Abstract
Chronic stress has been associated with an increased inflammatory profile in the brain, linking inflammation to the development of stress-related disorders. Although the detailed mechanism connecting chronic stress to inflammation remains unclear, as primary mediators of the immune response, it has been established that microglia play a role. We further investigated the effect of chronic stress on microglia reactivity by incorporating sex and multiple brain regions as variables in our analysis. We utilized the unpredictable chronic mild stress (UCMS) paradigm and then quantified the number of microglia, process arborization, process length, and the number of processes of microglia in the prefrontal cortex (PFC), nucleus accumbens (NAC), hypothalamus (HYPO), amygdala (AMY), and hippocampal CA1 and CA3. We did not observe a stress-induced change in the number of microglia in each region; however, chronic stress reduced microglia arborization, length, and number of processes in a brain region and sex-specific manner. Independent of stress, microglia exhibited a region-dependent reactive phenotype. Together, chronic stress affects microglia reactivity uniquely based on sex and brain region, while the different reactivity profile of microglia in males and females might underlie the sex-specific mechanism of the diseases.
Similar content being viewed by others
Introduction
Microglia serve as key regulators of the innate immune response in the brain1, participating in many major brain functions, including mediating the inflammatory response2. Microglia reactivity is triggered by recognizing the inflammatory signals generated by endogenous or exogenous stimuli3. Decades of research on microglia have revealed dual roles of microglia reactivity: repair and protect against inflammation, or drive inflammation and tissue damage4,5.
The complexity and heterogeneous nature of microglia lead to different functionalities and adaptable morphology6,7. The reactivity of microglia depends on the microenvironment and the functional state of the cell8. Under healthy conditions, the distribution and morphology of microglia are uniform and homeostatic9. Microglia conduct continuous immunosurveillance by extending processes to detect changes in the microenvironment10. When changes are detected, microglia secrete proinflammatory cytokines and undergo cellular remodeling by reducing process ramification and increasing cell body volume to gain mobility8,11,12. However, a chronic inflammatory state can lead to prolonged microglia reactivity and release of proinflammatory mediators, which can cause neuronal damage and contribute to disease13. This is evident in the progression of psychiatric disorders. Patients with depression and anxiety disorders, particularly females, have elevated levels of proinflammatory cytokines14,15. Interestingly, patients with autoimmune diseases also exhibit a higher prevalence of depression16, suggesting that elevated inflammation levels may also be associated with the onset of depressive symptoms17. Post-mortem analysis of depressed patients revealed an increased number of microglia and reactivity in the PFC and hippocampus18. Similar effects have been observed in rodent models of chronic stress that are used to study depression, including elevated proinflammatory cytokines, increased number of microglia, and increased microglia reactivity19,20,21, which occurs in parallel to behavioral and physiological effects22,23. Therefore, determining the distinct morphology of microglia in the context of chronic stress is crucial to understanding the link between chronic stress and inflammation.
Sex differences are prominent in chronic stress-related disorders. For example, women are more likely to be affected by depression and anxiety disorders compared to men24,25. Sex also affects the number of microglia in different brain regions; for example, females have more microglia in the hippocampus and PFC compared to males26,27,28. Microglia also secrete more proinflammatory cytokines and have increased reactivity in females compared to males29,30,31. These findings suggest that sex may affect the change in microglia reactivity due to chronic stress. Understanding sex differences in microglia reactivity provides important information on the unique attributes of stress-related disease progression in males and females.
While the role of microglia has been established in the inflammation and stress models, gaps in knowledge remain due to sex differences, the limited number of brain regions investigated, variability in stress protocols, and methods for defining morphological changes. In this study, we conduct a comprehensive investigation to measure changes in microglia reactivity due to chronic stress in multiple brain regions known to be affected by chronic stress: the PFC, NAC, HYPO, AMY, CA1, and CA3. To induce chronic stress, both male and female mice underwent the UCMS paradigm32. Microglia were detected using immunofluorescence and analyzed based on the process length, process arborization, the number of processes, and the number of microglia in each brain region included in this study. This study provides a comprehensive analysis of microglia reactivity across the brain and addresses the importance of sex differences in response to chronic stress.
Results
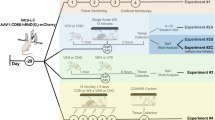
The UCMS paradigm was utilized to induce chronic stress according to the timeline in Fig. 1A. A total of 25 mice were randomly assigned to the NS and UCMS groups of the same sex. The UCMS paradigm was conducted by using a variety of randomly assigned physical and social stressors (Fig. 1B). To assess the effect of chronic stress, weight was measured at days 7 and 14 (Fig. 1C), locomotor activity was also evaluated in an open field test (OFT) on day 12 to ensure that chronic stress did not impact the overall activity of mice, and the light dark test (LDT) was conducted on day 13 to assess the avoidance behavior.
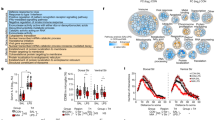
Chronic stress induced weight loss and avoidance behaviors in males. Independent of stress, the number of microglia was region specific. (A) Mice were randomly assigned to treatment groups based on sex: no stress (NS; male n = 6, female n = 6), stress (UCMS; male n = 7, female n = 6). Experimental design and timeline for the UCMS paradigm with the LDT performed on day 13. Experimental timeline generated using biorender.com. (B) Schematic illusion of stressors utilized in the UCMS paradigm. Stressors include isolation, light on/off, cage tile at 45°, wet bedding, stroboscope, static noise, food deprivation, restraint, cage shake, cold surface, new partner, and predator odor. Image generated using biorender.com. (C) mice were weighed on day 0, 7, and 14. Percent weight change is reported. A mixed-effect analysis revealed significant interactions of time and stress (F (2, 76) = 8.591, p = 0.0004), sex and stress (F (1, 76) = 18.99, p < 0.0001), sex, stress and time (F (2, 76) = 6.621, p = 0.0022). The mixed-effect analysis also indicated main effects of time (F (2, 76) = 28.38, p < 0.0001) and stress (F (1, 76) = 26.21, p < 0.0001) (D) In the OFT, total distance traveled is reported. Data was analyzed using two-way ANOVA followed by multiple comparison analysis: a significant interaction between stress and sex (F (1, 20) = 5.806, p = 0.0257) with only a main effect of sex (F (1, 20) = 10.61, p = 0.0039). (E) In the LDT, we measured the duration in the light zone. Two-way ANOVA of the total time spent in the light zone revealed a significant interaction between stress and sex (F (1, 20) = 5.080, p = 0.0356), with main effects of stress (F (1, 20) = 13.84, p = 0.0014) and sex (F (1, 20) = 7.845, p = 0.0110). F. The number of microglia was quantified using IBA1-positive cells in the PFC, NAC, HYPO, AMY, CA1, and CA3. Data analyzed using mixed-effect analysis and followed by multiple comparison analysis on the number of microglia: a significant effect of region (F (5, 66) = 4.484, p = 0.0014). Data presented as the mean with SEM. **p < 0.01, ****p < 0.0001.
Chronic stress induced weight loss and avoidance behavior in males
Weight loss is a common effect of the UCMS paradigm33 and has been observed in our previous studies34,35,36. A mixed-effect analysis revealed significant interactions of time and stress (F (2, 76) = 8.591, p = 0.0004), sex and stress (F (1, 76) = 18.99, p < 0.0001), sex, stress and time (F (2, 76) = 6.621, p = 0.0022). The mixed-effect analysis also indicated main effects of time (F (2, 76) = 28.38, p < 0.0001) and stress (F (1, 76) = 26.21, p < 0.0001). Multiple comparison analysis showed that the male UCMS group lost a significant amount of weight compared to the male NS group (p < 0.0001) and the female UCMS group (p = 0.0005), indicating that the UCMS paradigm induced the expected weight loss in males but not females.
To confirm that UCMS did not affect locomotor activity, the OFT was conducted to measure the total distance mice traveled during the test36,37,38 (Fig. 1D). The two-way ANOVA indicated a significant interaction between stress and sex (F (1, 20) = 5.806, p = 0.0257) with a main effect of sex (F (1, 20) = 10.61, p = 0.0039). Multiple comparison analysis showed a sex difference in the UCMS groups, where females traveled more distance compared to the males (p = 0.0024). The result from the OFT indicated that, as expected, chronic stress did not affect the overall locomotor activity of mice.
The LDT was conducted to measure changes in avoidance behavior due to the UCMS paradigm on day 13 of the UCMS protocol (Fig. 1E). Mice were placed in the dark zone, and the total time spent in the light zone was recorded for 15 min using the ANY-Maze system. Two-way ANOVA of the total time spent in the light zone revealed a significant interaction between stress and sex (F (1, 20) = 5.080, p = 0.0356), with main effects of stress (F (1, 20) = 13.84, p = 0.0014) and sex (F (1, 20) = 7.845, p = 0.0110). Multiple comparison analysis of the total time spent in the light zone indicated a significant difference between NS and UCMS in males only (p = 0.0022). A sex difference was also found between male and female UCMS groups, where UCMS males spent significantly less time in the light zone compared to UCMS females (p = 0.0094). The LDT results showed that chronic stress only increased the avoidance behavior in males.
Independent of chronic stress, microglia distribution is brain region specific.
At the completion of the UCMS paradigm on day 14, mice were perfused, and the brain tissue was collected for analysis. To investigate microglia reactivity in the PFC, NAC, HYPO, AMY, hippocampal CA1, and CA3, six IBA1-positive microglia from each brain region were randomly selected using Imaris software. The length and the number of microglial processes were then quantified and analyzed. The number of microglia was also counted manually by a blinded researcher. To accurately assess the number of microglia in each brain region, we normalized the data using the total number of DAPI-stained nuclei in each region (Fig. 1F).
The mixed-effect analysis only revealed a significant effect of region (F (5, 66) = 4.484, p = 0.0014) with no effect of stress on IBA1-positive microglia in different brain regions. Multiple comparison analysis showed that the PFC and AMY had significantly more microglia than HYPO (PFC: p = 0.0140, AMY: p = 0.0426). The PFC had significantly more microglia than the NAC (p = 0.0086), indicating that the highest number of microglia was found in the PFC and AMY, while the lowest number was found in the HYPO and NAC.
Chronic stress reduced microglia arborization, process length, and the number of processes, with notable sex differences
While studying changes in the number of microglia is a common method to detect changes in microglia reactivity, we did not find any change due to chronic stress. To conduct a comprehensive analysis on the effect of chronic stress on microglia, we evaluated the total length and total number of processes in microglia22,23, as well as process arborization using Sholl analysis39. The total length of the processes represents the sum of the length of all processes per microglia.
Prefrontal cortex
The representative immunofluorescent images showed a morphological difference between the NS and UCMS groups in the PFC (Fig. 2A). The total length of the PFC microglia processes (Fig. 2B) was quantified and analyzed using two-way ANOVA, which revealed a significant interaction between stress and sex in the PFC (F (1, 134) = 27.98, p < 0.0001), with main effects of stress (F (1, 134) = 15.06, p = 0.0002) and sex (F (1, 134) = 19.74, p < 0.0001). Multiple comparison analysis indicated the length of microglia processes from females was significantly shorter in UCMS compared to NS (p < 0.0001) in males only. Female NS also has significantly shorter microglial processes in total compared to male NS (p < 0.0001).
Chronic stress induced microglia reactivity in the PFC by decreasing the arborization, length, and number of processes. Independent of stress, females had shorter process length while males had more microglia processes. (A) Representative images of microglia in the PFC from NS and UCMS groups. Microglia were immunostained with IBA1 (red), nucleus was stained with DAPI (blue). 400× magnification. Scale bar: 50 μm. Nissl staining obtained from the Allen Mouse Brain Atlas and Allen Reference Atlas—Mouse Brain65. (B) Quantitative analysis of total length of processes in microglia from the PFC. Data analyzed using two-way ANOVA and followed by multiple comparison analysis: a significant interaction between stress and sex in the PFC (F (1, 134) = 27.98, p < 0.0001), with main effects of stress (F (1, 134) = 15.06, p = 0.0002) and sex (F (1, 134) = 19.74, p < 0.0001). (C) Quantitative analysis of number of processes in microglia from the PFC. Data analyzed using two-way ANOVA and followed by multiple comparison analysis: a significant interaction between stress and sex (F (1, 138) = 6.842, p = 0.0099), with a main effect of stress (F (1, 138) = 50.30, p < 0.0001). (D) Sholl analysis was conducted to measure the process arborization. Data analyzed using one-way ANOVA followed by multiple comparison analysis (F (3, 205) = 4.998, p = 0.0023). For each mouse, 6 microglia were randomly selected bilaterally for morphological quantification. Data presented as the mean with SEM. *p < 0.05, **p < 0.01, ****p < 0.0001.
Two-way ANOVA on the number of processes (Fig. 2C) indicated a significant interaction between stress and sex (F (1, 138) = 6.842, p = 0.0099), with a main effect of stress (F (1, 138) = 50.30, p < 0.0001). Using multiple comparison analysis, we found a significant difference between UCMS and NS (male: p = 0.0094, female: p < 0.0001), where UCMS had a lower number of processes compared to NS. Male NS also had a significantly lower number of processes compared to female NS (p = 0.0169).
To evaluate the process arborization (Fig. 2D), Sholl analysis was conducted on both sexes, and showed that chronic stress reduced the process arborization within 20 μm of the cell body in both sexes. One-way ANOVA revealed a significant difference between each group (F (3, 205) = 4.998, p = 0.0023). Multiple comparison analysis showed that chronic stress significantly reduced the process arborization in female UCMS compared to female NS (p = 0.0281).
Nucleus accumbens
In the NAC, the immunofluorescent images indicated a morphological difference between the NS and UCMS groups (Fig. 3A). Specifically, two-way ANOVA on the length of microglia processes (Fig. 3B) revealed a significant interaction between stress and sex (F (1, 121) = 12.12, p = 0.0007), with main effects of stress (F (1, 121) = 57.03, p < 0.0001) and sex (F (1, 121) = 19.13, p < 0.0001). Multiple comparison analysis showed a significant difference in process length; processes were significantly shorter in UCMS compared to NS (male: p = 0.0080; female: p < 0.0001). Although we did not observe a sex difference in the stress groups, male NS had significantly shorter processes than female NS (p < 0.0001), indicating a more reactive phenotype in males at the baseline.
Chronic stress induced microglia reactivity in the NAC by decreasing the arborization, length, and number of processes. Males exhibited a more reactive phenotype at baseline. (A) Representative images of the effect of stress on microglia in the NAC from NS and UCMS groups. Microglia were immunostained with IBA1 (red), nucleus was stained with DAPI (blue). 400× magnification. Scale bar: 50 μm. Nissl staining obtained from the Allen Mouse Brain Atlas and Allen Reference Atlas—Mouse Brain65. (B) Quantitative analysis of total length of processes in microglia from the NAC. Data analyzed using two-way ANOVA and followed by multiple comparison analysis: a significant interaction between stress and sex (F (1, 121) = 12.12, p = 0.0007), with main effects of stress (F (1, 121) = 57.03, p < 0.0001) and sex (F (1, 121) = 19.13, p < 0.0001). (C) Quantitative analysis of number of processes in microglia from the NAC. Data analyzed using two-way ANOVA and followed by multiple comparison analysis: an interaction between stress and sex (F (1, 135) = 6.605, p = 0.0113), with a significant effect of stress (F (1, 135) = 34.06, p < 0.0001) and sex (F (1, 135) = 25.61, p < 0.0001). (D) Sholl analysis was conducted to measure the process arborization. Data analyzed using one-way ANOVA followed by multiple comparison analysis (F (3, 203) = 3.237, p = 0.0232). For each mouse, 6 microglia were randomly selected bilaterally for morphological quantification. Data presented as the mean with SEM. **p < 0.01, ****p < 0.0001.
Using two-way ANOVA (Fig. 3C), we found an interaction between stress and sex (F (1, 135) = 6.605, p = 0.0113), with a significant effect of stress (F (1, 135) = 34.06, p < 0.0001) and sex (F (1, 135) = 25.61, p < 0.0001) on the number of processes. Through multiple comparison analysis, we found that female UCMS had a decreased number of processes compared to female NS (p < 0.0001). Independent of stress, NS males showed a more reactive phenotype by having a significantly lower number of processes compared to NS females (p < 0.0001).
Similar to the PFC, Sholl analysis showed that chronic stress reduced process arborization within 20 μm of the cell body in both sexes (Fig. 3D). By conducting one-way ANOVA, a significant difference was found among each group (F (3, 203) = 3.237, p = 0.0232). Multiple comparison analysis indicated that female UCMS had significantly lower process arborization compared to female NS (p = 0.0254).
Hypothalamus
The representative immunofluorescent images showed a morphological difference between the NS and UCMS groups in the HYPO (Fig. 4A). The total length of processes (Fig. 4B) was measured and analyzed using a two-way ANOVA. There was a significant interaction between stress and sex (F (1, 133) = 19.03, p < 0.0001), with the main effects of stress (F (1, 133) = 50.53, p < 0.0001) and sex (F (1, 133) = 33.87, p < 0.0001). Multiple comparison analysis revealed that microglia in female UCMS had significantly shorter processes compared to female NS (p < 0.0001). Similar to the PFC and NAC, NS males demonstrated a more reactive phenotype by having significantly shorter processes than NS females (p < 0.0001).
Chronic stress induced microglia reactivity in the HYPO by decreasing the arborization, the length, and number of processes. Males exhibited a more reactive phenotype at baseline. (A) Representative images of the effect of stress on microglia in the HYPO from NS and UCMS groups. Microglia were immunostained with IBA1 (red), nucleus was stained with DAPI (blue). 400× magnification. Scale bar: 50 μm. Nissl staining obtained from the Allen Mouse Brain Atlas and Allen Reference Atlas—Mouse Brain65. (B) Quantitative analysis of total length of processes in microglia from the HYPO. Data analyzed using two-way ANOVA and followed by multiple comparison analysis: a significant interaction between stress and sex (F (1, 133) = 19.03, p < 0.0001) with the main effects of stress (F (1, 133) = 50.53, p < 0.0001) and sex (F (1, 133) = 33.87, p < 0.0001). (C) Quantitative analysis of number of processes in microglia from the HYPO. Data analyzed using two-way ANOVA and followed by multiple comparison analysis: a significant interaction between stress and sex (F (1, 143) = 15.29, p = 0.0001) with main effects of stress (F (1, 143) = 110.5, p < 0.0001) and sex (F (1, 143) = 36.10, p < 0.0001). (D) Sholl analysis was conducted to measure the process arborization. Data analyzed using one-way ANOVA followed by multiple comparison analysis (F (3, 200) = 16.02, p < 0.0001). For each mouse, 6 microglia were randomly selected bilaterally for morphological quantification. Data presented as the mean with SEM. ***p < 0.001, ****p < 0.0001.
By conducting a two-way ANOVA on the number of processes in the HYPO microglia (Fig. 4C), a significant interaction was also identified between stress and sex (F (1, 143) = 15.29, p = 0.0001) with main effects of stress (F (1, 143) = 110.5, p < 0.0001) and sex (F (1, 143) = 36.10, p < 0.0001). With multiple comparison analysis, we found that the UCMS groups had a significant decrease in the number of processes compared to the NS groups in both sexes (p < 0.0001). Independent of stress, a sex difference was also observed in the NS groups, where males had a significantly lower number of processes compared to females, showing a more reactive phenotype (p < 0.0001).
In the HYPO, Sholl analysis indicated a chronic stress-induced decrease in process arborization within 28 μm of the cell body for females, and 36 μm of the cell body for males (Fig. 4D). By conducting one-way ANOVA, we found a significant difference between each group (F (3, 200) = 16.02, p < 0.0001). Multiple comparison analysis revealed that female UCMS had a significant reduce in process arborization compared to female NS (p = 0.0002). A sex difference was also found at baseline, where male NS had significantly reduced process arborization compared to female NS (p = 0.0003).
Amygdala
Similar to the HYPO, the fluorescent images from the AMY also indicated a morphological difference between the NS and UCMS groups (Fig. 5A). Two-way ANOVA on the length of processes in the AMY (Fig. 5B) revealed a significant interaction between stress and sex (F (1, 136) = 18.44, p < 0.0001) with main effects of stress (F (1, 136) = 11.34, p < 0.0001) and sex (F (1, 136) = 17.47, p < 0.0001). Using the multiple comparison analysis, we found a significant difference between UCMS and NS in females, where the length of microglial processes in UCMS was significantly shorter compared to NS (p < 0.0001). A sex difference in the NS groups showed that male microglia had shorter process length compared to females (p < 0.0001).
Chronic stress induced microglia reactivity in the AMY by decreasing the arborization, the length and number of processes. Males exhibited a more reactive phenotype at baseline. (A) Representative images of the effect of stress on microglia in the AMY from NS and UCMS groups. Microglia were immunostained with IBA1 (red), nucleus was stained with DAPI (blue). 400× magnification. Scale bar: 50 μm. Nissl staining obtained from the Allen Mouse Brain Atlas and Allen Reference Atlas—Mouse Brain65. (B) Quantitative analysis of total length of processes in microglia from the AMY. Data analyzed using two-way ANOVA and followed by multiple comparison analysis: a significant interaction between stress and sex (F (1, 136) = 18.44, p < 0.0001) with main effects of stress (F (1, 136) = 11.34, p < 0.0001) and sex (F (1, 136) = 17.47, p < 0.0001). (C) Quantitative analysis of number of processes in microglia from the AMY. Data analyzed using two-way ANOVA and followed by multiple comparison analysis: a significant interaction between stress and sex (F (1, 134) = 26.38, p < 0.0001) with main effects of stress (F (1, 134) = 73.27, p < 0.0001) and sex (F (1, 134) = 16.84, p < 0.0001). (D) Sholl analysis was conducted to measure the process arborization. Data analyzed using one-way ANOVA followed by multiple comparison analysis (F (3, 206) = 12.21, p < 0.0001). For each mouse, 6 microglia were randomly selected bilaterally for morphological quantification. Data presented as the mean with SEM. ** p < 0.01, ****p < 0.0001.
Two-way ANOVA conducted on the number of processes (Fig. 5C) showed a significant interaction between stress and sex (F (1, 134) = 26.38, p < 0.0001) with main effects of stress (F (1, 134) = 73.27, p < 0.0001) and sex (F (1, 134) = 16.84, p < 0.0001). Through multiple comparison analysis, a significant difference between UCMS and NS in females was identified, where the UCMS group had a significantly lower number of processes compared to the NS group (p < 0.0001). A sex difference was also found in the NS groups, where males had a significantly lower number of processes compared to females (p < 0.0001). Together with a shorter and reduced number of processes, NS males exhibited a more reactive phenotype in the AMY than NS females.
A notable decrease in process arborization was observed using Sholl analysis within 20 μm of the cell body in males, and within 26 μm of the cell body in females (Fig. 5D). One-way ANOVA revealed a significant difference among each group (F (3, 206) = 12.21, p < 0.0001). The result of multiple comparison analysis showed that female UCMS had a significant reduce in process arborization compared to female NS (p < 0.0001). A sex difference was also observed in the NS groups, where male NS showed a significant decrease in process arborization compared to female NS (p = 0.0279).
Hippocampal CA1
In the CA1, the fluorescent image showed a morphological difference between the NS and UCMS groups (Fig. 6A). The length of processes in the CA1 was quantified and analyzed using two-way ANOVA (Fig. 6B). We found a significant interaction between stress and sex (F (1, 137) = 11.34, p = 0.0010) with only a main significant effect of stress (F (1, 137) = 25.50, p < 0.0001). Multiple comparison analysis only indicated a significant difference in the process length between the UCMS and NS groups in males (p < 0.0001), where UCMS had significantly shorter processes compared to NS. A sex difference was observed in the NS groups, where females had a shorter process length compared to males, indicating a more reactive phenotype in NS females (p = 0.0294).
Chronic stress induced microglia reactivity in the CA1 by decreasing the arborization, the length, and number of processes. Females exhibited a more reactive phenotype at baseline. (A) Representative images of the effect of stress on microglia in the CA1 from NS and UCMS groups. Microglia were immunostained with IBA1 (red), nucleus was stained with DAPI (blue). 400× magnification. Scale bar: 50 μm. Nissl staining obtained from the Allen Mouse Brain Atlas and Allen Reference Atlas—Mouse Brain65. (B) Quantitative analysis of total length of processes in microglia from the CA1. Data analyzed using two-way ANOVA and followed by multiple comparison analysis: a significant interaction between stress and sex (F (1, 137) = 11.34, p = 0.0010) with only a main significant effect of stress (F (1, 137) = 25.50, p < 0.0001). (C) Quantitative analysis of number of processes in microglia from the CA1. Data analyzed using two-way ANOVA and followed by the multiple comparison analysis: a significant a main significant effects of stress (F (1, 137) = 55.36, p < 0.0001). (D) Sholl analysis was conducted to measure the process arborization. Data analyzed using one-way ANOVA followed by multiple comparison analysis (F (3, 206) = 10.95, p < 0.0001). For each mouse, 6 microglia were randomly selected bilaterally for morphological quantification. Data presented as the mean with SEM. *p < 0.05, ***p < 0.001, ****p < 0.0001.
Two-way ANOVA was also conducted on the number of processes to determine the microglia reactivity in the CA1(Fig. 6C). The result indicated a main significant effect of stress (F (1, 137) = 55.36, p < 0.0001). Multiple comparison analysis revealed a significant difference between the UCMS and NS groups in both sexes, where the UCMS groups had a decreased number of processes compared to the NS groups (p < 0.0001).
In the CA1, Sholl analysis showed that chronic stress decreased the process arborization within 22 μm of the cell body in both sexes (Fig. 6D). One-way ANOVA revealed a significant difference in each group (F (3, 206) = 10.95, p < 0.0001). Multiple comparison analysis showed that female UCMS had a significant decrease in process arborization compared to female NS (p < 0.0001). In the NS groups, males had a significant decrease in process arborization compared to females (p = 0.0008), indicating a sex difference.
Hippocampal CA3
Similar to the CA1, the NS and UCMS groups were also morphologically different (Fig. 7A). Two-way ANOVA on the length of processes (Fig. 7B) revealed a significant interaction between stress and sex (F (1, 140) = 22.98, p < 0.0001), with main significant effects of stress (F (1, 140) = 39.30, p < 0.0001) and sex (F (1, 140) = 6.391, p = 0.0126). Multiple comparison analysis indicated a significant difference between NS and UCMS in males only, where UCMS had significantly reduced length of processes compared to NS (p < 0.0001). A sex difference was also found in the NS groups, where females had significantly shorter microglia processes compared to males (p < 0.0001).
Chronic stress induced microglia reactivity in the CA3 by decreasing the arborization, the length, and number of processes. Females exhibited a more reactive phenotype at baseline. (A) Representative images of the effect of stress on 11microglia in the CA3 from NS and UCMS groups. Microglia were immunostained with IBA1 (red), nucleus was stained with DAPI (blue). 400 × magnification. Scale bar: 50 μm. Nissl staining obtained from the Allen Mouse Brain Atlas and Allen Reference Atlas—Mouse Brain65. (B) Quantitative analysis of total length of processes in microglia from the CA3. Data analyzed using two-way ANOVA and followed by multiple comparison analysis: a significant interaction between stress and sex (F (1, 140) = 22.98, p < 0.0001), with main significant effects of stress (F (1, 140) = 39.30, p < 0.0001) and sex (F (1, 140) = 6.391, p = 0.0126). (C) Quantitative analysis of number of processes in microglia from the CA3. Data analyzed using two-way ANOVA and followed by multiple comparison analysis: a significant interaction between stress and sex (F (1, 141) = 15.32, p = 0.0001) with main effects of stress (F (1, 141) = 39.92, p < 0.0001) and sex (F (1, 141) = 4.058, p = 0.0459). (D) Sholl analysis was conducted to measure the process arborization. Data analyzed using one-way ANOVA followed by multiple comparison analysis (F (3, 206) = 9.078, p < 0.0001). For each mouse, 6 microglia were randomly selected bilaterally for morphological quantification. Data presented as the mean with SEM. **p < 0.01, ***p < 0.001, ****p < 0.0001.
Two-way ANOVA conducted on the number of microglia processes in the CA3 (Fig. 7C) indicated a significant interaction between stress and sex (F (1, 141) = 15.32, p = 0.0001) with main effects of stress (F (1, 141) = 39.92, p < 0.0001) and sex (F (1, 141) = 4.058, p = 0.0459). Based on the multiple comparison analysis, male UCMS had significantly less processes in microglia compared to male NS (p < 0.0001). A sex difference was found in the UCMS groups where males had a significantly lower number of processes compared to females (p = 0.0002).
In the CA3, Sholl analysis showed that chronic stress promoted a decrease within 25 μm of the cell body in both sexes (Fig. 7D). One-way ANOVA revealed a significant difference between each group (F (3, 206) = 9.078, p < 0.0001). Multiple comparison analysis further indicated a sex difference; the male UCMS group had a significant decrease in process arborization compared to female UCMS (p = 0.0031).
Discussion
In this study, we conducted a comprehensive analysis of chronic stress-induced microglia reactivity and reported a sex and region-specific effect on microglia reactivity due to chronic stress. We first analyzed the number of IBA1-positive microglia and then evaluated morphology based on the arborization, length, and the number of processes from individual microglia in the PFC, NAC, HYPO, AMY, CA1, and CA3. Our analysis focused on the regional heterogeneity and the sex differences of microglia in chronic stress.
With increased reactivity, microglia become mobile and migrate to the site of inflammation40,41. Therefore, quantifying the number of microglia is a common way to evaluate the regional inflammatory response in the brain42. There are conflicting reports indicating that microglia increase or decrease in number due to stress; however, it is possible that the variability between studies, such as the model of stress, animal species or strain, and the duration of stress protocol, can affect the number of microglia43. For example, Tynan et al., Hellwig et al., and Tong et al. reported an increased number of microglia in the PFC, NAC, and hippocampus19,44,45, while Bollinger et al. reported no changes in the number of microglia due to chronic stress31. Here, we found that chronic stress did not affect the number of microglia in any of the brain regions investigated; however, there were notable changes in the number of microglia when comparing brain regions and sex. Among the six brain regions investigated, the PFC and AMY had the highest number of microglia, while the HYPO had the lowest number, which is consistent with other reports on the number of microglia27,46. Even though these brain regions have the most differential numbers of microglia, it did not relate to the reactivity changes due to chronic stress. One of the major differences in the method of microglia quantification from Tynan et al.19 and Hellwig et al.45 compared to ours, is that high magnification images were utilized in our study. Using high magnification images reduces the field of view and the area of each brain region that is analyzed, which may account for the discrepancies in our results from previously published studies.
A change in the number of microglia in a brain region may be associated with the severity of the disease. For example, an increased number of microglia is observed in patients with schizophrenia18, suicide47, stroke48, and multiple sclerosis49. These diseases arguably have more severe effects on the brain than mild chronic stressors and may lead to heightened microglia reactivity, resulting in not only a reactive phenotype but also increased microglia. Therefore, only measuring a change in the number of microglia may lead to oversights in understanding microglia reactivity in some disease models. By utilizing multiple measures of reactivity, our study supports the importance of investigating microglia reactivity through multiple parameters.
In our study, we observed that brain regions with higher total microglia were often accompanied by shorter process length in the no stress control groups. For example, females had more microglia in the CA1 than males, with shorter process length compared to males. This correlation between the microglia number and microglia phenotype was also observed in the female CA1 from other studies26,28. There may be a link between the increased number of microglia and reduced length of processes. We speculate that a higher density of microglia in a given region may result in shorter process length because the surveillance area is distributed to a greater number of microglia.
In models of inflammation, microglia adopt a morphology with shortened and reduced numbers of processes to increase cell motility3. Microglia morphology is largely dependent on the neurons50; neurons control microglia reactivity by releasing specific cytokines or neurotransmitters based on the change in the local microenvironment51. Although we found that chronic stress induced morphological changes, it did not have a uniform effect on microglial arborization, length, or the number of processes across all brain regions. Though it was not possible to link a specific microglia morphology to a certain neuron subtype in our study, the brain region variation in microglia morphology may reflect the negative impact of chronic stress on local neuronal activity. It is well established that neuron dysregulation leads to behavioral changes52, and based on the relationship between neurons and microglia, our findings provide a rationale to investigate how microglia reactivity is directly associated with the behavioral effects of chronic stress.
Microglia exhibited a more reactive phenotype in the NS males compared to NS females in the AMY. At the same time, chronic stress increased microglia reactivity in both sexes and all regions except the AMY in males. While the OFT has been used to evaluate locomotor and anxiety-like behaviors37,53, the LDT is commonly used to assess anxiety-like behaviors in rodents54, and the AMY plays a primary role in regulating behaviors associated with anxiety55,56, Only male UCMS mice exhibited avoidance behaviors associated with anxiety in the LDT, and there were no observed behavioral effects in the OFT due to stress. Overall, females traveled a greater distance than males, as others have also reported in the OFT57,58. Interestingly, the AMY was more responsive to negative stimuli in females, but more responsive to positive stimuli in males59 in human studies. Furthermore, the AMY was more sensitive to persistent negative stimulation60, and increased cortisol levels were linked to increased AMY activity in females61. Although we did not measure corticosterone levels in mice, chronic stress increases corticosterone levels in mice62, similar to cortisol in humans63. We observed that females were affected by chronic stress in the context of cellular reactivity, yet, we and others examining rodent behaviors associated with anxiety show reduced or no effect in females compared to males64. Here, we show that females are impacted by chronic stress at the cellular level through stress-induced microglia reactivity. The inconsistency between the behavioral and cellular effects of chronic stress in females not only indicates potential sex differences in mechanisms of disease but also supports the importance of pairing behavioral testing with molecular analysis.
This study contributes to the current body of literature that supports the effect of chronic stress on microglia reactivity. Importantly, others have identified that prenatal exposure to glucocorticoids, followed by chronic stress, initiates a sex-specific effect on microglia morphology, which may affect microglia plasticity in long-term stress exposures23. Additionally, studies from this group have also demonstrated that the duration of chronic stress affected microglia morphology in the dentate gyrus (DG) and the NAC22. Our finding aligns with Gasper et al. that a 2-week chronic stress reduced the length and number of processes in the NAC microglia in females; however, we observed that chronic stress reduced the process length in the NAC microglia in males22,23. For the hippocampus, an earlier study conducted by Hellwig et al. showed that stress increased the number of processes in the DG microglia45. Gasper et al. reported that a 6-week chronic stress increased the process length and number in microglia from females only22,23. Here in our study, although we did not include the DG in our analysis, we investigated the CA1 and CA3 hippocampal subregions. We observed a stress-induced decrease in the number of processes in the CA1 in females within 2 weeks of chronic stress, but we also reported decreases in both length and number of processes in the CA1 and CA3 microglia in males. Although the comparative studies included mice from both sexes22,23,45, our study utilized sex as one of the variables in every morphological quantification to offer further statistical comparison between sex and chronic stress groups.
Given the complementary and contrasting results in the field of microglia reactivity, we suspect that brain regions, the duration of stress, animal species, parameters for microglia morphology quantification, and other experimental factors play a role in microglia plasticity. Here, we contribute unique data to this field of research by conducting a thorough investigation of microglia morphology utilizing multiple analysis parameters, comparing six relevant brain regions, and incorporating sex as a biological variable. We also assessed and compared the behavioral and cellular changes due to chronic stress and explored the confounding effect of sex on microglia number and reactivity in the brain. Our comprehensive analysis of multiple brain regions complements and expands upon this current knowledge. Furthermore, our findings contribute to the field of chronic stress research, where chronic stress could promote a unique microglia reactivity profile that is region and sex specific.
Methods and materials
Animals
Male and female C57B6/J mice (n = 25) were purchased from Jackson Labs. Mice were 8–10 weeks old, and group housed with food and water access ad libitum. The colony was maintained in a thermoregulated and humidity-controlled room on a 12:12 h light/dark cycle (light on from 6:00 AM to 6:00 PM). Mice were randomly assigned to the following treatments: no stress (NS) and stress (UCMS) groups. Mice were age and sex-matched to behaviorally naïve controls.
Unpredictable chronic mild stress paradigm
The UCMS groups for both sexes underwent the UCMS paradigm for 14 days, while NS mice were gently handled every three days. The UCMS protocol was adapted from our previous studies34,35,36. For 14 consecutive days, mice were subjected to three stressors a day in the morning, afternoon, and overnight in a separate procedure room. Stressors lasted for 1–2 h in the morning and afternoon, and for 13 h overnight. Stressors included: cage on a cold surface, interruption of light/dark cycle, food deprivation for 14 h, exposure to predator urine odor, new cage partners of the same sex, a 45° cage tilt, cage shaking at 100 rotations per minute, restraint, social isolation, wet bedding, stroboscope, and static noise. Throughout the UCMS protocol, stressors remained random as no stressor was repeated within the same day or on two consecutive days, and novelty was maintained by pairing stressors such as the cage on a cold surface and cage shaking. Mice were returned to the animal colony room between stressors.
Body weight measurement
The body weight (Fig. 1D) of each mouse was evaluated the day prior to the UCMS procedure (baseline weight) and then recorded every 7 days for the remainder of the study. The percent change in weight from baseline weight was reported.
The open field test (OFT)
The OFT was conducted on day 12 of the UCMS paradigm. Mice were placed in the testing room an hour before the test for acclimation, During the test, individual mouse was placed in the testing chamber (40 × 40 × 35 cm) with ambient room light (325 lx). Each OFT test lasted for an hour, and all tests were recorded using the ANY-Maze system (Stoelting Co, version 7.49). The total distance traveled was measured and analyzed.
The light dark test (LDT)
The LDT was conducted on day 13 for all groups (Fig. 1E). The testing chamber (ANY-Box) was equally divided into two areas: the dark zone (20 × 40 × 35 cm) with black painted walls and a lid to prevent any lighting; the light zone (20 × 40 × 35 cm) with clear walls and no lid. A bright light with a light intensity of 1000 lx was shined on the light side only. Mice were placed in the testing room an hour before testing for acclimation. Each test was recorded for 15 min. The time spent in the light zone and the number of entries made to the light zone were analyzed using the ANY-Maze system (Stoelting Co, version 7.49).
Tissue collection
Mice were deeply anesthetized with an intraperitoneal injection of 12.5 mg/kg xylazine hydrochloride, 87.5 mg/kg ketamine. They were then transcardially perfused with phosphate buffered saline and fixed with 4% paraformaldehyde (pH 7.4). Whole brains were extracted and post-fixed with 4% paraformaldehyde (pH 7.4) for 24 h and stored in 30% sucrose at 4C for processing.
Immunohistochemistry (IHC)
The immunohistochemistry protocol was adapted from our previous work34. Prior to sectioning, perfused brains were embedded in optimal cutting temperature reagent (ThermoFisher) and cryosectioned into 30-micron tissue sections using a cryostat (HM 525NX, ThermoFisher). Sections were blocked using a blocking buffer containing 0.1% Triton-X100 and 3% normal donkey serum in 1X PBS for an hour at room temperature, then incubated overnight at 4C with goat anti-IBA1 (1:800, PA5-18039, Invitrogen). After washing three times with 1 × PBST and blocking for an hour at room temperature, tissues were incubated with secondary antibody donkey anti-goat Alexa Fluor 568 (1:1000, A-11057, Invitrogen) for 1 h at room temperature. Sections were washed three times with 1X PBST and twice with 1X PBS, then incubated at room temperature for 5 min for DAPI staining (1:3000, ref62248, ThermoScientific), following a 5-min wash in 1X PBS. Tissues were mounted with SlowFade Gold (S36936, Invitrogen) for imaging.
Image acquisition morphological analysis
Image acquisition and morphological analysis were performed by a blinded researcher. For each mouse, images were captured bilaterally from the prelimbic region of the PFC, the core of the NAC, the paraventricular hypothalamus (HYPO), the basolateral amygdala (AMY), the CA1 and CA3 regions of the hippocampus. Images were acquired using a 40 × oil-based objective on a Leica SP8 confocal fluorescence microscope. Each image was captured in a 30-micron deep z-stack (20 z-steps, 1.5 micron/step) with 1024 X 1024 resolution and 8-bit color graphics. 3D reconstruction was then carried out using Imaris (Version 10.1, Oxford Instruments, Concord, MA USA). The Imaris “Filament” module was used to generate 3D reconstruction of microglia using IBA1-positive cells. For each image, 6 IBA1-positive cells were randomly selected using Imaris to quantify the process arborization, the total number of processes and total length of processes per microglia. The number of microglia per region was also counted bilaterally by hand. Cells were identified as microglia when it was both positive for DAPI and IBA1. For each region, the number of microglia was counted bilaterally while blinded to the treatment and the sex of the mice.
Statistical evaluation
Statistical analysis was conducted using GraphPad Prism software. One-way ANOVA was used to evaluate the process arborization in Sholl analysis for all brain regions. Two-way ANOVA analysis was used to evaluate the effect of chronic stress in the LDT, the effect of treatment, and sex in the total number of microglia per region, total number, and length of processes per microglia. Three-way ANOVA was used to analyze the percentage of weight change and microglia density. Multiple analysis was conducted using Tukey’s post hoc test. A post-hoc power analysis was conducted using the G* Power (version 3.1). There was a large effect size, f = 0.96, with 6 mice per group, which provides a power of 0.8 for p < 0.05. The Kruskal–Wallis test was conducted to confirm that the data were normally distributed. In Sholl analysis, due to the large variation in the distance away from the cell body, a cutoff point of 55 μm was applied to all microglia. The cutoff point was calculated by averaging the furthest distance away from the cell body in each group. Statistical outliers were calculated using the two-sided Grubbs’ test (α = 0.05) and excluded from the analysis. Excluded data points: Weight: day 14: 1 Female NS, 1 male NS. Region: 1 female UCMS in NAC, 1 male UCMS in CA3, 1 male UCMS in CA1.PFC length of process: 7 male NS, 1 female NS, 1 male UCMS, 1 female UCMS. PFC total number of processes: 2 male NS, 2 female NS, 2 female UCMS. NAC length of process: 10 female NS, 2 female UCMS, 2 male UCMS. NAC total number of processes: 1 male NS, 1 male UCMS, 3 female NS. HYPO length of process: 1 female UCMS. HYPO total number of processes: 3 female UCMS. AMY number of processes: 2 male NS, 2 female NS, 1 female UCMS. AMY length of process: 2 male NS, 1 female NS, 1 male UCMS, 3 female UCMS. AMY total number of processes: 2 male NS, 2 female NS, 1 female UCMS. CA1 length of process: 1 male NS, 2 female NS, 2 female UCMS. CA1 total number of processes: 1 female NS, 4 male NS. CA3 length of process: 1 male NS, 1 female NS, 1 male UCMS, 3 female UCMS. CA3 number of processes: 3 male NS, 2 male UCMS.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gomez Perdiguero, E. et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551. https://doi.org/10.1038/nature13989 (2015).
Paolicelli, R. C. et al. Microglia states and nomenclature: A field at its crossroads. Neuron 110, 3458–3483. https://doi.org/10.1016/j.neuron.2022.10.020 (2022).
Woodburn, S. C., Bollinger, J. L. & Wohleb, E. S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflammation 18, 258. https://doi.org/10.1186/s12974-021-02309-6 (2021).
Cunningham, C. Microglia and neurodegeneration: The role of systemic inflammation. Glia 61, 71–90. https://doi.org/10.1002/glia.22350 (2013).
Prinz, M. & Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 20, 136–144 (2017).
Ransohoff, R. M. How neuroinflammation contributes to neurodegeneration. Science 353, 777–783. https://doi.org/10.1126/science.aag2590 (2016).
Savage, J. C., Carrier, M. & Tremblay, M.-È. in Microglia: Methods and Protocols (eds Olga Garaschuk & Alexei Verkhratsky) 13–26 (Springer, New York, 2019).
Guo, S., Wang, H. & Yin, Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front. Aging Neurosci. 14, 815347. https://doi.org/10.3389/fnagi.2022.815347 (2022).
Hume, D. A., Perry, V. H. & Gordon, S. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: Phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J. Cell Biol. 97, 253–257. https://doi.org/10.1083/jcb.97.1.253 (1983).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. https://doi.org/10.1126/science.1110647 (2005).
Wang, Y. J., Monteagudo, A., Downey, M. A., Ashton-Rickardt, P. G. & Elmaleh, D. R. Cromolyn inhibits the secretion of inflammatory cytokines by human microglia (HMC3). Sci. Rep. 11, 8054. https://doi.org/10.1038/s41598-021-85702-8 (2021).
Madry, C. et al. Microglial ramification, surveillance, and interleukin-1β release are regulated by the two-pore domain K+ channel THIK-1. Neuron 97, 299-312.e296. https://doi.org/10.1016/j.neuron.2017.12.002 (2018).
Lyman, M., Lloyd, D. G., Ji, X., Vizcaychipi, M. P. & Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 79, 1–12. https://doi.org/10.1016/j.neures.2013.10.004 (2014).
Pallavi, P. et al. Serum cytokines and anxiety in adolescent depression patients: Gender effect. Psychiatry Res. 229, 374–380. https://doi.org/10.1016/j.psychres.2015.06.036 (2015).
Manczak, E. M., Williams, D. & Chen, E. The role of family routines in the intergenerational transmission of depressive symptoms between parents and their adolescent children. J. Abnorm. Child Psychol. 45, 643–656. https://doi.org/10.1007/s10802-016-0187-z (2017).
Glanville, K. P., Coleman, J. R. I., O’Reilly, P. F., Galloway, J. & Lewis, C. M. Investigating pleiotropy between depression and autoimmune diseases using the UK biobank. Biol. Psychiatry Glob. Open Sci. 1, 48–58. https://doi.org/10.1016/j.bpsgos.2021.03.002 (2021).
Mao, L., Ren, X., Wang, X. & Tian, F. Associations between autoimmunity and depression: Serum IL-6 and IL-17 have directly impact on the HAMD scores in patients with first-episode depressive disorder. J. Immunol. Res. 2022, 6724881. https://doi.org/10.1155/2022/6724881 (2022).
Steiner, J. et al. Immunological aspects in the neurobiology of suicide: Elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr Res. 42, 151–157. https://doi.org/10.1016/j.jpsychires.2006.10.013 (2008).
Tynan, R. J. et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav. Immun. 24, 1058–1068. https://doi.org/10.1016/j.bbi.2010.02.001 (2010).
Wang, H.-T. et al. Early-life social isolation-induced depressive-like behavior in rats results in microglial activation and neuronal histone methylation that are mitigated by minocycline. Neurotox. Res. 31, 505–520. https://doi.org/10.1007/s12640-016-9696-3 (2017).
de Pablos, R. M. et al. Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J. Neuroinflammation 11, 34. https://doi.org/10.1186/1742-2094-11-34 (2014).
Gaspar, R. et al. The duration of stress determines sex specificities in the vulnerability to depression and in the morphologic remodeling of neurons and microglia. Front. Behav. Neurosci. 16, 834821. https://doi.org/10.3389/fnbeh.2022.834821 (2022).
Gaspar, R. et al. Resilience to stress and sex-specific remodeling of microglia and neuronal morphology in a rat model of anxiety and anhedonia. Neurobiol. Stress 14, 100302. https://doi.org/10.1016/j.ynstr.2021.100302 (2021).
Kessler, R. C., Petukhova, M., Sampson, N. A., Zaslavsky, A. M. & Wittchen, H. U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 21, 169–184. https://doi.org/10.1002/mpr.1359 (2012).
Altemus, M., Sarvaiya, N. & Neill Epperson, C. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 35, 320–330. https://doi.org/10.1016/j.yfrne.2014.05.004 (2014).
Mouton, P. R. et al. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 956, 30–35. https://doi.org/10.1016/S0006-8993(02)03475-3 (2002).
Lawson, L. J., Perry, V. H., Dri, P. & Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170. https://doi.org/10.1016/0306-4522(90)90229-W (1990).
Schwarz, J. M., Sholar, P. W. & Bilbo, S. D. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 120, 948–963. https://doi.org/10.1111/j.1471-4159.2011.07630.x (2012).
Crain, J. M., Nikodemova, M. & Watters, J. J. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J. Neurosci. Res. 91, 1143–1151. https://doi.org/10.1002/jnr.23242 (2013).
Crain, J. M. & Watters, J. J. Microglial P2 purinergic receptor and immunomodulatory gene transcripts vary by region, sex, and age in the healthy mouse CNS. Transcr. Open Access https://doi.org/10.4172/2329-8936.1000124 (2015).
Bollinger, J. L., Bergeon Burns, C. M. & Wellman, C. L. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav. Immun. 52, 88–97. https://doi.org/10.1016/j.bbi.2015.10.003 (2016).
White, A. G., Elias, E., Orozco, A., Robinson, S. A. & Manners, M. T. Chronic stress-induced neuroinflammation: Relevance of rodent models to human disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms25105085 (2024).
Pothion, S., Bizot, J.-C., Trovero, F. & Belzung, C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav. Brain Res. 155, 135–146. https://doi.org/10.1016/j.bbr.2004.04.008 (2004).
Zhang, A. Y., Elias, E. & Manners, M. T. Sex-dependent astrocyte reactivity: Unveiling chronic stress-induced morphological changes across multiple brain regions. Neurobiol. Dis. 200, 106610. https://doi.org/10.1016/j.nbd.2024.106610 (2024).
Elias, E., Zhang, A. Y., White, A. G., Pyle, M. J. & Manners, M. T. Voluntary wheel running promotes resilience to the behavioral effects of unpredictable chronic mild stress in male and female mice. Stress 26, 2203769. https://doi.org/10.1080/10253890.2023.2203769 (2023).
Manners, M. T. et al. CREB deletion increases resilience to stress and downregulates inflammatory gene expression in the hippocampus. Brain Behav. Immun. 81, 388–398. https://doi.org/10.1016/j.bbi.2019.06.035 (2019).
Seibenhener, M. L. & Wooten, M. C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. https://doi.org/10.3791/52434 (2015).
Elias, E., Zhang, A. Y. & Manners, M. T. Novel pharmacological approaches to the treatment of depression. Life (Basel) https://doi.org/10.3390/life12020196 (2022).
Sholl, D. A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 87, 387–406 (1953).
Parkhurst, C. N. & Gan, W. B. Microglia dynamics and function in the CNS. Curr. Opin. Neurobiol. 20, 595–600. https://doi.org/10.1016/j.conb.2010.07.002 (2010).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758. https://doi.org/10.1038/nn1472 (2005).
Davis, B. M., Salinas-Navarro, M., Cordeiro, M. F., Moons, L. & De Groef, L. Characterizing microglia activation: A spatial statistics approach to maximize information extraction. Sci. Rep. 7, 1576. https://doi.org/10.1038/s41598-017-01747-8 (2017).
Schramm, E. & Waisman, A. Microglia as central protagonists in the chronic stress response. Neurol. Neuroimmunol. Neuroinflamm. https://doi.org/10.1212/nxi.0000000000200023 (2022).
Tong, L. et al. Microglia loss contributes to the development of major depression induced by different types of chronic stresses. Neurochem. Res. 42, 2698–2711. https://doi.org/10.1007/s11064-017-2270-4 (2017).
Hellwig, S. et al. Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain Behav. Immun. 55, 126–137. https://doi.org/10.1016/j.bbi.2015.11.008 (2016).
Tan, Y.-L., Yuan, Y. & Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 25, 351–367. https://doi.org/10.1038/s41380-019-0609-8 (2020).
Schnieder, T. P. et al. Microglia of prefrontal white matter in suicide. J. Neuropathol. Exp. Neurol. 73, 880–890. https://doi.org/10.1097/nen.0000000000000107 (2014).
Jawaid, A., Krajewska, J., Pawliczak, F., Kandra, V. & Schulz, P. E. A macro role for microglia in poststroke depression. J. Am. Geriatr. Soc. 64, 459–461. https://doi.org/10.1111/jgs.13974 (2016).
Yong, V. W. Microglia in multiple sclerosis: Protectors turn destroyers. Neuron 110, 3534–3548. https://doi.org/10.1016/j.neuron.2022.06.023 (2022).
van Rossum, D. & Hanisch, U.-K. Microglia. Metab. Brain Dis. 19, 393–411. https://doi.org/10.1023/B:MEBR.0000043984.73063.d8 (2004).
Biber, K., Neumann, H., Inoue, K. & Boddeke, H. W. G. M. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 30, 596–602. https://doi.org/10.1016/j.tins.2007.08.007 (2007).
Ma, J. & Tang, J. A review for dynamics of collective behaviors of network of neurons. Sci. China Technol. Sci. 58, 2038–2045. https://doi.org/10.1007/s11431-015-5961-6 (2015).
Kraeuter, A. K., Guest, P. C. & Sarnyai, Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol. Biol. 1916, 99–103. https://doi.org/10.1007/978-1-4939-8994-2_9 (2019).
Bourin, M. & Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 463, 55–65. https://doi.org/10.1016/S0014-2999(03)01274-3 (2003).
Kim, M. J. et al. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav. Brain Res. 223, 403–410. https://doi.org/10.1016/j.bbr.2011.04.025 (2011).
Davis, M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 15, 353–375. https://doi.org/10.1146/annurev.ne.15.030192.002033 (1992).
Knight, P. et al. Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacol. Biochem. Behav. 204, 173168. https://doi.org/10.1016/j.pbb.2021.173168 (2021).
Fritz, A. K., Amrein, I. & Wolfer, D. P. Similar reliability and equivalent performance of female and male mice in the open field and water-maze place navigation task. Am. J. Med. Genet. C Semin. Med. Genet. 175, 380–391. https://doi.org/10.1002/ajmg.c.31565 (2017).
Hamann, S., Herman, R. A., Nolan, C. L. & Wallen, K. Men and women differ in amygdala response to visual sexual stimuli. Nat. Neurosci. 7, 411–416. https://doi.org/10.1038/nn1208 (2004).
Andreano, J. M., Dickerson, B. C. & Barrett, L. F. Sex differences in the persistence of the amygdala response to negative material. Soc. Cogn. Affect. Neurosci. 9, 1388–1394. https://doi.org/10.1093/scan/nst127 (2014).
Kogler, L. et al. Sex differences in the functional connectivity of the amygdalae in association with cortisol. Neuroimage 134, 410–423. https://doi.org/10.1016/j.neuroimage.2016.03.064 (2016).
Dallman, M. F. et al. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann. N. Y. Acad. Sci. 1018, 141–150. https://doi.org/10.1196/annals.1296.017 (2004).
Russell, E., Koren, G., Rieder, M. & Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 37, 589–601. https://doi.org/10.1016/j.psyneuen.2011.09.009 (2012).
Donner, N. C. & Lowry, C. A. Sex differences in anxiety and emotional behavior. Pflügers Arch. Eur. J. Physiol. 465, 601–626. https://doi.org/10.1007/s00424-013-1271-7 (2013).
Allen Institute for Brain Science (2004). Allen Mouse Brain Atlas. Available from mouse.brain-map.org. Allen Institute for Brain Science (2011).
Acknowledgements
None
Funding
This work was supported by startup funds granted to Melissa T. Manners from Rowan University and National Institutes of Mental Health R15MH130900. This work was also supported by resources from National Institutes of Health award- 1S10OD032124.
Author information
Authors and Affiliations
Contributions
MTM conceived and designed experiments, and acquired funding. AYZ, EE, AGW, LGJ, TH performed the experiments. AYZ performed data visualization and statistical analysis. AYZ prepared the original manuscript draft. MTM reviewed and edited the final manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The animal study was reviewed and approved by the Animal Ethics Committee of Cooper University Hospital. All experimental procedures were approved by Cooper University Hospital’s Institutional Animal Care and Use Committee. All methods were performed in accordance with the relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org) for the reporting of animal experiments.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, A.Y., Elias, E., White, A.G. et al. Microglia reactivity is brain region and sex specific in the context of chronic stress. Sci Rep 15, 33285 (2025). https://doi.org/10.1038/s41598-025-18000-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18000-2