Abstract
Postpartum hemorrhage (PPH) is a serious complication of hypertensive disorders of pregnancy (HDP) that can severely endanger maternal life. However, clinicians lack an HDP-specific, pre-delivery tool to quantify individual PPH risk. We aimed to develop and internally validate a nomogram for predicting PPH in patients with HDP. This retrospective single-center study included 480 women with HDP admitted between January 2022 and January 2025. PPH was defined as blood loss ≥ 500 mL after vaginal delivery or ≥ 1000 mL after cesarean section within 24 h. Patients were randomly assigned to a training set (n = 336) and a validation set (n = 144) in a 7:3 ratio. Clinical and laboratory variables, including systolic blood pressure (SBP), proteinuria, activated partial thromboplastin time (APTT), fibrinogen (FIB), 25-hydroxyvitamin D [25(OH)D], hepatocyte growth factor (HGF), and endothelin (ET), were analyzed by logistic regression to identify independent predictors for inclusion in the nomogram. Model performance was evaluated by area under the receiver operating characteristic curve (AUC), sensitivity, specificity, calibration curves, and decision curve analysis (DCA). Multivariate analysis indicated that SBP, proteinuria, APTT, and ET were independent risk factors for PPH in HDP patients, whereas FIB, 25(OH)D, and HGF were independent protective factors (P <0.05). The nomogram yielded an AUC of 0.895 (95% CI: 0.859–0.931; sensitivity 75.6%, specificity 86.2%) in the training set and 0.882 (95% CI: 0.811–0.953; sensitivity 78.9%, specificity 83.0%) in the validation set, with Hosmer-Lemeshow χ2 = 2.114, p = 0.977 and χ2 = 10.093, p = 0.259, respectively, indicating excellent discrimination and calibration. DCA indicated clinical net benefit across a wide range of threshold probabilities. This nomogram demonstrated high discrimination and good calibration for predicting PPH risk in women with HDP, with possible applicability in pre-delivery risk stratification. External validation is warranted before its application in routine clinical practice.
Similar content being viewed by others
Introduction
Hypertensive disorders of pregnancy (HDP), including gestational hypertension, preeclampsia, and eclampsia, are among the most serious complications affecting maternal and perinatal health1. Globally, HDP affects approximately 5–10% of pregnancies and is a leading cause of maternal morbidity and mortality2. These disorders are characterized by systemic vasoconstriction, endothelial dysfunction, and a pro-inflammatory state, which not only jeopardize fetal well-being but also significantly increase the risk of postpartum complications3. Among the serious complications associated with HDP, postpartum hemorrhage (PPH) poses a particularly grave threat, accounting for a significant proportion of maternal deaths, especially in low-and middle-income countries4,5. Accurate early prediction of PPH risk in HDP patients is critical for optimizing peripartum management and improving clinical outcomes. In addition to systemic vascular and endothelial dysfunction, impaired uteroplacental blood flow is a key feature of HDP. In normal pregnancy, spiral artery remodeling increases vessel diameter and reduces resistance, ensuring adequate placental perfusion, whereas in preeclampsia this process is deficient, leading to high vascular impedance and reduced capacitance6. Prospective data also show that total uterine artery volume blood flow is inversely correlated with uterine and umbilical artery pulsatility indices and positively associated with birth weight in term pregnancies7, highlighting the central role of uteroplacental perfusion in HDP pathophysiology and its potential contribution to PPH risk.
Recent studies have highlighted the potential roles of several biochemical markers in the pathogenesis of pregnancy-related complications. Among these, 25-hydroxyvitamin D [25(OH)D], a stable circulating form of vitamin D, has been linked to endothelial function and calcium-mediated uterine contractility. Deficiency in 25(OH)D may impair myometrial tone and coagulation, both of which are critical in the prevention of PPH8,9. Hepatocyte growth factor (HGF), a pleiotropic cytokine involved in vascular repair and angiogenesis, may reflect the body’s capacity to recover from endothelial injury commonly seen in HDP. Low HGF levels may indicate poor vascular resilience during placental separation, increasing the risk of hemorrhage10,11. Endothelin (ET), a potent vasoconstrictor elevated in preeclampsia, has been associated with microvascular dysfunction, uteroplacental hypoperfusion, and impaired uterine contractility, all of which may contribute to increased PPH risk12,13. Given these pathophysiological associations, 25(OH)D, HGF, and ET were selected as candidate predictors in our model.
Despite the individual relevance of these biomarkers, few studies have integrated them into a predictive model for postpartum outcomes in HDP. A nomogram is a visual tool used for disease prediction that integrates multiple predictive factors to provide individualized risk assessments for patients. In many fields, nomogram models have been proven effective in predicting disease prognosis and treatment outcomes14,15. Therefore, this study aims to develop and validate a nomogram model based on 25(OH)D, HGF, and plasma ET levels to predict the risk of PPH in patients with HDP. By identifying high-risk individuals through a simple and clinically applicable tool, this model has the potential to facilitate timely intervention and enhance maternal safety.
Materials and methods
Study subjects
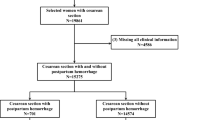
We retrospectively analyzed data from patients with HDP who were treated at our hospital between January 2022 and January 2025, and a total of 480 patients were finally included in the study. Using a random number table method, all patients were divided into a training set and a validation set in a ratio of 7:3. After successful delivery, patients were further categorized into the PPH group and the non-PPH group based on whether PPH occurred—defined as blood loss ≥ 500 mL within 24 h after vaginal delivery or ≥ 1000 mL after cesarean section. Inclusion Criteria: (1) Patients met the diagnostic criteria for HDP16; (2) Singleton pregnancy; (3) Patients provided written informed consent after being informed of the study details. Exclusion Criteria: (1) Incomplete clinical data; (2) Patients with a history of primary hypertension; (3) Patients with coagulation disorders or recent use of anticoagulant medications; (4) Patients with cognitive impairment or psychiatric disorders; (5) Patients with dysfunction of vital organs such as the heart, liver, or kidneys; (6) Patients with autoimmune diseases or malignancies. Initially, 518 patients with HDP were screened. A total of 38 patients were excluded, of whom 22 (4.2%) had incomplete clinical data. Specifically, 25(OH)D was missing in 8 cases (1.5%), HGF in 11 cases (2.1%), ET in 13 cases (2.5%), and coagulation parameters in 3 cases (0.6%), with some patients missing more than one variable. The remaining exclusions were based on other predefined criteria (see Fig. 1). Ultimately, 480 patients were enrolled. All included patients were treated at a tertiary-level academic teaching hospital in Zhengzhou City, which serves as a referral center for high-risk pregnancies. The patient population comprised predominantly Han ethnicity and covered a wide age range (23–40 years). Body mass index (BMI) among the patients ranged from 16 to 33 kg/m². Participants were from both urban and rural areas, with approximately 52% residing in urban regions and 48% from rural settings.
Observational indicators
All clinical and laboratory data used in the model were collected during routine assessments within 24–48 h prior to delivery. This included measurement of blood pressure, proteinuria, coagulation parameters, and levels of 25(OH)D, HGF, and ET. The timing of data collection was consistent across all patients, regardless of delivery mode.
Basic clinical data
The following clinical data were collected: age, systolic blood pressure (SBP), diastolic blood pressure (DBP), BMI, gestational age, mode of delivery, number of pregnancies, proteinuria (measured by 24-hour urinary protein quantification), gestational diabetes mellitus, and fetal weight. Proteinuria was defined as urinary protein excretion ≥ 0.3 g/24 h or a spot urine protein-to-creatinine ratio ≥ 0.3, consistent with the diagnostic criteria for HDP.
Coagulation parameters
During the pre-delivery hospitalization period, fasting venous blood samples were routinely collected in the early morning as part of standardized clinical protocols for patients with HDP. A 5 mL blood sample was centrifuged at 2000 r/min for 10 min to obtain platelet-poor plasma. Coagulation function was assessed using a C2000-A fully automated coagulation analyzer (Mindray, China) to measure prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), and fibrinogen (FIB) levels. An additional 5 mL of fasting venous blood was collected, and platelet count (PLT) was measured using an XS900i fully automated hematology analyzer (Sysmex, Japan).
Detection of 25(OH)D, HGF and ET
Fasting venous blood samples collected during hospitalization were processed according to the hospital’s standard operating procedures. After centrifugation at 3000 r/min for 10 min, plasma samples were stored at − 80 °C in the hospital laboratory. For the present study, plasma levels of 25(OH)D, HGF and ET were retrospectively measured from these stored samples using enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., China). All assays were performed in batch by trained laboratory personnel, strictly following the manufacturer’s instructions. For elective caesarean sections, blood samples were collected 24–48 h before the scheduled surgery during routine preoperative assessment. For vaginal deliveries, samples were obtained upon hospital admission for induction or during the latent phase of spontaneous labor, prior to the onset of active labor, to minimize acute labor-related changes in biomarker levels. Laboratory personnel were blinded to patient outcomes and clinical information; all samples were anonymized and processed in batch after outcome data collection was complete. The three selected biomarkers were pre-specified in the study protocol and subsequently confirmed to be statistically significant in univariate analysis.
Statistical analysis
Categorical variables were expressed as frequencies and percentages [n (%)] and compared using the Chi-square test. Continuous variables were presented as mean ± standard deviation \((\bar x \pm s)\) and compared using the independent t-test. Variables with statistical significance in univariate analysis (P < 0.05) were included in the multivariate logistic regression model to identify independent risk factors for PPH in patients with HDP. Although penalized regression methods such as LASSO were not applied, the risk of overfitting was addressed by (1) splitting the dataset into training (70%) and validation (30%) sets, (2) performing internal validation using 1000 bootstrap resamples, and (3) assessing model discrimination via the area under the receiver operating characteristic (ROC) curve (AUC). Model calibration was evaluated with calibration plots and the Hosmer–Lemeshow goodness-of-fit test. Additionally, the Nagelkerke R2 was calculated to estimate the model’s explanatory power. Variance inflation factor (VIF) analysis was used to evaluate multicollinearity among predictors; variables with VIF > 5 were considered to have high collinearity and excluded from the final model. Clinical utility was assessed using decision curve analysis (DCA). In addition to the original training-validation split, a 10-fold cross-validation was conducted on the entire dataset to further assess the internal generalisation performance of the nomogram model. The mean AUC and its 95% confidence interval (CI) were calculated across folds. At the optimal probability cut-off determined by the Youden index, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were computed. To assess the robustness of the model, we conducted sensitivity analyses using reduced variable sets, a model excluding non-routine biomarkers (HGF and ET).
All statistical analyses were performed using R software version 4.3.1. The “rms” package was used to build the nomogram, generate calibration curves, and obtain the Nagelkerke R2. The ROC curves were generated using the “pROC” package. The Hosmer–Lemeshow goodness-of-fit test was conducted using the “ResourceSelection” package, and DCA was carried out with the “rmda” package. Multicollinearity analysis was performed using the “car” package. A P value < 0.05 was considered statistically significant.
Results
Distribution of HDP subtypes and onset timing
As shown in Supplementary Table 1, in the training cohort, gestational hypertension was the most common subtype (167 cases, 49.7%), followed by preeclampsia without severe features (72 cases, 21.4%), severe preeclampsia (81 cases, 24.1%), and eclampsia (16 cases, 4.8%). In the validation cohort, the proportions were 47.2% (68 cases), 24.3% (35 cases), 23.6% (34 cases), and 4.9% (7 cases), respectively. Regarding onset timing, early-onset HDP (< 34 weeks) occurred in 84 cases (25.0%) in the training cohort and 40 cases (27.8%) in the validation cohort. Chi-square tests showed no statistically significant differences between the training and validation cohorts for either HDP subtype distribution or onset timing (P > 0.05).
Comparison of clinical data between the training set and the validation set
There were no statistically significant differences between the training and validation sets in terms of PPH, age, SBP, DBP, BMI, gestational age, mode of delivery, number of pregnancies, proteinuria, gestational diabetes mellitus, fetal weight, PT, APTT, FIB, TT, PLT, 25(OH)D, HGF, and ET (P > 0.05). See Table 1.
Univariate analysis of PPH in patients with HDP
As shown in Table 2, there were no statistically significant differences between the PPH group and the non-PPH group in terms of age, DBP, BMI, gestational age, mode of delivery, number of pregnancies, gestational diabetes mellitus, fetal weight, PT, TT, and PLT (P > 0.05). However, significant differences were observed in SBP, proteinuria, APTT, FIB, 25(OH)D, HGF, and ET between the two groups (P <0.05).
Multivariate analysis of PPH in patients with HDP
SBP, proteinuria, APTT, FIB, 25(OH)D, HGF, and ET screened in the univariate analysis were used as independent variables, and the occurrence of PPH in patients with HDP was used as the dependent variable in a multivariate logistic regression analysis, and the values assigned to the variables are shown in Table 3. The analysis revealed that SBP (OR = 1.048, 95% CI: 1.019–1.077, P = 0.001), proteinuria (OR = 2.494, 95% CI: 1.085–5.730, P = 0.031), APTT (OR = 1.212, 95% CI: 1.116–1.316, P < 0.001), and ET (OR = 1.067, 95% CI: 1.035-1.100, P < 0.001) were independent risk factors for PPH in HDP patients. In contrast, FIB (OR = 0.656, 95% CI: 0.459–0.937, P = 0.020), 25(OH)D (OR = 0.883, 95% CI: 0.836–0.933, P < 0.001), and HGF (OR = 0.908, 95% CI: 0.876–0.942, P < 0.001) were identified as independent protective factors (Table 4).
Multicollinearity among the variables included in the multivariable logistic regression model was assessed using the VIF. The results showed that all VIF values were less than 5 (SBP = 1.05, proteinuria = 1.03, APTT = 1.06, FIB = 1.01, 25(OH)D = 1.09, HGF = 1.08, ET = 1.07), indicating no significant multicollinearity. These findings suggest that the variables are relatively independent and that multicollinearity is unlikely to have adversely affected the results of the multivariable logistic regression analysis. The Nagelkerke R2 of the final model was 0.522, indicating moderate to good explanatory power.
Construction of the nomogram model
A nomogram model was constructed based on the eight independent factors identified through multivariate logistic regression analysis to predict the risk of PPH in patients with HDP. As shown in Fig. 2, each independent factor was assigned a score, and the total score was calculated to estimate the risk of PPH. A higher total score indicates greater predictive accuracy. For example, a patient with the following characteristics: SBP of 160 mmHg, positive proteinuria, APTT of 34 s, FIB of 1.5 g/L, 25(OH)D of 15 ng/mL, HGF of 55 ng/mL, and ET of 100 pg/mL would be assigned approximately 30,14, 49, 26, 69, 46, and 51 points respectively. The total score of 285 would correspond to a predicted PPH risk of approximately 85%. This example demonstrates how the nomogram can be applied to an individual patient to estimate the probability of PPH and assist with clinical decision-making. A simplified integer-based scoring table was provided in Supplementary Table 2, which enable rapid scoring without referring directly to the graphical nomogram. To facilitate rapid clinical decision-making, we further translated the total nomogram score into a simplified three-tier classification system (Supplementary Fig. 1).
Internal validation of the prediction model
The robustness of the nomogram was evaluated through a 10-fold cross-validation procedure. Predictions were first generated by repeatedly training the model on nine-tenths of the data and testing it on the remaining one-tenth. This process was repeated ten times so that each subset served once as a validation set and nine times as part of the training set. After aggregating the prediction results from all folds, a pooled ROC curve was constructed. The model achieved a mean AUC of 0.874 (95% CI: 0.838–0.910), indicating consistently high discrimination across the resampling cycles and confirming the stability of its predictive performance (Supplementary Fig. 2).
Evaluation of the calibration of the nomogram model
In the training and validation sets, the C-index values of the nomogram model were 0.895 and 0.882, respectively. Calibration curves were generated using 1000 bootstrap resamples in both datasets. As shown in Fig. 3, the predicted curves closely matched the observed curves in both datasets. The Hosmer–Lemeshow goodness-of-fit test further supported the model’s calibration performance, with χ2 = 2.114, P = 0.977 in the training set and χ2 = 10.093, P = 0.259 in the validation set. These findings suggest that the nomogram possesses favorable calibration and reliability in predicting PPH risk among patients with HDP.
Evaluation of the discrimination ability of the nomogram model
ROC curve analysis was performed to evaluate the discriminatory power of the nomogram model. The AUC was 0.895 (95% CI: 0.859–0.931, P < 0.001) in the training set, with a sensitivity of 75.6%, specificity of 86.2%, PPV of 63.9%, and NPV of 91.6%. In the validation set, the corresponding metrics were an AUC of 0.882 (95% CI: 0.811–0.953, P < 0.001), sensitivity of 78.9%, specificity of 83.0%, PPV of 62.5%, and NPV of 91.7% (Fig. 4). These results indicate that the nomogram model exhibited strong discriminative ability in both datasets.
Sensitivity analyses with smaller variable sets
In the model excluding non-routine biomarkers (HGF and ET), the AUC was 0.827 (95% CI: 0.781–0.873, P < 0.001) in the training set and 0.841 (95% CI: 0.762–0.920, P < 0.001) in the validation set, with good calibration (Hosmer–Lemeshow p > 0.05 in both). Sensitivity analyses using reduced variable sets also retained acceptable discrimination and calibration, confirming the robustness of the model while demonstrating the added predictive value of the non-routine biomarkers HGF and ET. Detailed results are provided in Supplementary Tables 3 and Supplementary Fig. 3.
Clinical utility assessment of the nomogram model via DCA
DCA was performed to evaluate the clinical utility of the nomogram. In the training set, the model provided a net benefit across a threshold probability range of 3–95% (Fig. 5A). In the validation set, the model demonstrated clinical benefit within a threshold probability range of 7–96% (Fig. 5B). These findings suggest that the nomogram offers clinical net benefit and practical value in predicting PPH among patients with HDP.
Discussion
The occurrence of PPH in patients with HDP can be life-threatening. Therefore, understanding the risk factors for PPH in HDP patients is crucial for implementing early interventions and tailoring individualized treatment strategies. Our study found that SBP, proteinuria, APTT, FIB, 25(OH)D, HGF, and ET were associated with the development of PPH in patients with HDP. Using the above factors, we constructed a Nomogram model. This model provides clinicians with a tool that is both intuitive and efficient for predicting the risk of PPH in patients with HDP in order to provide more individualized and precise medical care.
In our study, the overall incidence of PPH was approximately 25%, which is higher than most reported rates. The relatively high incidence of PPH observed in our cohort may be explained by several factors. First, our hospital is a tertiary referral center for high-risk pregnancies, where many women present with severe preeclampsia or multiple complications. Second, we applied strict diagnostic thresholds (≥ 500 mL for vaginal delivery, ≥ 1000 mL for cesarean delivery) and carefully reviewed clinical records, which may have captured more cases than routine clinical estimation. Finally, regional differences in obstetric practice and patient characteristics may also have contributed. Given this high baseline risk of PPH in our population, it is particularly important to understand which clinical and laboratory variables contribute most strongly to hemorrhage. SBP is a key clinical indicator reflecting the severity of HDP17. As the severity of HDP progresses from gestational hypertension to preeclampsia and then to severe preeclampsia or eclampsia, SBP levels tend to rise accordingly. In severe HDP, systolic hypertension tends to be more pronounced than diastolic hypertension and is associated with a higher risk of maternal complications such as cerebral hemorrhage, pulmonary edema, and renal impairment18,19. A study by Cagino et al. that included 8,357 singleton pregnancies found that preeclampsia with severe features can increase the risk of PPH4. In our study, we also found that elevated SBP is an independent risk factor for PPH in patients with HDP. High SBP reflects not only increased vascular tone but also underlying endothelial damage and systemic inflammation, both of which are hallmarks of preeclampsia and other HDP subtypes. These pathological changes may lead to poor uterine contractility, impaired platelet aggregation, and coagulation abnormalities, thereby predisposing patients to excessive postpartum bleeding. Moreover, elevated SBP can exacerbate vascular fragility, increasing the likelihood of microvascular rupture during or after delivery20,21. Increased proteinuria is associated with impaired renal perfusion, coagulation disorders, and uterine dystocia, all of which are major contributors to PPH22,23. Zhong et al. identified proteinuria as a risk factor for postpartum hemorrhage in patients with hypertensive disorders of pregnancy (HDP), which is consistent with our findings24. These findings suggest that close monitoring of proteinuria levels in hypertensive pregnancies could help identify high-risk patients, enabling timely interventions such as uterotonics or blood product preparation to mitigate PPH risk.
During normal pregnancy, the maternal hemostatic system undergoes physiological changes towards a hypercoagulable state25. PT, APTT, TT and FIB are the key indicators for assessing coagulation function. PT reflects the exogenous coagulation pathway, APTT embodies endogenous coagulation, TT detects anticoagulant abnormalities in the process of fibrin formation, and FIB, as a glycoprotein, forms fibrin under the action of prothrombin, which is the final common pathway of the coagulation process26. A study investigating risk factors for PPH in vaginal deliveries found that prolonged APTT was associated with an increased risk of PPH27. In addition, Vermeulen et al. noted lower FIB levels as a predictor of PPH. For severe PPH, concentrated FIB can be used for treatment28. Our multivariate logistic regression analysis demonstrated that prolonged APTT was an independent risk factor for PPH in HDP patients, whereas higher FIB levels showed a protective effect, which aligns with the findings of the above study. Therefore, routine prenatal coagulation testing in HDP patients enables early identification of high-risk populations, providing evidence for timely blood transfusion, coagulation factor supplementation, or surgical decision-making.
The inclusion of 25(OH)D, HGF, and ET in the nomogram is supported by their plausible mechanistic roles in the pathophysiology of HDP and PPH, including vascular regulation, coagulation, and uterine contractility. Vitamin D deficiency can directly impair calcium absorption, leading to reduced levels of calcium ions in the body. Low calcium ion levels can affect uterine contractility, resulting in uterine atony and the inability to compress blood vessels in the uterine wall to stop bleeding29. Additionally, as calcium ions serve as coagulation factor IV, insufficient levels may impair the coagulation process and increase blood loss30. Li et al. reported that low serum levels of 25(OH)D were a risk factor for PPH31. Consistently, our findings indicated that elevated 25(OH)D levels were associated with a reduced risk of PPH in patients with HDP. HGF plays a key role in angiogenesis, tissue regeneration, and anti-inflammatory responses32. Reduced HGF levels may increase the risk of pregnancy complications and adverse pregnancy outcomes33. Our multivariate analysis also revealed that for each unit increase in HGF, the risk of postpartum hemorrhage (PPH) decreased by 9.2%. ET, a potent vasoconstrictive peptide produced by endothelial cells, plays a significant role in vascular homeostasis and has been increasingly implicated in the pathophysiology of HDP, including preeclampsia34. ET can strongly constrict blood vessels, leading to increased vascular resistance and a further rise in blood pressure. We found that elevated ET levels are associated with an increased risk of PPH. We propose that a possible explanation is that, in patients with HDP, elevated ET levels may lead to adverse outcomes by promoting endothelial dysfunction, increasing vascular resistance, and impairing uterine contractility. These mechanisms may interfere with effective postpartum hemostasis, thereby increasing the risk of PPH.
To facilitate a more convenient and accurate prediction of the risk of PPH in patients with HDP, we developed a nomogram prediction model based on independent risk factors identified through univariate and multivariate analyses. Comprehensive evaluations of the training and validation sets, including calibration curves and ROC curve analyses, demonstrated the model’s high predictive accuracy. Although the calibration plots demonstrated good overall agreement, slight deviation was observed in the low-risk range of the validation set. This likely reflects the limited number of patients classified as very low risk, leading to less stable estimates in this subgroup. While the overall Hosmer-Lemeshow test supported good calibration, the reliability of risk classification for patients predicted to be at very low risk may be somewhat reduced. Therefore, predictions in this range should be interpreted with caution, and further external validation in larger cohorts will be required to confirm the robustness of the model in low-risk populations. Further DCA revealed that the nomogram provides a net clinical benefit across a broad range of threshold probabilities (3–95% in the training set and 7–96% in the validation set). In clinical practice, if the risk threshold is set at 30% (The choice of 30% as an illustrative threshold was based on clinical experience), only patients exceeding this predicted risk would receive enhanced monitoring or proactive management (e.g., preparing blood products, early use of uterotonics, involvement of senior obstetric staff). Lower thresholds (e.g., 10–20%) would increase sensitivity and capture more at-risk patients but at the cost of more unnecessary interventions, whereas higher thresholds (e.g., > 50%) would improve specificity but risk missing some high-risk cases. This trade-off illustrates the balance between benefit and harm in real-world decision-making, and the DCA supports the clinical utility of the nomogram in guiding tailored, evidence-based interventions. In addition to providing individualized risk estimates, the nomogram may also inform clinical management strategies. For patients predicted to be at high risk, clinicians could consider adjusting delivery planning (e.g., ensuring delivery in a facility with access to intensive care and senior obstetric staff), preparing blood products in advance, and initiating prophylactic measures such as early administration of uterotonics or placement of additional intravenous access. Intensified intrapartum and postpartum monitoring may also be warranted to enable prompt recognition and management of hemorrhage. Conversely, patients predicted to be at low risk may continue with routine obstetric management, which can help avoid unnecessary interventions and allow more efficient allocation of healthcare resources. By linking predicted probabilities to concrete decisions regarding delivery planning, blood product preparation, and prophylactic interventions, the nomogram has the potential to strengthen clinical decision-making and improve maternal safety. The nomogram we constructed not only shows promising application potential in clinical practice but also offers a powerful tool to enhance individualized management of patients with HDP. Although the final model included seven predictors for 120 events, we implemented several strategies to reduce the risk of overfitting, including random splitting into training and validation sets, internal validation with 1,000 bootstrap resamples, and multicollinearity assessment using VIF. In terms of model construction, we did not employ penalized regression techniques such as LASSO because the number of candidate variables after univariate screening was small (seven independent predictors), all of which were both clinically plausible and statistically significant. The events-per-variable ratio was approximately 17, exceeding the commonly recommended threshold of 10, and no significant multicollinearity was detected (all VIF values < 5). Under these conditions, standard multivariable logistic regression was deemed appropriate, and the model’s stability was further confirmed by internal validation using 1000 bootstrap resamples and 10-fold cross-validation. From a clinical perspective, the predictors included in the nomogram—SBP, proteinuria, APTT, FIB, 25(OH)D, HGF, and ET—are either part of standard obstetric evaluation or can be measured using commercially available ELISA kits. We acknowledge that HGF and ET are not routinely measured in standard obstetric practice. However, their inclusion in our model is based on their strong statistical predictive power and their mechanistic relevance to endothelial dysfunction and vascular regulation in HDP and PPH. Although ET, HGF, and 25(OH)D enhanced model performance, they are not part of routine obstetric testing. By contrast, the simplified model excluding ET and HGF relies only on readily available clinical parameters such as blood pressure, proteinuria, and coagulation tests. This substantially improves feasibility by eliminating the need for specialized assays, reducing costs and turnaround time, and enhancing applicability across different healthcare settings. For these reasons, the simplified model may in fact have greater translational value in everyday obstetric practice. The full model, which incorporates ET, HGF, and 25(OH)D, may be more suitable for tertiary hospitals or research institutions where biomarker assays are available and high predictive accuracy is particularly critical. In tertiary hospitals and research institutions where access to these assays is available, the nomogram can already be used to stratify high-risk patients and guide preventive strategies. Moreover, with the increasing adoption of multiplex ELISA and point-of-care testing platforms, we anticipate that measuring these biomarkers will become increasingly feasible, even in resource-limited environments. The nomogram is intended for use in the immediate pre-delivery period, typically 24 to 48 h before childbirth, to identify HDP patients at high risk for PPH. This timeframe corresponds to the routine hospitalization for labor or scheduled cesarean section, providing a 24-48-hour window in which clinicians can implement preventive interventions, such as administration of uterotonics, arrangement of blood products, intensified monitoring, or mobilization of surgical and anesthesia teams, to reduce the risk of adverse maternal outcomes. The scoring criteria for the nomogram are illustrated in Fig. 2. Although an online calculator is not currently available, we intend to develop a user-friendly tool in the future to facilitate clinical integration. Barriers such as access to specific biomarkers and the need for staff training should be considered in real-world implementation.
Our study also has several limitations. First, this study was conducted in a single tertiary hospital in China and used a retrospective design, which may limit the generalisability of the findings. The biomarker profiles observed in our cohort may not fully reflect those of populations in other geographic regions, ethnic groups, or healthcare contexts, particularly in resource-limited settings where nutritional status and disease patterns may differ. Second, although the predictors included in the model were selected based on statistical significance and clinical relevance, some biomarkers such as HGF and ET are not widely available in routine obstetric practice, which may limit the model’s immediate clinical feasibility. In tertiary or research settings where these assays are available, the current model could be applied as is. For broader adoption, future work will explore reduced models that exclude HGF and ET while retaining acceptable predictive performance. With the increasing availability of point-of-care and multiplex assay platforms, incorporating these biomarkers into routine practice may become more feasible over time. Third, while internal validation using a split-sample approach demonstrated good discrimination and calibration, the potential risk of overfitting cannot be entirely excluded given the number of predictors used. Fourth, the incidence of PPH in our cohort (approximately 25%) was higher than most reported rates, even among high-risk obstetric populations. This may have influenced the apparent performance of the model and could restrict its generalizability to lower-risk populations. External validation in different clinical settings will be essential to confirm its broader applicability. Fifth, the outcome definition of PPH relied on retrospective estimation of blood loss from clinical records. Visual estimation and chart abstraction are prone to misclassification, which may have introduced measurement bias. Although we attempted to minimize this by using standardized documentation and careful review of operative and nursing notes, residual error cannot be excluded. Sixth, the strategy for variable selection in this study was based on univariate significance prior to multivariate modeling, rather than penalized regression methods such as LASSO that are often recommended to reduce overfitting. We chose this approach because the number of candidate predictors was small, the events-per-variable ratio was adequate, and internal validation confirmed the stability of the model. Nonetheless, we acknowledge that this choice may have introduced some bias, and future studies should further validate the findings using penalized regression approaches. Lastly, external validation using independent cohorts from other institutions was not performed, and real-world calibration and clinical net benefit analyses in diverse clinical practice settings remain necessary. To address these gaps, we plan to conduct a multicenter prospective study to externally validate, calibrate, and refine the model for broader clinical application.
In conclusion, this study evaluated the risk factors for PPH in patients with HDP and identified SBP, proteinuria, APTT, and ET as risk factors, while FIB, 25(OH)D, and HGF were protective factors. The nomogram constructed from these variables showed high discrimination and good calibration, with possible applicability in pre-delivery risk stratification. Given the limited availability of some biomarkers and the single-center retrospective design, external validation is warranted before routine adoption.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Wu, P., Green, M. & Myers, J. E. Hypertensive disorders of pregnancy. Bmj 381, e071653 (2023).
Narang, K. & Szymanski, L. M. Multiple gestations and hypertensive disorders of pregnancy: what do we know?? Curr. Hypertens. Rep. 23 (1), 1 (2020).
Metoki, H. et al. Hypertensive disorders of pregnancy: definition, management, and out-of-office blood pressure measurement. Hypertens. Res. 45 (8), 1298–1309 (2022).
Cagino, K. A. et al. Risk of postpartum hemorrhage in hypertensive disorders of pregnancy: stratified by severity. Am. J. Perinatol. 41 (15), 2165–2174 (2024).
Aimagambetova, G., Bapayeva, G., Sakhipova, G. & Terzic, M. Management of Postpartum Hemorrhage in Low- and Middle-Income Countries: Emergency Need for Updated Approach Due to Specific Circumstances, Resources, and Availabilities. J Clin Med. 13 (23). (2024).
de Ganzo Suárez, T., de Paco Matallana, C. & Plasencia, W. Spiral, uterine artery doppler and placental ultrasound in relation to preeclampsia. Best Pract. Res. Clin. Obstet. Gynaecol. 92, 102426 (2024).
La Verde, M. et al. The association between fetal doppler and uterine artery blood volume flow in term pregnancies: a pilot study. Ultraschall Med. 45 (2), 184–189 (2024).
Gallo, S. et al. Vitamin D supplementation during pregnancy: an evidence analysis center systematic review and Meta-Analysis. J. Acad. Nutr. Diet. 120 (5), 898–924e4 (2020).
Vivanti, A. J. et al. Vitamin D and pregnancy outcomes: overall results of the FEPED study. J. Gynecol. Obstet. Hum. Reprod. 49 (8), 101883 (2020).
Kreicberga, I., Junga, A. & Pilmane, M. Assessment of apoptosis and appearance of hepatocyte growth factor in placenta at different gestational ages: A cross-sectional study. Int. J. Reprod. Biomed. 19 (6), 505–514 (2021).
Watanabe, T. et al. Changes in plasma levels of hepatocyte growth factor and its associated factors during pregnancy. J. Obstet. Gynaecol. Res. 32 (1), 10–14 (2006).
Qu, H. & Khalil, R. A. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 319 (3), H661–h81 (2020).
Tomimatsu, T., Mimura, K., Endo, M., Kumasawa, K. & Kimura, T. Pathophysiology of preeclampsia: an angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens. Res. 40 (4), 305–310 (2017).
Tong, C., Miao, Q., Zheng, J. & Wu, J. A novel nomogram for predicting the decision to delayed extubation after thoracoscopic lung cancer surgery. Ann. Med. 55 (1), 800–807 (2023).
Wu, J. et al. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun. (Lond). 40 (7), 301–312 (2020).
Gestational Hypertension and Preeclampsia. ACOG practice bulletin, number 222. Obstet. Gynecol. 135 (6), e237–e60 (2020).
Garovic, V. D. et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: A scientific statement from the American heart association. Hypertension 79 (2), e21–e41 (2022).
Bian, Y., Zheng, S., Liu, X. & Jiang, X. Prediction model for severe maternal morbidity in pregnant women with hypertensive disorders of pregnancy. Pak J. Med. Sci. 40 (6), 1054–1062 (2024).
Panda, S. et al. Maternal and perinatal outcomes in hypertensive disorders of pregnancy and factors influencing it: A prospective Hospital-Based study in Northeast India. Cureus 13 (3), e13982 (2021).
Sutton, E. F. et al. Early pregnancy blood pressure elevations and risk for maternal and neonatal morbidity. Obstet. Gynecol. 136 (1), 129–139 (2020).
Ngene, N. C. & Moodley, J. Blood pressure measurement in pregnancy and in hypertensive disorders of pregnancy: devices, techniques and challenges. Cardiovasc. J. Afr. 30 (2), 120–129 (2019).
Erez, O. et al. Preeclampsia and eclampsia: the conceptual evolution of a syndrome. Am. J. Obstet. Gynecol. 226 (2s), S786–s803 (2022).
Jiao, Y. et al. Value of proteinuria in evaluating the severity of HELLP and its maternal and neonatal outcomes. BMC Pregnancy Childbirth. 23 (1), 591 (2023).
Zhong, X. & Zhang, P. Analysis of risk factors associated with different degrees of postpartum hemorrhage in patients with pregnancy-induced hypertension and construction of a prediction model using line graph. J. Matern Fetal Neonatal Med. 36 (2), 2239983 (2023).
Thornton, P. & Douglas, J. Coagulation in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 24 (3), 339–352 (2010).
Winter, W. E., Flax, S. D. & Harris, N. S. Coagulation testing in the core laboratory. Lab. Med. 48 (4), 295–313 (2017).
Bihan, L. et al. Development and validation of a predictive tool for postpartum hemorrhage after vaginal delivery: A prospective cohort study. Biology (Basel). 12 (1), 54 (2022).
Vermeulen, T. & Van de Velde, M. The role of fibrinogen in postpartum hemorrhage. Best Pract. Res. Clin. Anaesthesiol. 36 (3–4), 399–410 (2022).
Sörsjö Stevenazzi, A., Pihl, S., Blomberg, M. & Axelsson, D. The association between maternal vitamin D deficiency and postpartum hemorrhage and uterine Atony. Acta Obstet. Gynecol. Scand. 103 (2), 286–293 (2024).
Amzajerdi, A., Keshavarz, M., Ghorbali, E., Pezaro, S. & Sarvi, F. The effect of vitamin D on the severity of dysmenorrhea and menstrual blood loss: a randomized clinical trial. BMC Womens Health. 23 (1), 138 (2023).
Li, W. J., Chen, K. H., Huang, L. W., Tsai, Y. L. & Seow, K. M. Low maternal serum 25-Hydroxyvitamin D concentration is associated with postpartum hemorrhage: A retrospective observational study. Front. Endocrinol. (Lausanne). 13, 816480 (2022).
Libetta, C. et al. Hepatocyte growth factor (HGF) and hemodialysis: physiopathology and clinical implications. Clin. Exp. Nephrol. 20 (3), 371–378 (2016).
Huang, S. J. et al. Osteoprotegerin, Interleukin and hepatocyte growth factor for prediction of diabetes and hypertension in the third trimester of pregnancy. World J. Clin. Cases. 8 (22), 5529–5534 (2020).
Saleh, L., Danser, J. A. & van den Meiracker, A. H. Role of endothelin in preeclampsia and hypertension following antiangiogenesis treatment. Curr. Opin. Nephrol. Hypertens. 25 (2), 94–99 (2016).
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conception and design: Jingli Zhang and Haiying Wu. Method: Bingqian Zhi. Data Collection: Bingqian Zhi. Manuscript Writing: Jingli Zhang. Manuscript revision: Haiying Wu. Research supervision: Haiying Wu. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethical approval and consent to participate
The study was approved by the Ethics Committee of The Henan Provincial People’s Hospital (No. 2025HPPH-0211521), and informed consent was obtained from all patients. This study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Zhi, B. & Wu, H. Nomogram for predicting postpartum hemorrhage in women with hypertensive disorders of pregnancy. Sci Rep 15, 32845 (2025). https://doi.org/10.1038/s41598-025-18133-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18133-4