–286. https://doi.org/10.1038/nature04969 (2006).
Mattevi, C., Kim, H. & Chhowalla, M. A review of chemical vapour deposition of graphene on copper. J. Mater. Chem. 21(10), 3324–3334. https://doi.org/10.1039/C0JM02126A (2011).
Tetlow, H. et al. Growth of epitaxial graphene: Theory and experiment. Phys. Rep. 542(3), 195–295. https://doi.org/10.1016/j.physrep.2014.03.003 (2014).
Shah, J., Lopez-Mercado, J., Carreon, M. G., Lopez-Miranda, A. & Carreon, M. L. Plasma synthesis of graphene from mango peel. ACS Omega 3(1), 455–463. https://doi.org/10.1021/acsomega.7b01825 (2018).
Kumar, N. et al. Top-down synthesis of graphene: A comprehensive review. FlatChem 27, 100224. https://doi.org/10.1016/j.flatc.2021.100224 (2021).
Tyurnina, A. V. et al. Ultrasonic exfoliation of graphene in water: A key parameter study. Carbon 168, 737–747. https://doi.org/10.1016/j.carbon.2020.06.029 (2020).
Zhao, W. et al. Preparation of graphene by exfoliation of graphite using wet ball milling. J. Mater. Chem. 20(28), 5817. https://doi.org/10.1039/c0jm01354d (2010).
Biccai, S. et al. Exfoliation of 2D materials by high shear mixing. 2D Mater. 6(1), 015008. https://doi.org/10.1088/2053-1583/aae7e3 (2018).
Wu, H. et al. High pressure homogenization of graphene and carbon nanotube for thermal conductive polyethylene composite with a low filler content. J. Appl. Polym. Sci. 139(12), 51838. https://doi.org/10.1002/app.51838 (2022).
Chen, X. et al. A review on application of graphene-based microfluidics. J. Chem. Technol. Biotechnol. 93(12), 3353–3363. https://doi.org/10.1002/jctb.5710 (2018).
Liu, F. et al. Synthesis of graphene materials by electrochemical exfoliation: Recent progress and future potential. Carbon Energy 1(2), 173–199. https://doi.org/10.1002/cey2.14 (2019).
Muir, K., Liggat, J. J. & O’Keeffe, L. Thermal volatilisation analysis of graphite intercalation compound fire retardants. J. Therm. Anal. Calorim. 148(5), 1905–1920. https://doi.org/10.1007/s10973-022-11804-8 (2023).
Zhang, L., Li, J., Huang, Y., Zhu, D. & Wang, H. Synergetic effect of ethyl methyl carbonate and trimethyl phosphate on BF4– intercalation into a graphite electrode. Langmuir 35(11), 3972–3979. https://doi.org/10.1021/acs.langmuir.9b00262 (2019).
Li, X. X., Zhao, J. J. & Ma, D. Y. Preparation and characteristics of highly expandable graphite intercalation compounds by two-step chemical intercalation. Key Eng. Mater. 703, 278–283. https://doi.org/10.4028/www.scientific.net/KEM.703.278 (2016).
Liu, J. et al. Improved synthesis of graphene flakes from the multiple electrochemical exfoliation of graphite rod. Nano Energy 2(3), 377–386. https://doi.org/10.1016/j.nanoen.2012.11.003 (2013).
Nobuhara, K., Nakayama, H., Nose, M., Nakanishi, S. & Iba, H. First-principles study of alkali metal-graphite intercalation compounds. J. Power Sources 243, 585–587. https://doi.org/10.1016/j.jpowsour.2013.06.057 (2013).
Xu, J. et al. Recent progress in graphite intercalation compounds for rechargeable metal (Li, Na, K, Al)-ion batteries. Adv. Sci. 4(10), 1700146. https://doi.org/10.1002/advs.201700146 (2017).
Lenchuk, O., Adelhelm, P. & Mollenhauer, D. New insights into the origin of unstable sodium graphite intercalation compounds. Phys. Chem. Chem. Phys. 21(35), 19378–19390. https://doi.org/10.1039/C9CP03453F (2019).
Rajapakse, M. et al. “Intercalation as a versatile tool for fabrication, property tuning, and phase transitions in 2D materials. Npj 2D Mater. Appl. 5(1), 30. https://doi.org/10.1038/s41699-021-00211-6 (2021).
Wan, J. et al. Tuning two-dimensional nanomaterials by intercalation: Materials, properties and applications. Chem. Soc. Rev. 45(24), 6742–6765. https://doi.org/10.1039/C5CS00758E (2016).
Iyo, A., Ogino, H., Ishida, S. & Eisaki, H. Dramatically accelerated formation of graphite intercalation compounds catalyzed by sodium. Adv. Mater. https://doi.org/10.1002/adma.202209964 (2023).
Toyoda, M., Hou, S., Huang, Z.-H. & Inagaki, M. Exfoliated graphite: Room temperature exfoliation and their applications. Carbon Lett. 33(2), 335–362. https://doi.org/10.1007/s42823-022-00450-7 (2023).
Wei, X. H., Liu, L., Zhang, J. X., Shi, J. L. & Guo, Q. G. The preparation and morphology characteristics of exfoliated graphite derived from HClO4–graphite intercalation compounds. Mater. Lett. 64(9), 1007–1009. https://doi.org/10.1016/j.matlet.2009.11.025 (2010).
Inagaki, M., Tashiro, R., Washino, Y. & Toyoda, M. Exfoliation process of graphite via intercalation compounds with sulfuric acid. J. Phys. Chem. Solids 65(2–3), 133–137. https://doi.org/10.1016/j.jpcs.2003.10.007 (2004).
Stark, M. S., Kuntz, K. L., Martens, S. J. & Warren, S. C. Intercalation of layered materials from bulk to 2D. Adv. Mater. 31(27), 1808213. https://doi.org/10.1002/adma.201808213 (2019).
Nicolosi, V., Chhowalla, M., Kanatzidis, M. G., Strano, M. S. & Coleman, J. N. Liquid exfoliation of layered materials. Science 340(6139), 1226419. https://doi.org/10.1126/science.1226419 (2013).
Saikam, L., Arthi, P., Senthil, B. & Shanmugam, M. A review on exfoliated graphite: Synthesis and applications. Inorg. Chem. Commun. 152, 110685. https://doi.org/10.1016/j.inoche.2023.110685 (2023).
Goshadrou, A. & Moheb, A. Continuous fixed bed adsorption of C.I. Acid Blue 92 by exfoliated graphite: An experimental and modeling study. Desalination 269(1–3), 170–176. https://doi.org/10.1016/j.desal.2010.10.058 (2011).
Forsman, W. C., Vogel, F. L., Carl, D. E. & Hoffman, J. Chemistry of graphite intercalation by nitric acid. Carbon 16(4), 269–271. https://doi.org/10.1016/0008-6223(78)90040-4 (1978).
Petitjean, D., Furdin, G., Herold, A. & Pavlovsky, N. D. New data on graphite intercalation compounds containing HClO 4: Synthesis and exfoliation. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. Mol. Cryst. Liq. Cryst. 245(1), 213–218. https://doi.org/10.1080/10587259408051691 (1994).
Zhao, J. J., Li, X. X., Guo, Y. X. & Ma, D. Y. Preparation and microstructure of exfoliated graphite with large expanding volume by two-step intercalation. Adv. Mater. Res. 852, 101–105. https://doi.org/10.4028/www.scientific.net/AMR.852.101 (2014).
Melezhyk, A., Galunin, E. & Memetov, N. Obtaining graphene nanoplatelets from various graphite intercalation compounds. IOP Conf. Ser. Mater. Sci. Eng. 98, 012041. https://doi.org/10.1088/1757-899X/98/1/012041 (2015).
Hossain, S. T. & Wang, R. Electrochemical exfoliation of graphite: Effect of temperature and hydrogen peroxide addition. Electrochim. Acta 216, 253–260. https://doi.org/10.1016/j.electacta.2016.09.022 (2016).
Gulnura, N., Kenes, K., Yerdos, O., Zulkhair, M. & Di Capua, R. Preparation of Expanded Graphite Using a Thermal Method. IOP Conf. Ser. Mater. Sci. Eng. 323, 012012. https://doi.org/10.1088/1757-899X/323/1/012012 (2018).
Zhang, F. et al. Rapid preparation of expanded graphite by microwave irradiation for the extraction of triazine herbicides in milk samples. Food Chem. 197, 943–949. https://doi.org/10.1016/j.foodchem.2015.11.056 (2016).
Liu, T. et al. One-step room-temperature preparation of expanded graphite. Carbon 119, 544–547. https://doi.org/10.1016/j.carbon.2017.04.076 (2017).
Rajendran, J. et al. Thermally expanded graphite incorporated with PEDOT:PSS based anode for microbial fuel cells with high bioelectricity production. J. Electrochem. Soc. 169(1), 017515. https://doi.org/10.1149/1945-7111/ac4b23 (2022).
Gantayat, S., Prusty, G., Rout, D. R. & Swain, S. K. Expanded graphite as a filler for epoxy matrix composites to improve their thermal, mechanical and electrical properties. New Carbon Mater. 30(5), 432–437. https://doi.org/10.1016/S1872-5805(15)60200-1 (2015).
Yakovlev, A. V., Finaenov, A. I., Zabudkov, S. L. & Yakovleva, E. V. Thermally expanded graphite: Synthesis, properties, and prospects for use. Russ. J. Appl. Chem. 79(11), 1741–1751. https://doi.org/10.1134/S1070427206110012 (2006).
Murugan, P., Nagarajan, R. D., Shetty, B. H., Govindasamy, M. & Sundramoorthy, A. K. Recent trends in the applications of thermally expanded graphite for energy storage and sensors: A review. Nanoscale Adv. 3(22), 6294–6309. https://doi.org/10.1039/D1NA00109D (2021).
Wei, X. H., Liu, L., Zhang, J. X., Shi, J. L. & Guo, Q. G. HClO4–graphite intercalation compound and its thermally exfoliated graphite. Mater. Lett. 63(18–19), 1618–1620. https://doi.org/10.1016/j.matlet.2009.04.030 (2009).
Sykam, N., Gautam, R. K. & Kar, K. K. Electrical, mechanical, and thermal properties of exfoliated graphite/phenolic resin composite bipolar plate for polymer electrolyte membrane fuel cell. Polym. Eng. Sci. 55(4), 917–923. https://doi.org/10.1002/pen.23959 (2015).
Sykam, N., Jayram, N. D. & Mohan Rao, G. Exfoliation of graphite as flexible SERS substrate with high dye adsorption capacity for Rhodamine 6G. Appl. Surf. Sci. 471, 375–386. https://doi.org/10.1016/j.apsusc.2018.11.082 (2019).
Wei, Q. et al. High-performance expanded graphite from flake graphite by microwave-assisted chemical intercalation process. J. Ind. Eng. Chem. 122, 562–572. https://doi.org/10.1016/j.jiec.2023.03.020 (2023).
Sykam, N., Jayram, N. D. & Rao, G. M. Highly efficient removal of toxic organic dyes, chemical solvents and oils by mesoporous exfoliated graphite: Synthesis and mechanism. J. Water Process Eng. 25, 128–137. https://doi.org/10.1016/j.jwpe.2018.05.013 (2018).
Wei, X. H., Liu, L., Zhang, J. X., Shi, J. L. & Guo, Q. G. Mechanical, electrical, thermal performances and structure characteristics of flexible graphite sheets. J. Mater. Sci. 45(9), 2449–2455. https://doi.org/10.1007/s10853-010-4216-y (2010).
Yang, Y. F., Zhang, X. J. & Xu, X. Preparation and characteristics of expanded graphite. Adv. Mater. Res. 189–193, 2695–2698. https://doi.org/10.4028/www.scientific.net/AMR.189-193.2695 (2011).
Yu, X.-J., Wu, J., Zhao, Q. & Cheng, X.-W. Preparation and characterization of sulfur-free exfoliated graphite with large exfoliated volume. Mater. Lett. 73, 11–13. https://doi.org/10.1016/j.matlet.2011.11.078 (2012).
Elbidi, M., Resul, M. F. M. G., Rashid, S. A. & Salleh, M. A. M. Preparation of eco-friendly mesoporous expanded graphite for oil sorption. J. Porous Mater. 30(4), 1359–1368. https://doi.org/10.1007/s10934-023-01428-0 (2023).
Chen, Y.-L., Hsiao, C.-H., Ya, J.-Y. & Hsieh, P.-Y. Preparation of expanded graphite with (NH4)2S2O8 and H2SO4 by using microwave irradiation. J. Taiwan Inst. Chem. Eng. https://doi.org/10.1016/j.jtice.2023.105026 (2023).
Li, J., Liu, Q. & Da, H. Preparation of sulfur-free exfoliated graphite at a low exfoliation temperature. Mater. Lett. 61(8–9), 1832–1834. https://doi.org/10.1016/j.matlet.2006.07.142 (2007).
He, J., Song, L., Yang, H., Ren, X. & Xing, L. Preparation of sulfur-free exfoliated graphite by a two-step intercalation process and its application for adsorption of oils. J. Chem. 2017, 1–8. https://doi.org/10.1155/2017/5824976 (2017).
Hou, B., Sun, H., Peng, T., Zhang, X. & Ren, Y. Rapid preparation of expanded graphite at low temperature. New Carbon Mater. 35(3), 262–268. https://doi.org/10.1016/S1872-5805(20)60488-7 (2020).
Sykam, N. & Kar, K. K. Rapid synthesis of exfoliated graphite by microwave irradiation and oil sorption studies. Mater. Lett. 117, 150–152. https://doi.org/10.1016/j.matlet.2013.12.003 (2014).
Tang, X. et al. Study on the mechanism of expanded graphite to improve the fading resistance of the non-asbestos organic composite braking materials. Tribol. Int. 180, 108278. https://doi.org/10.1016/j.triboint.2023.108278 (2023).
Kumar, R., Nirwan, A., Mondal, B., Kumar, R. & Dixit, A. Study on thermophysical properties of pentadecane and its composites with thermally expanded graphite as shape-stabilized phase change materials. J. Therm. Anal. Calorim. 147(16), 8689–8697. https://doi.org/10.1007/s10973-021-11180-9 (2022).
He, J., Yuan, M., Ren, H., Song, T. & Zhang, Y. The electrochemical preparation and characterization of sulfur-free expanded graphite. J. Chem. Sci. 135(1), 17. https://doi.org/10.1007/s12039-023-02138-5 (2023).
Xiang, N. et al. The in situ preparation of Ni–Zn ferrite intercalated expanded graphite via thermal treatment for improved radar attenuation property. Molecules 28(10), 4128. https://doi.org/10.3390/molecules28104128 (2023).
Li, J. et al. Thermal performance analysis of composite phase change material of myristic acid-expanded graphite in spherical thermal energy storage unit. Energies 16(11), 4527. https://doi.org/10.3390/en16114527 (2023).
Ye, X. et al. Review: Photothermal effect of two-dimensional flexible materials. J. Mater. Sci. 60(4), 1797–1825. https://doi.org/10.1007/s10853-025-10593-3 (2025).
Murray, P. T. & Peeler, D. T. Pulsed-laser interactions with graphite. AIP Conf. Proc. 288(1), 359–364. https://doi.org/10.1063/1.44921 (1993).
Ultrafast laser ablation of graphite. Phys. Rev. B. Accessed: Apr. 01, 2025. https://doi.org/10.1103/PhysRevB.79.184105
Xu, J. et al. A micron-sized laser photothermal effect evaluation system and method. Sensors https://doi.org/10.3390/s21155133 (2021).
Khalil, A. A. I. A spectroscopic analysis study of graphite using laser technique. Laser Phys. 20(1), 238–244. https://doi.org/10.1134/S1054660X10010081 (2010).
“Current and Future Trends in Laser Medicine - Parrish - 1991 - Photochemistry and Photobiology - Wiley Online Library.” Accessed: Apr. 01, 2025. https://doi.org/10.1111/j.1751-1097.1991.tb09885.x
Petitjean, D., Furdin, G., Herold, A. & Pavlovsky, N. D. New data on graphite intercalation compounds containing HClO4: Synthesis and exfoliation. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. Mol. Cryst. Liq. Cryst. https://doi.org/10.1080/10587259408051691 (1994).
Besenhard, J. O., Minderer, P. & Bindl, M. Hydrolysis of perchloric acid and sulfuric acid graphite intercalation compounds. Synth. Met. 34(1), 133–138. https://doi.org/10.1016/0379-6779(89)90376-7 (1989).
Li, Z. Q., Lu, C. J., Xia, Z. P., Zhou, Y. & Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 45(8), 1686–1695. https://doi.org/10.1016/j.carbon.2007.03.038 (2007).
Saikia, B. K., Boruah, R. K. & Gogoi, P. K. A X-ray diffraction analysis on graphene layers of Assam coal. J. Chem. Sci. 121(1), 103–106. https://doi.org/10.1007/s12039-009-0012-0 (2009).
Choudhury, D., Das, B., Sarma, D. D. & Rao, C. N. R. XPS evidence for molecular charge-transfer doping of graphene. Chem. Phys. Lett. 497(1–3), 66–69. https://doi.org/10.1016/j.cplett.2010.07.089 (2010).
Blyth, R. I. R. et al. XPS studies of graphite electrode materials for lithium ion batteries. Appl. Surf. Sci. 167(1–2), 99–106. https://doi.org/10.1016/S0169-4332(00)00525-0 (2000).
Światowska, J. et al. XPS, XRD and SEM characterization of a thin ceria layer deposited onto graphite electrode for application in lithium-ion batteries. Appl. Surf. Sci. 257(21), 9110–9119. https://doi.org/10.1016/j.apsusc.2011.05.108 (2011).
Ni, Z., Wang, Y., Yu, T. & Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 1(4), 273–291. https://doi.org/10.1007/s12274-008-8036-1 (2008).
Ferrari, A. C. & Basko, D. M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 8(4), 235–246. https://doi.org/10.1038/nnano.2013.46 (2013).
Ferrari, A. C. et al. Raman spectrum of graphene and graphene Layers. Phys. Rev. Lett. 97(18), 187401. https://doi.org/10.1103/PhysRevLett.97.187401 (2006).
Wang, Y., Alsmeyer, D. C. & McCreery, R. L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 2(5), 557–563. https://doi.org/10.1021/cm00011a018 (1990).
Liu, D., Lan, G., Hu, S. & Wang, H. X-ray diffraction and raman scattering studies of HClO4-graphite intercalation compounds. Carbon 30(2), 251–254. https://doi.org/10.1016/0008-6223(92)90087-D (1992).
Li, Z., Deng, L., Kinloch, I. A. & Young, R. J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 135, 101089. https://doi.org/10.1016/j.pmatsci.2023.101089 (2023).
Calizo, I., Balandin, A. A., Bao, W., Miao, F. & Lau, C. N. Temperature dependence of the Raman spectra of graphene and graphene multilayers. Nano Lett. 7(9), 2645–2649. https://doi.org/10.1021/nl071033g (2007).
Ferrari, A. C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 143(1–2), 47–57. https://doi.org/10.1016/j.ssc.2007.03.052 (2007).
Hernandez, Y. et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 3(9), 563–568. https://doi.org/10.1038/nnano.2008.215 (2008).
Karagiannidis, P. G. et al. Microfluidization of graphite and formulation of graphene-based conductive inks. ACS Nano 11(3), 2742–2755. https://doi.org/10.1021/acsnano.6b07735 (2017).
Morton, J. A. et al. An eco-friendly solution for liquid phase exfoliation of graphite under optimised ultrasonication conditions. Carbon 204, 434–446. https://doi.org/10.1016/j.carbon.2022.12.070 (2023).
Robertson, A. W. & Warner, J. H. Hexagonal Single Crystal Domains of Few-Layer Graphene on Copper Foils. Nano Lett. 11(3), 1182–1189. https://doi.org/10.1021/nl104142k (2011).
Kikuma, J. & Tonner, B. P. XANES spectra of a variety of widely used organic polymers at the C K-edge. J. Electron Spectrosc. Relat. Phenom. 82(1–2), 53–60. https://doi.org/10.1016/S0368-2048(96)03049-6 (1996).
Shahzad, F. et al. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 353(6304), 1137–1140. https://doi.org/10.1126/science.aag2421 (2016).
Li, B., Luo, S., Anwer, S., Chan, V. & Liao, K. Heterogeneous films assembled from Ti3C2T MXene and porous double-layered carbon nanosheets for high-performance electromagnetic interference shielding. Appl. Surf. Sci. 599, 153944. https://doi.org/10.1016/j.apsusc.2022.153944 (2022).
Xia, T. Graphenization of graphene oxide films for strongly anisotropic thermal conduction and high electromagnetic interference shielding (2023).
Ahmed, S., Li, B., Luo, S. & Liao, K. Heterogeneous Ti 3 C 2 T x MXene-MWCNT@MoS 2 film for enhanced long-term electromagnetic interference shielding in the moisture environment. ACS Appl. Mater. Interfaces 15(42), 49458–49467. https://doi.org/10.1021/acsami.3c08279 (2023).
Verma, R., Thakur, P., Chauhan, A., Jasrotia, R. & Thakur, A. A review on MXene and its’ composites for electromagnetic interference (EMI) shielding applications. Carbon 208, 170–190. https://doi.org/10.1016/j.carbon.2023.03.050 (2023).
Gao, Q., Wang, X., Schubert, D. W. & Liu, X. Review on polymer/MXene composites for electromagnetic interference shielding applications. Adv. Nanocompos. 1(1), 52–76. https://doi.org/10.1016/j.adna.2023.11.002 (2024).
Acknowledgements
This work was supported by Khalifa University of Science and Technology under the project FSU-2023-010, 2D Materials for Space Applications. The authors gratefully acknowledge the financial and technical support provided by Khalifa University, as well as the facilities and resources made available by the Advanced Research and Innovation Center (ARIC) and INTRATOMIC.
Author information
Authors and Affiliations
Contributions
R.E. wrote the manuscript, performed the characterizations, and analyzed the results. B.L., S.S., Z.A., and D.A. contributed to the material characterization. B.A. assisted with results analysis and characterization. Y.A. contributed to the results, manuscript review, and provided supervision. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elkaffas, R., Li, B., Shajahan, S. et al. Unprecedented expansion of graphite with low power laser for high-quality liquid phase exfoliated graphene. Sci Rep 15, 32733 (2025). https://doi.org/10.1038/s41598-025-17947-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17947-6