Abstract
Essential oils offer a viable alternative to traditional agrochemicals, mitigating problems such as environmental contamination and weed resistance. However, application faces challenges such as low solubility in water and rapid environmental degradation. To overcome these limitations, nanoemulsions containing essential oils from Piper amalago L. and Piper dilatatum Rich were formulated using ultrasonic homogenization. These nanoemulsions were investigated for their herbicidal properties through phytotoxic and cytogenotoxic activity assays using weed plants Bidens pilosa L. and Lolium multiflorum Lam., and model plant Lactuca sativa L. The results indicated that the nanoemulsions presented nanometric-sized droplets (~ 10 nm), PDI close to 0, and greater thermal stability compared to essential oils. The nanoemulsions exerted an inhibitory effect on germination and growth of plants studied, possibly due to cytogenotoxic and mutagenic damages observed. Furthermore, content of photosynthetic pigments was affected, indicating oxidative damage and changes in chlorophyll metabolism.
Similar content being viewed by others
Introduction
The prevalent usage of synthetic herbicides for weed control to meet agricultural food needs, raises concerns due to environmental impacts and human health risks stemming from off-target toxicity and the environmental persistence of these products1,2. Furthermore, the excessive use of herbicides with the same mode of action and the low number of new modes of action introduced into the market in recent decades has contributed to the widespread evolution of weed resistance in many cropping systems2,3. Aligned with the United Nations (UN) 2030 Agenda for Sustainable Development, which highlights the importance of sustainable agriculture and food security, developing more effective and ecologically responsible technologies for controlling unwanted plants4,5.
Essential oils have emerged as potential bioherbicides6,7,8. Formed by complex mixtures of volatile compounds, essential oils are structurally diverse and have multiple sites of action9. Due to their high biodegradability, low toxicity for mammals, and diverse modes of action hindering the development of resistance to agricultural pests, essential oils present a viable alternative for biopesticide production10.
Piperaceae is a family of aromatic plants known for the production of essential oils. The genus Piper L. is the largest within this family, containing approximately 2000 species11. Piper essential oils have a great diversity of phytochemical compounds and exhibit diverse biological activities, such as antioxidant, anti-inflammatory antibacterial, insecticidal, cytotoxic, and phytotoxic action12,13,14,15,16. Studies have shown that the essential oils of some Piper species, such as P. dilatatum Rich. and P. amalago L., have suppressive effects on weeds, negatively interfering with their germination, growth, and photosynthetic machinery14,17. Although Piper essential oils show potential for weed control, challenges common to essential oils, such as limited water solubility and susceptibility to degradation under exposure to environmental factors, such as oxidation under exposure to heat, light, and oxygen, restrict their application18,19,20.
Recent studies point to nanoemulsions as a resource to increase aqueous solubility, and stability and prevent the effects of the environment in essential oils with herbicidal properties8,21,22,23. The small size of nanoemulsions makes them kinetically stable over long periods, reducing susceptibility to physical changes23,24, and improving penetration and translocation into plant tissues, thereby increasing bioactivity25. The development of essential oil-based nanoemulsions has gained momentum in recent years, particularly in applications such as edible coatings and films for food preservation, and as antifungal, antibacterial, antioxidant, and insecticidal agents for disease vectors26,27,28,29.
Considering the herbicidal potential, but also the low water solubility and high degradation rate of essential oils from P. amalago and P. dilatatum, we hypothesize that formulating these oils as nanoemulsions will improve their stability and herbicidal effectiveness in controlling the weed species Bidens pilosa L. (eudicot) and Lolium multiflorum Lam. (monocot). These species are considered problematic in economically important crops such as corn, sugarcane, sorghum, wheat, rice, and barley30,31,32. To our knowledge, this is the first study to propose the use of nanoemulsions containing essential oils from P. amalago and P. dilatatum for bioherbicidal purposes, contributing to the development of sustainable, essential oil-based weed management alternatives.
The nanoemulsions were evaluated for their physicochemical properties, their effectiveness as pre- and post-emergence herbicides, and their effects on photosynthetic pigment levels. The mode of action of the formulations was determined based on the cytogenotoxic effects observed in the cell cycle of the model plant Lactuca sativa L.
Material and methods
Plant material
Samples of P. amalago and P. dilatatum were collected from plants grown in an Atlantic Forest fragment located in southeastern Brazil (Castelo-ES, Brazil 20° 36′ 42″ S 41° 10′ 14″ W). The plant material was stored in paper bags and dried in an oven with air circulation at 40 °C. For herbicidal activity tests, seeds of the weed B. pilosa were collected within a radius of 400 m2 of a coffee crop (Coffea arabica L.) on a site in Córrego dos Pilões, located in the municipality of Iúna, Espírito Santo, Brazil (20° 36′ 61.40″ S 41° 72′ 26.28″ W). All the collected species were previously identified under the taxonomist approach by Prof. Tatiana Tavares Carrijo, and the voucher specimens are housed in the Herbarium CAP (acronyms according to Thiers33, continuously updated), under numbers CAP000005 (P. amalago), CAP000013 (P. dilatatum) and CAP009966 (B. pilosa). The authors confirm that the necessary permissions for sample collection were obtained in accordance with the State Institute for the Environment and Water Resources. The other weed used in the experiments, L. multiflorum (cultivar BRS Ponteio, BRseeds), was purchased from an agribusiness store. For cytogenotoxicity tests, L. sativa seeds (cultivar Grandes Lagos Americana, Isla seeds) were used.
Extraction and chromatographic profile of the essential oils
Approximately 520 g and 860 g of dry leaves of P. dilatatum and P. amalago, respectively, were hydrodistilled using a Clevenger-type apparatus for 4 h per batch. For each extraction, 60 g of plant material were distilled in approximately 1.5 L of distilled water, and successive extractions were performed until the total amount of plant material was processed34. The extracted oil was centrifuged at 5000 rpm for 5 min to remove the aqueous phase and stored in a freezer at 4 °C. The yield of essential oils was determined, in triplicate, through the ratio between the mass of essential oil and the mass of dry plant material, expressed in % (m/m).
The chemical composition of essential oils was analyzed by gas chromatography with flame ionization detection (GC-FID, Shimadzu QP2010SE, Kyoto, Japan) and gas chromatography coupled to mass spectrometry (GC–MS, Shimadzu QP2010SE, Japan) according to the methodology described by Mendes et al.35 and Souza et al.36 GC–MS analysis utilized a 30 m × 0.25 mm fused silica capillary column with 0.25 μm Rtx®-5MS stationary phase, with helium used as the carrier gas for both detectors. Briefly, 1 μL of essential oil solution with a concentration of 3% in 99.9% acetonitrile was injected. The oven temperature was programmed from 40 °C to 180 °C at a rate of 3 °C/min. The FID and MS detectors were set at 240 °C and 200 °C, respectively. GC–MS analyzes employed electron impact ionization at 70 eV, while GC-FID analyzes used a 300 °C flame formed by H2 and atmospheric air. Identification of the components was done by comparing mass spectra with databases and using retention index (RI). The RI was calculated with a mixture of C7-C40 alkanes. Retention times were adjusted using GC-FID and compared with literature values37,38,39. The relative percentage of each compound present in the EOs was determined by calculating the ratio between the integrated peak area and the total area of all sample constituents with a relative area greater than 2%.
Preparation of nanoemulsions
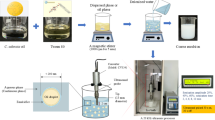
The nanoemulsions were prepared using 1% (m v-1) of essential oil, which corresponds to 10.000 µg mL-1, in addition to 14% (m v-1) of Tween 80 in aqueous medium. Initially, the essential oil and surfactant were weighed in a Falcon tube and maintained at 500 rpm on a magnetic stirrer (Thermo Scientific, Cimarec, China) for 5 min. Then, 20 mL of type 1 water were added to this mixture and subsequently taken to a high-power ultrasonic homogenizer (IKA T Ultra-Turrax) for 5 min at 7000 rpm, under an ice bath. The nanoemulsions were prepared using the high energy method, according to the methodology adapted from Azevedo et al.40.
Characterization and stability of nanoemulsions
The characterization of the hydrodynamic diameter, Zeta potential and polydispersity index was carried out by dynamic light scattering (DLS) in triplicate (Zetasizer Nano ZS90, Malvern Zen Instrument Ltd, United Kingdom (25 ± 2 °C), 633 nm laser, reading angle of 173°, following Mendes et al.41 To examine the thermal stability of P. amalago and P. dilatatum essential oils, Tween 80, and nanoemulsions, we performed thermogravimetric analyzes using a DTG thermal analyzer (SHIMADZU, model 60H, Kyoto, Japan). Approximately 3 mg of each sample was placed in alumina containers and heated from 25 to 450 °C at 10 °C.min-1 under a nitrogen flow of 50 mL.min-1.
Phytotoxic activity on germination and initial development
The pre-emergent effects of essential oils and nanoemulsions from P. amalago and P. dilatatum were evaluated against the plants B. pilosa and L. multiflorum in bioassays under laboratory conditions. The seeds were previously disinfected with 5% sodium hypochlorite and washed several times with distilled water. The experiment was carried out in 15 cm Petri dishes with filter paper. Five concentrations (187.5, 375, 750, 1500 and 3000 µg mL-1) were prepared by dissolving the nanoemulsions in distilled water. These concentrations are common in phytotoxicity experiments with essential oils41,42. Tween 80 (4.2%, v v-1) was used as a negative control. Tween 80 used as a surfactant in the preparation of nanoemulsions has biocompatibility, non-toxic nature and GRAS (Generally Recognized as Safe) status43. Furthermore, it does not present phytotoxic and cytogenotoxic effects for plants44,45. The herbicide glyphosate (1 mL L-1) was used as a positive control. Twenty-five seeds of B. pilosa or L. multiflorum were distributed in each Petri dish and moistened with 3 mL of the respective treatments. The plates were closed, sealed with plastic film and kept in a germination chamber at (25 ± 2) °C with a photoperiod of 16 h of light/8 h of dark. Five replicates were prepared for each treatment. After 7 days, the germination rate and root and shoot length were determined46.
Phytotoxic activity on weeds in a greenhouse
The herbicidal activity of nanoemulsions was studied on 21-day-old B. pilosa and L. multiflorum plants under controlled conditions in a greenhouse according to the methodology adapted from Singh et al.46 Fifteen seeds of each plant were sown in polypropylene tubes (19.5 cm long × 5 cm wide) filled with 230 g of vegetable soil (substrate enriched with organic compound). Fifteen days after seedling emergence, the plants were thinned, keeping only 4 healthy plants of similar size in each tube. Three replications were carried out per treatment, totaling 12 plants. Plants were watered daily until the 2–4 true leaf stage. At 21 days, the plants were watered with the two highest concentrations of nanoemulsions (1500 and 3000 µg mL-1) using a Pasteur pipette. 4 mL of treatments were applied per tube (1 mL per plant). As in the pre-emergence experiments, a solution of Tween 80 (4.2%, v v-1) in distilled water was used as a negative control and glyphosate (1 mL L-1) was applied as a positive control. During the experiment, the temperature of the greenhouse was maintained below 28 °C (ranging between 25 and 28 °C). Humidity was maintained at approximately 90–95%, remaining above 70% during the hottest days. After 15 days of application of the nanoemulsions, the length of the roots and shoots of the plants was measured and recorded, and the leaves were examined for visible lesions in their morphology.
Quantification of photosynthetic pigments
The effect of P. amalago and P. dilatatum nanoemulsions on photosynthetic pigments was investigated in plants subjected to pre-emergence phytotoxic activity tests. For this, L. multiflorum and B. pilosa plants exposed to concentrations of 1500 and 3000 µg mL-1 of nanoemulsions and the negative control Tween 80 (4.2%, v v-1) were lyophilized (K108, Liotop) and crushed (MagNA Lyser, Roche). The plant tissue (0.1 g) was homogenized without direct incidence of light in an ethanolic series, using solutions of 250 µL of ethanol: distilled water of 98, 80, and 50% (v/v) at 80ºC for 20 min, followed by centrifugation at 14,000 rpm for 5 min. Six replicates were prepared for each treatment. Photosynthetic pigment levels were obtained by measuring absorbance with a UV/Vis spectrophotometer (Multiskan FC Microplate Photometer, Thermo Scientific) at 665 nm (chlorophyll a), 649 nm (chlorophyll b) and 470 nm (carotenoids). Ethanol (76%) was used for the white solution. The pigments concentrations were calculated according to the modified Lichtenthaler and Wellburn equation47 and were expressed in µg mg-1 dry weight (DW), as follow:
A represents the absorbance at each respective wavelength, m is the dry mass of the plant tissue in mg (100 mg), V is the total volume of the extract in milliliters (mL).
Cytogenotoxic activity
The cytogenotoxic and mutagenic effects of nanoemulsions from P. amalago and P. dilatatum were evaluated through analysis of the cell cycle of L. sativa (2n = 18). Twenty-five seeds were subjected to germination and exposed to the highest concentrations of nanoemulsions (1500 and 3000 µg mL-1) in Petri dishes, under the same experimental conditions as the pre-emergence tests. Tween 80 (4.2%, v v-1) in distilled water was used as a negative control. Five replicates were prepared for each treatment. After germination, when the rootlets reached approximately 2 cm in length, the roots were collected, fixed in Carnoy’s solution (ethanol: acetic acid, 3:1) and stored at − 18 °C in a freezer. To prepare the slides, the roots were washed in distilled water and stained with 2% acetic orcein. 1000 cells per slide were evaluated, totaling 5000 cells per concentration. To determine the different mitotic stages, the slides were observed under an optical microscope coupled to a digital camera. 1000 cells per slide were evaluated, totaling 5000 cells per concentration34. The cytotoxicity of EO was evaluated in terms of mitotic index and phase index. Genotoxicity was determined by combining the frequencies of cells with chromosomal and nuclear alterations. Mutagenicity was determined by the frequency of micronuclei42,48.
Statistical analysis
Initially, the normality of errors and homogeneity of variances were verified using the Shapiro–Wilk and Bartlett tests, respectively. Subsequently, the mean values of the experimental treatments were analyzed by analysis of variance (ANOVA) and compared by the Tukey test (p ≤ 0.05). All tests were carried out in a completely randomized design. The experiments were analyzed using the ExpDes.pt package of the R software (version 4.3.2)49.
Results and discussion
Yield and chemical profile of essential oils
Chemical analysis allowed the identification of 11 compounds present in the essential oil of P. amalago and 9 compounds in the essential oil of P. dilatatum, comprising 86.7% and 83.7% of the total, respectively. The yields of essential oils from P. amalago and P. dilatatum were 0.15 and 0.10% (m m-1), respectively (Table 1, Fig. 1). These yields are considered low when compared to those reported for other brazilian ecotypes of these species. Studies on P. amalago native to Brazil have shown yields ranging from 0.20% to 1.02%, while P. dilatatum has exhibited yields between 0.2% and 1.50%50,51,52,53,54,55. Research involving species of the Piper genus has shown that essential oil productivity can vary in response to environmental factors such as light intensity, precipitation, humidity and seasonality16. In this context, the environmental conditions to which the plants were exposed may explain the low yields observed in this study.
Gas chromatograms of the essential oils from the leaves of (A) P. amalago and (B) P. dilatatum. (1) α-pinene; (2) β-pinene; (3) linalool; (4) δ-elemene; (5) β-elemene; (6) β-caryophyllene; (7) α-humulene; (8) γ-gurjunene; (9) β-selinene; (10) α-selinene; (11) germacrene A; (12) γ-cadinene; (13) germacrene D; (14) viridiflorene; (15) α-farnesene; (16) spathulenol; (17) α-eudesmol.
The constituents of the essential oils were classified into monoterpene hydrocarbons, oxygenated monoterpenes, oxygenated sesquiterpenes, and, predominantly, sesquiterpene hydrocarbons. The latter accounted for 66.2% in P. amalago and 72.3% in P. dilatatum. Other chemical characterizations of brazilian ecotypes have also identified hydrocarbon sesquiterpenes as the major class of compounds in the essential oil of these species. However, the percentage of hydrocarbon sesquiterpenes found in the essential oil of P. amalago in this study was 20% to 100% higher than those previously reported for these ecotypes, which ranged from 0% to 45.4%50,51,55. Regarding the essential oil of P. dilatatum, the percentage of hydrocarbon sesquiterpenes also stood out, ranking among the highest values reported for Brazilian ecotypes of the species, with a range between 31.5% and 87.7%52,53. Studies with essential oils from Piper and other species have attributed the phytotoxic effects found to the presence of sesquiterpenes12,16,41,42. The high levels of these compounds in the P. amalago and P. dilatatum plants used in this study indicate a distinct sesquiterpene chemical profile for these Brazilian ecotypes. These findings reinforce the potential use of P. amalago and P. dilatatum essential oils as natural herbicidal agents, especially considering the known biological activity of sesquiterpenes.
The major compounds (relative area > 10%) identified in P. amalago were the sesquiterpene hydrocarbons β-elemene (17.8%) and germacrene A (17.6%), followed by the oxygenated monoterpene linalool (12.1%). Linalool was also mainly found, together with β-caryophyllene and guaiol, in the essential oil of P. amalago54. On the other hand, our findings differ from other chemical characterizations for the essential oil from P. amalago leaves50,55. In the essential oil of P. dilatatum, the main compounds identified were the sesquiterpene hydrocarbons viridiflorene (22.9%) and germacrene D (20.8%). Our findings are in line with Silva et al.52 who identified germacrene D and viridiflorol as some of the main compounds in the essential oil of P. dilatatum. Andrade et al.53 also identified germacrene D among the major compounds in the essential oil of P. dilatatum. Other characterizations for the species showed delta-3-carene, bicyclogermacrene, β-caryophyllene, apiol, β-caryophyllene, spathulenol and γ-cadinene as the majority56,57.
Differences in yield and chemical compositions reported for P. amalago and P. dilatatum can be attributed to climatic, seasonal, genetic variations, harvest stage and extraction method. These factors can affect the chemical constituents of essential oils21. Perigo et al.55 reported that although terrain elevation and harvest time did not significantly affect the yield of essential oils from Piper species, including P. amalago, the chemical composition is influenced, presenting qualitative and quantitative differences between the species and intraspecifically for different locations/environmental conditions.
Characterization of nanoemulsions
The droplet hydrodynamic diameter, PDI (polydispersity index) and Zeta potential value of the P. amalago and P. dilatatum nanoemulsion formulation were determined by dynamic light scattering (DLS) (Table 2). The droplet size of the nanoemulsions was around ~ 10 nm, a suitable size for oil-in-water nanoemulsions (10–100 nm)58,59. The PDI of the nanoemulsions was 0.17 for P. dilatatum and 0.30 for P. amalago, indicating good uniformity and stability of the droplets. PDI is a factor that reflects the uniformity and stability of nanoemulsions. PDI values close to 1 indicate a polydisperse system, while 0 or close values indicate a monodisperse system, more uniform and stable during long periods of storage60,61,62.
The Zeta potential of nanoemulsions presented a low value (in modulus), − 3.55 for P. amalago and − 4.73 mV for P. dilatatum. Values above 30 mV indicate a stable nanoemulsion due to strong electrostatic repulsion that prevents particle coalescence, while values below 30 mV indicate a tendency to globulation/flocculation due to electrostatic attraction due to Van der Waals energy63,64. Mayer et al.65 suggested that nonionic surfactants, such as Tween 80, may favor negative electrical charges due to the presence of anionic impurities, such as free fatty acids, in the oil or surfactant. Although the Zeta potential allows predictions to be made about the storage stability of a colloidal dispersion, it is a limit based on experiments, is not the only indicator of the stability of a nanoemulsion and can be optimized in statistical projects21.
Essential oils are recognized as volatile substances with low chemical and physical resistance to adverse environmental conditions such as temperature, oxygen and humidity, which correspondingly reduces their bioactivity with increasing temperature. In this context, we examined the thermal stability of essential oils from P. dilatatum and P. amalago, the surfactant Tween 80 and nanoemulsions based on the corresponding essential oils. The results of this study are presented in Fig. 2.
It was observed through thermogravimetric curves that there was an abrupt loss of mass of the essential oils of P. amalago and P. dilatatum, at maximum decomposition temperatures of 168 and 187 °C, respectively. The thermogravimetric curves of nanoemulsions show a marked mass loss event between 50 and 90 °C, and this is associated with the evaporation of water from the surface of the samples, as the formulation is composed of 90% water. After this event, it was observed that protection via the formation of nanoemulsions increased the maximum decomposition temperature of P. amalago and P. dilatatum essential oil to 386 and 402 °C, respectively (Fig. 2A). These results demonstrate the increase in the thermal stability of essential oils by nanoencapsulation through nanoemulsions. According to Barradas et al.66, the aqueous formulation and steric repulsion between the surfactant chains form a thin layer of water on the surface of the system. As essential oils are poorly soluble in water, their evaporation is delayed when essential oils are formulated into nanoemulsions.
The potential protective effect of essential oils becomes more evident when two peaks are discerned in the P. dilatatum DTG nanoemulsion at 360 and 414 °C, implying the presence of two different decomposing substances (Fig. 2B). In contrast, the P. amalago nanoemulsion showed a significant shift (6 °C) in its DTG peak compared to Tween 80, indicating the presence of an additional substance (e.g., the essential oil itself), which interacts with o Tween 80, modifying its thermal stability.
Comparing the thermal stability of both essential oils protected by nanoemulsions, we noticed a consistent loss of mass for the P. dilatatum nanoemulsion from 200 °C, while the P. amalago nanoemulsion remained relatively stable until reaching its maximum decomposition at 386 °C. This result suggests the presence of P. dilatatum essential oil in its free form, since Tween 80 remains stable in the temperature range of 200–300 °C. The presence of free P. dilatatum essential oil may result in potent herbicidal activity in the short term, but this activity may be reduced in the long term due to its limited chemical stability. The development of nanoemulsions containing the essential oils of P. amalago and P. dilatatum has the potential to improve their thermal stability, allowing for a wider range of applications and an extended shelf life. However, confirmation of these stability improvements requires additional bioactivity testing after subjecting the nanoemulsions to temperatures that exceed their decomposition limits (168 and 187 °C). This validation will ensure the effectiveness preservation of the herbicide.
Phytotoxic activity on germination and initial development
The P. amalago nanoemulsion reduced the germination and root length of B. pilosa by 46.29% and 36.06% at a concentration of 3000 µg mL-1 in relation to the negative control. This same concentration also reduced the shoot length of B. pilosa by 33.89% in relation to the negative control, with an inhibitory effect similar to the herbicide glyphosate. In L. multiflorum, the concentration of 3000 µg mL-1 of P. amalago nanoemulsion reduced germination by 52.72% in relation to the negative control. Concentrations of 187.5, 1500 and 3000 µg mL-1 negatively affected root length, with the two highest concentrations causing the most pronounced reductions in relation to the negative control, 53.07% and 46.49%, respectively. These concentrations also significantly reduced shoot length by 48.88% and 44.07% when compared to the negative control. The effect of concentrations 1500 and 3000 µg mL-1 on the initial development (length of roots and shoots) of L. multiflorum was the same as that of glyphosate (Fig. 3A).
Effect of nanoemulsions containing (A) P. amalago essential oil (NE_Amalago) and (B) P. dilatatum (OE_Dilatatum) on germination, root length and shoot length of B. pilosa and L. multiflorum. Bars (mean ± SD; n = 5) followed by the same letter within each species do not differ from each other using the Tukey test (p > 0.05). NC, negative control [Tween 80 (4.2%, v/v)]; GLY, glyphosate.
The concentration of 3000 µg mL-1 of the P. dilatatum nanoemulsion affected the root length of L. multiflorum in a similar way to glyphosate, causing a reduction of 53.50% in relation to the negative control. This same concentration also caused a reduction of 30.73% in the roots length and 29.23% in the length of the shoots of B. pilosa in relation to the negative control, the latter reduction being statistically equal to that caused by glyphosate (Fig. 3B).
Nanoemulsions of P. amalago and P. dilatatum showed a herbicide effect on seed germination and seedling growth of B. pilosa and L. multiflorum, with the concentration of 3000 µg mL-1 being responsible for the greatest inhibitory potential. A previous study showed that P. dilatatum essential oil had strong herbicidal potential on root and aerial growth of L. perenne and L. sativa.13 Our results indicate similarity in the phytotoxic effect of the nanoemulsion with P. dilatatum essential oil on the root and shoot growth of weeds. Additionality, a study carried out by our research group with essential oil from P. amalago leaves described P. amalago essential oil as an inhibitor of root and aerial growth of B. pilosa with activity similar to glyphosate at a concentration of 1500 µg mL-116. These same effects were observed for the P. amalago essential oil nanoemulsion in the present study. Thus, we can suggest that there was a similarity in the effect of the nanoemulsion with P. amalago essential oil on initial growth.
In the present study, it was observed that the essential oils of P. amalago and P. dilatatum were composed of sesquiterpenes and monoterpenes. These compounds are known to be involved in allelopathic interactions and inhibit seed germination and plant growth67,68. The compounds β-elemene, germacrene A and linalool, main compounds found in the essential oil of P. amalago, were associated with the inhibitory effects of P. amalago on the germination and initial growth of B. pilosa in a previous study16. Other studies have also associated the phytotoxic activity of essential oils with these compounds69,70,71. Among these, linalool stands out as the main compound that may have led to the phytotoxic effects of the P. amalago nanoemulsion. Linalool is recognized for its herbicidal activity, being reported to inhibit root elongation, germination, root hair formation, and metaxylem development of weeds71. Oxygenated monoterpenes, as linalool is classified, can cause the rupture of mitochondrial membranes, leading to an imbalance in cellular respiration and oxidative damage72.
About the P. dilatatum nanoemulsion, the main compound of the essential oil, germacrene D, may also have contributed to the observed effects, since previous studies have associated this compound with reduced germination, growth of both roots and shoot of plants73,74,75. Other compounds found mainly in the essential oils of P. amalago and P. dilatatum, such as β-pinene, β-caryophyllene and α-farnesene, may also have contributed to the phytotoxic activity of the nanoemulsions. The sesquiterpene α-farnesene induces the accumulation of reactive oxygen species (ROS), alters auxin transport and causes malformations in microtubules, affecting cell division in the root meristem leading to an abnormal shape76,77. The monoterpene β-pinene, for example, induces anatomical and physiological changes in seeds, in addition to affecting DNA synthesis78. β-caryophyllene exerts allelopathic activity that impacts the germination and growth of several weeds, including B. pilosa41.
The nanoemulsions of P. amalago and P. dilatatum caused a reduction in the germination of the species studied, a reduction that was not observed in studies with the essential oil of these species. This result can be attributed to the reduced size of the droplets, which facilitate penetration into plant tissues79. As the droplets shrink, the biological activity of the encapsulated lipophilic compounds increases, due to facilitated transport across biological membranes and the greater surface-to-volume ratio80. Thus, bioactive compounds can be absorbed on the surface of seeds, roots, stems and leaves, or penetrate the cuticle and epidermis, reaching the vascular tissues and being transported to other parts of the plant63. Overcoming limiting factors such as evaporation and oxidation of essential oil components by nanoemulsions may also have increased the allelochemical potential in germination21,81. Improved efficacy of nanoemulsions compared to essential oils has been reported in other studies18. Its formulation improves the properties of the active substances of essential oils, allowing their controlled release and rapid interaction with plant cells after application to seeds or leaf surfaces18,21. Thus, the synergism between the major and minor components of essential oils, the smaller droplet size and the reduction in evaporation and oxidation may have contributed to the inhibitory effects of P. amalago and P. dilatatum nanoemulsions on B. pilosa and L. multiflorum.
The ideal scenario is that P. amalago and P. dilatatum nanoemulsions act selectively against weeds, without negatively affecting crop species that coexist in the same environment. Other nanoemulsions, such as those derived from Foeniculum vulgare Mill and Schinus terebinthifolia Raddi essential oils demonstrated herbicidal activity against weeds without harming the crop to be protected (non-target plant)18,82. In this context, given the bioherbicidal potential of P. amalago and P. dilatatum, it is essential to assess their effects on non-target species to ensure their safe and effective use in agricultural settings. This would allow the establishment of concentrations that are both selective—effective against weeds—and safe for crop plants.
Phytotoxic activity on weeds in a greenhouse
Based on the responses from the pre-emergence experiment, the two concentrations considered most effective in the initial growth of B. pilosa and L. multiflorum, 1500 and 3000 µg mL-1, were evaluated for post-emergence herbicidal activity (Fig. 4). A differential effect of the P. amalago nanoemulsion was observed, affecting the growth of only B. pilosa. Concentrations 1500 and 3000 µg mL-1 reduced root length by 5.5% and 11.0%, respectively, concerning the negative control. The 3000 µg mL-1 concentration also caused a 3.4% reduction in area length (Fig. 4A). The P. dilatatum nanoemulsion did not affect the growth of any of the weeds studied (Fig. 4B).
Post-emergence phytotoxic effect of nanoemulsions containing (A) P. amalago essential oil (NE_Amalago) and (B) P. dilatatum (OE_Dilatatum) on root and shoot length of B. pilosa and L. multiflorum. Bars (mean ± SD; n = 3) followed by the same letter within each species do not differ from each other using the Tukey test (p > 0.05). NC, negative control [Tween 80 (4.2%, v/v)]; GLY, glyphosate.
Piper amalago and P. dilatatum nanoemulsions did not affect the growth of L. multiflorum. This species was identified for its high risk of developing herbicide resistance in wheat and barley fields in New Zealand31. Recently, Buddenhagen et al.32 identified L. multiflorum as one of the weeds that survived the application of different post-emergent herbicides with different modes of action, before the harvest period on 38 farms visited. In this study, the authors confirmed the resistance of species of the genus Lolium to herbicides that inhibit acetolactate synthase (ALS), acetyl-CoA carboxylase (ACCase) and glyphosate. Therefore, the non-susceptibility of L. multiflorum to P. amalago and P. dilatatum nanoemulsions is understandable due to the difficult control and resistance to post-emergent herbicides already documented for the species.
The greater post-emergence herbicidal activity of the P. amalago nanoemulsion when compared to the P. dilatatum nanoemulsion may be associated with the free P. dilatatum essential oil content found in the DTG analysis. The long-term phytotoxic activity of the P. dilatatum nanoemulsion may have been reduced due to the high volatility and low physical stability of the free essential oil to factors such as temperature, humidity, light and oxygen in the environment83. Thus, the balance between free essential oil and essential oil content in nanoemulsions can contribute to sustained herbicidal control, combining the initial herbicidal action with the prolonged efficacy provided by the physicochemical stability of nanoemulsions.
Considering the visual effects of toxicity on weeds, the induction of leaf burn, chlorosis, wilting and necrosis by the P. amalago nanoemulsion and P. dilatatum was not observed (Fig. 5A,B). The absence of these rapid damages suggests that the nanoemulsions does not act as a contact herbicide, but rather as a systemic herbicide. In other words, its molecules translocate from deposition sites and are distributed throughout the plant84.
Although the phytotoxic effect of P. amalago nanoemulsion on the growth of B. pilosa was observed, its inhibitory activity was low. The performance of herbicides largely depends on their absorption by weeds. Unlike pre-emergent herbicides that can be absorbed by seeds, roots, hypocotyls, cotyledons, coleoptiles or leaves, post-emergent herbicides enter plants primarily through the leaves. The presence of waxes and very long-chain fatty acid derivatives in the leaf cuticle can offer a barrier to systemic post-emergent herbicides, hindering their absorption. Factors such as leaf shape and length, trichome density, presence of stomata and leaf microclimate can also influence the absorption of these herbicides84.
Furthermore, lipophilic and small-sized molecules favor absorption, while greater solubility in water favors translocation within the plant to the site of action/molecular target of the herbicide. These last characteristics were achieved for P. amalago essential oil through the nanoemulsion formulation. In this sense, the greater herbicidal efficacy of the P. amalago nanoemulsion can be achieved through the optimization of physicochemical parameters seeking to favor the penetration and translocation of the essential oil in the target plant. A single post-emergence herbicide application generally does not control weeds efficiently, multiple applications and sequential pre- and post-emergence treatments are recommended for managing these plants84. Thus, the sequential application of P. amalago nanoemulsion during pre- and post-emergence of B. pilosa may be an alternative to increase its herbicidal activity.
Quantification of photosynthetic pigments
The content of photosynthetic pigments in the target plants was influenced only by the P. amalago nanoemulsion (Fig. 6A,B). Treatment with a concentration of 3000 µg mL-1 resulted in a significant decrease of 24.5% in chlorophyll a, 18.1% in chlorophyll b, and 33.9% in carotenoids in L. multiflorum compared to the negative control (Fig. 6A). Chlorophylls are essential pigments for electron capture in photosystem II, while carotenoids act as antioxidants against photooxidative damage85,86. Thus, these pigments play a crucial role in light energy absorption during photosynthesis82. Changes in the biosynthesis and content of these pigments can affect chemical energy production and allow the escape of free radicals produced during photosynthesis, compromising plant growth and development8. Therefore, the lower levels of photosynthetic pigments found in L. multiflorum seedlings treated with the P. amalago nanoemulsion support the observed reduction in L. multiflorum root growth exposed to the same treatment in early development assays.
Chlorophyll a, b and carotenoid content in B. pilosa and L. multiflorum dry seedlings treated with nanoemulsions containing (A) P. amalago (NE_Amalago) and (B) P. dilatatum essential oil (NE_Dilatatum). Bars (mean ± SD; n = 6) followed by the same letter within each species do not differ from each other using the Tukey test (p > 0.05). NC, negative control [Tween 80 (4.2%, v/v)].
The decline in photosynthetic pigments observed in the present study is consistent with the results of Fernandes et al.82 and Somala et al.8 These authors reported a reduction in the content of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids in L. sativa plants and the weeds Urochloa brizantha, Echinochloa crus-galli, and Amaranthus tricolor treated with nanoemulsions of essential oils from Schinus terebinthifolia Raddi and Cymbopogon nardus L. Rys et al.87 also demonstrated that a nanoemulsion containing Carum carvi L. essential oil can disrupt the electron transport chain and affect the photosynthetic efficiency of E. crus-galli by measuring the rapid kinetics of chlorophyll a fluorescence.
In B. pilosa, unlike in L. multiflorum, the 3000 µg mL-1 concentration of the P. amalago nanoemulsion caused an 18.9% increase in chlorophyll content compared to the negative control (Fig. 6A). This increase may be due to the action of the P. amalago nanoemulsion on chlorophyll metabolism regulation. Chlorophylls and their derivatives are highly photosensitizing molecules, meaning that when present in excess, they can generate ROS, which in turn cause growth retardation or plant death88. In this sense, the increase in chlorophyll a level and the consequent generation of ROS may have led to the reduction in the growth of roots and shoots of B. pilosa observed in the pre-and post-emergence tests.
Although the exact mechanism by which essential oils affect the rate of photosynthetic pigments is not fully understood, it is suggested that the molecules of these pigments may react with ROS in plant tissue produced in response to essential oils85. In this context, the imbalance in chlorophyll and carotenoid content observed in the weeds tested in this study may be attributed to the monoterpenes present in P. amalago essential oil, and the greater quantity compared to the nanoemulsion of P. dilatatum. These compounds can disrupt the photosynthetic machinery, consequently facilitating ROS production and inducing oxidative stress89. The sesquiterpene β-caryophyllene present only in P. amalago essential oil may also have contributed to the reduction of photosynthetic pigments. Studies analyzing chlorophyll a fluorescence in Physalis ixocarpa plants exposed to this compound showed inhibition of photosystem II by transforming active reaction centers into “heat sinks” and also inducing chlorosis in leaves90.
Cytogenotoxic activity
Cell cycle analysis can provide information about the mode of action of the tested phytotoxic substance, since plant growth is closely related to the increase in the number of dividing cells. Thus, changes in the growth of roots and shoots are a reflection of the action of the compound in mitotic division. Therefore, nanoemulsions of P. amalago and P. dilatatum were analyzed for their cytotoxic, genotoxic and mutagenic potential in meristematic cells of L. sativa roots (Table 3 and Table 4). This bioassay is widely used in cytogenotoxicity studies due to its sensitivity, large and well-defined chromosomes and rapid cell proliferation42,74,91.
The P. amalago essential oil nanoemulsion reduced the mitotic index of L. sativa in the two concentrations tested. The mitotic index is a parameter of cytotoxicity and its decrease indicates the inability of cells to enter prophase or a slowdown in the rate of cell proliferation92. In the present study, P. amalago nanoemulsion increased the frequency of interphase cells and decreased the frequency of prophase cells compared to the negative control (Table 3). This result indicates a prolonged S phase, which may have occurred due to the blockade of G1 and G2 phases by inhibition of DNA synthesis93,94,95. The decrease in IM caused by the P. amalago nanoemulsion may be related to the monoterpenes linalool and α-pinene present in its essential oil. Monoterpenes inhibit DNA synthesis, preventing cells from entering the mitotic phase, and at higher concentrations can completely inhibit MI due to apoptosis and/or cell death78,94.
Although no decrease in the mitotic index of cells exposed to the P. dilatatum nanoemulsion was observed, the nanoemulsion also decreased the frequency of cells in prophase, suggesting the prolongation of the S phase (Table 4). The sesquiterpenes β-caryophyllene and β-elemene present in both nanoemulsions may also be related to the decrease in IM, as they have already been reported for their cytotoxic effects against cancer cell lines93,96. Studies with β-elemene have shown that it is capable of arresting the cell cycle in the G0/G1 phase, leading to the phosphorylation of p38 of MAPK causing the death of glioblastoma cells through an apoptotic trigger97. In this sense, it is possible that these compounds may be interfering with the cell division of L. sativa, preventing the cells from entering mitosis or even leading them to apoptosis or cell death. Thus, the pre-emergent inhibitory effects of the P. amalago and P. dilatatum nanoemulsion on the initial growth of B. pilosa and L. multiflorum may have occurred due to interference in the cell division of the tested plants, through the reduction of cell transition for mitosis.
The presence of micronuclei in L. sativa meristematic cells was used to detect the mutagenic effect of nanoemulsions. Micronucleus is an inherited mutation from incorrectly repaired or unrepaired DNA damage in parental cells98. The concentration of 3000 µg mL-1 of the P. dilatatum nanoemulsion induced an increase in this change in relation to the negative control, demonstrating the mutagenic potential of the nanoemulsion. Chromosomal alterations were considered as a genotoxicity parameter. Both nanoemulsions increased the frequency of these changes in relation to the negative control, indicating their genotoxic potential (Tables 3 and 4).
The mechanism of action of genotoxic agents can be classified as clastogenic and aneugenic. Clastogenicity is characterized by direct damage to DNA, such as chromosomal breaks. Aneugenicity is caused by changes in cellular components, such as malformation of the mitotic spindle91. In the present study, different chromosomal changes were induced and observed (Figs. 7 and 8), such as adhesion (Fig. 8A), chromosomal loss (Fig. 8B), delays in anaphase (Fig. 8C), c-metaphase (Fig. 8D) and micronuclei (Fig. 8E,F). Nanoemulsions of P. amalago and P. dilatatum induced an increase in cells in c-metaphase (Fig. 7A,B). C-metaphases are aneugenic changes that occur when chromosomal centromeres are not fixed to the mitotic spindle, due to their malfunction or inactivation, paralyzing the cell cycle at metaphase82. These results explain the increase in the frequency of metaphases caused by nanoemulsions (Table 3 and Table 4).
Frequency of chromosomal anomalies observed in meristematic cells of Lactuca sativa roots exposed to nanoemulsions of (A) P. amalago (NE_Amalago) and (B) P. dilatatum (NE_dilatatum). Bars (mean ± SD; n = 5) followed by the same letter within each species do not differ from each other using the Tukey test (p > 0.05). NC, negative control [Tween 80 (4.2%, v/v)]. Adherence: metaphase with adherence. Delay: anaphase delay. Loss: metaphase with chromosome loss.
Chromosomal abnormalities observed in meristematic cells of L. sativa exposed to nanoemulsions containing essential oils from P. amalago and P. dilatatum. (A) metaphase with chromosome adhesion, (B) metaphase with chromosome loss, (C) anaphase delay, (D) c-metaphase, (E) c-methaphase and micronuclei, (F) micronuclei. Scale bar = 20 μm.
The concentration of 1500 µg mL-1 of the P. dilatatum nanoemulsion also increased the frequency of chromosomes lost in metaphase (Fig. 7B), highlighting the aneugenic mechanism of this nanoemulsion99. This result may be associated with the induction of micronuclei observed in cells exposed to P. dilatatum nanoemulsion, since micronuclei are formed by fragments or entire chromosomes that were lost during mitosis100.
The P. amalago nanoemulsion also led to increased chromosome adhesion at a concentration of 1500 µg mL-1 (Fig. 7A). Chromosomal adhesion was observed at the metaphase stage. This aneugenic change is caused by degradation and depolymerization of chromosomal DNA, improper folding of chromosomal fibers and removal of the protein covering the DNA. This viscosity is irreversible and can cause cell death and consequently a decline in cell division99. The cytotoxic, genotoxic and mutagenic effects found in L. sativa may be associated with the phytotoxic activity of P. amalago and P. dilatatum nanoemulsions on B. pilosa and L. multiflorum. The decrease in cell cycle progression and consequently in the number of differentiating meristematic cells may have impaired the initial development of these plants, causing a reduction in the growth of roots and shoots.
In conclusion, we were successful in producing nanoemulsions containing the essential oils of P. amalago and P. dilatatum, evidenced by the size of the nanoscale droplets, low PDI, in addition to the improvement in aqueous solubility and the increase in the maximum decomposition temperature of the essential oils. Regarding herbicidal activity, nanoemulsions had a significant impact on the germination and initial development of B. pilosa and L. multiflorum, presenting promising potential for controlling these weeds in pre-emergence, possibly due to the absorption of phytotoxic compounds such as β-elemene germacrene A, linalool, β-caryophyllene and germacrene D by plant tissues due to the reduced droplet size of nanoemulsions. The P. amalago essential oil nanoemulsion can also be considered promising as a post-emergence herbicide for B. pilosa. Furthermore, the P. amalago nanoemulsion affected the content of photosynthetic pigments, it is a possible inducer of oxidative stress. The cytogenotoxicity tests demonstrated that the genotoxic and mutagenic cytotoxic effects observed in L. sativa resulting from the aneugenic and clastogenic mode of action of nanoemulsions may have occurred in B. pilosa and L. multiflorum, affecting the progression of the cell cycle and leading to reduction of the initial growth of these plants. This strategy of forming nanoemulsions through the ultrasonic homogenization method with the surfactant Tween 80 has the potential to improve sustainable and effective agricultural approaches with natural bioactive compounds. Future studies could evaluate the effectiveness of nanoemulsions of P. amalago and P. dilatatum essential oils in field contexts and investigate their impacts on non-target species.
Despite these promising results, it is important to recognize that results obtained under controlled (laboratory) and semi-controlled (greenhouse) conditions may not fully represent the complexities of field environments. Variables such as soil composition, climate, microbial interactions, and application methods can significantly influence the efficacy and environmental impact of nanoemulsions. Therefore, field trials are essential to validate the herbicidal potential, assess long-term effects, and ensure the safety of these formulations for non-target organisms and ecosystems. Additionally, to enable the industrial application of essential oil-based Piper nanoemulsions as herbicides, it is essential to ensure large-scale production of plant material with high essential oil content. Strategies aimed at increasing biomass and oil yield, such as the selection and propagation of superior genotypes through genetic improvement, in vitro germination, tissue culture, and micropropagation, can be aligned to support this goal.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Qu, R. et al. Where are the new herbicides?. Pest Manag. Sci. 77(6), 2620–2625 (2021).
Motta, E. V. S. & Moran, N. A. The effects of glyphosate, pure or in herbicide formulation, on bumble bees and their gut microbial communities. Sci. Total Environ. 872, 162102–162102 (2023).
Dayan, F. E. & Duke, S. O. Natural compounds as next-generation herbicides. Plant Physiol. 166(3), 1090–1105 (2014).
Katila, P. et al. Sustainable Development Goals (Cambridge University Press, 2019).
Balah, M. A. & AbdelRazek, G. M. Pesticidal activity of Solanum elaeagnifolium Cav. leaves against nematodes and perennial weeds. Acta Ecol. Sin. 40(5), 373–379 (2020).
Maes, C. et al. Cinnamomum zeylanicum essential oil formulation with poly (propylene imine) dendrimers with surface-grafted glycerol: Release kinetics of trans-cinnamaldehyde and germination inhibition effects. J. Agric. Food Chem. 70(16), 5177–5185 (2022).
Li, J. et al. Artemisia argyi essential oil exerts herbicidal activity by inhibiting photosynthesis and causing oxidative damage. Ind. Crop. Prod. 194, 116258–116258 (2023).
Somala, N., Laosinwattana, C., Chotsaeng, N. & Teerarak, M. Citronella essential oil-based nanoemulsion as a post-emergence natural herbicide. Sci. Rep. 13, 20851 (2023).
Aghbash, B. N. et al. Chemical composition, antibacterial and radical scavenging activity of essential oils from Satureja cacrantha C.A. Mey. at different growth stages. Foods 9(4), 494 (2020).
Pavela, R. & Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 21(12), 1000–1007 (2016).
Asmarayani, R. Phylogenetic relationships in Malesian-pacific Piper (Piperaceae) and their implications for systematics. Taxon 67(4), 693–724 (2018).
Andriana, Y., Xuan, T. D., Quy, T. N., Tran, H. D. & Le, Q. T. Biological activities and chemical constituents of essential oils from Piper cubeba Bojer and Piper nigrum L. Molecules 24(10), 1876 (2019).
Salehi, B. et al. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 24(7), 1364 (2019).
Jaramillo-Colorado, B. E., Pino-Benitez, N. & González-Coloma, A. Volatile composition and biocidal (antifeedant and phytotoxic) activity of the essential oils of four Piperaceae species from choco-colombia. Ind. Crop. Prod. 138, 111463–111463 (2019).
Cavalcante, D. N., Corrêa, R. F., Campelo, P. H., Sanches, E. A. & Bezerra, J. A. Essential oils from unconventional food plants (Murraya spp., Ocimum spp., Piper spp.) as alternative food flavorings. Food Chem. Adv. 3, 100481–100481 (2023).
Vasconcelos, L. C. et al. Influence of seasonal variation on the chemical composition of Piper amalago essential oils and their phytocytogenotoxic activity in model plants and weeds. Bragantia 83, e20240090 (2024).
Pavela, R. et al. Microemulsions for delivery of apiaceae essential oils—towards highly effective and eco-friendly mosquito larvicides?. Ind. Crop. Prod. 129, 631–640 (2019).
Kaur, P. et al. Nanoemulsion of Foeniculum vulgare essential oil: A propitious striver against weeds of Triticum aestivum. Ind. Crops Prod. 168, 113601 (2021).
Kumar, A., Kanwar, R. & Mehta, S. K. Recent development in essential oil-based nanocarriers for eco-friendly and sustainable agri-food applications: A review. ACS Agric. Sci. Technol. 2(5), 823–837 (2022).
Pavoni, L. et al. Microemulsions: An effective encapsulation tool to enhance the antimicrobial activity of selected EOs. Int. J. Drug Deliv. Technol. 53, 101101 (2019).
Somala, N., Laosinwattana, C. & Teerarak, M. Formulation process, physical stability and herbicidal activities of Cymbopogon nardus essential oil-based nanoemulsion. Sci. Rep. 12(1), 10280 (2022).
Fernandez, C. et al. Allelochemicals of Pinus Halepensis as drivers of biodiversity in mediterranean open mosaic habitats during the colonization stage of secondary succession. J. Chem. Ecol. 39, 298–311 (2013).
Komaiko, J. S. & McClements, D. J. Formation of food-grade nanoemulsions using low-energy preparation methods: A review of available methods. Compr. Rev. Food Sci. Food Saf. 15(2), 331–352 (2016).
Gupta, A., Narsimhan, V., Hatton, T. A. & Doyle, P. S. Kinetics of the change in droplet size during nanoemulsion formation. Langmuir 32(44), 11551–11559 (2016).
Kala, S. et al. Analogous foliar uptake and leaf-to-root translocation of micelle nanoparticles in two dicot plants of diverse families. NanoImpact 28, 100431 (2022).
Erfanifar, Z., Majdinasab, M. & Shaghaghian, S. Production and characterization of sage seed gum bioactive film containing Zataria multiflora essential oil nanoemulsion. Food Chem. 408, 134871 (2023).
Çiçek, S., Korkmaz, Y. B. & Işik, S. A New economical route for the grapefruit peel essential oil: Nanoemulsion form and insecticidal activity. S. Afr. J. Bot. 169, 56–65 (2024).
Lou, S., Ni, X., Xiao, W., Li, Y. & Gao, Z. Physical stability, microstructure and antimicrobial properties of Konjac Glucomannan coatings enriched with Litsea cubeba essential oil nanoemulsion and its effect on citruses preservation. Int. J. Biol. Macromol. 256, 128306 (2024).
Romano, C. A., Oliveira Neto, J. R., Cunha, L. C., Santos, A. H. & Paula, J. R. Essential oil-based nanoemulsion of Murraya koenigii is an efficient larvicidal against Aedes aegypti under field conditions. Ind. Crop. Prod. 208, 117836 (2024).
Galinato, M. I., Moody, K., Piggin, C. M. Upland Rice Weeds of South and Southeast Asia; International Rice Research Institute: Makati Cty, 156 (Philippines, 1999).
Ngow, Z., Chynoweth, R. J., Gunnarsson, M., Rolston, P. & Buddenhagen, C. E. A herbicide resistance risk assessment for weeds in wheat and barley crops in New Zealand. PLoS ONE 15(6), e0234771 (2020).
Buddenhagen, C. E. et al. Resistance to post-emergent herbicides is becoming common for grass weeds on New Zealand wheat and barley farms. PLoS ONE 16(10), e0258685–e0258685 (2021).
Thiers, B. Index Herbariorum: A global directory of public herbaria and associated staff. (New York Botanical Garden’s Virtual Herbarium, New York, 2024). Available at: http://sweetgum.nybg.org/ih/. Accessed on: Dec. 18, 2022.
Pinheiro, P. F. et al. Phytotoxicity and cytotoxicity of essential oil from leaves of Plectranthus amboinicus, carvacrol, and thymol in plant bioassays. J. Agric. Food Chem. 63(41), 8981–8990 (2015).
Mendes, L. A. et al. Larvicidal effect of essential oils from Brazilian cultivars of guava on Aedes aegypti L. Ind. Crops Prod. 108, 684–689 (2017).
Souza, T. S. et al. Essential oil of Psidium guajava: Influence of genotypes and environment. Sci. Hortic. 216, 38–44 (2017).
Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry 5th edn. (Texensis Publishing, 2017).
El-Sayed, A. M. The Pherobase: Database of Pheromones and Semiochemicals; (2014). http://www.pherobase.com.
National Institute of Standards and Technology. NIST Chemistry WebBook—SRD 69. Nist.gov. https://webbook.nist.gov/chemistry/.
Azevedo, M. M. B. et al. Croton cajucara essential oil nanoemulsion and its antifungal activities. Processes 9(11), 1872 (2021).
Mendes, L. A. et al. Herbicide and cytogenotoxic activity of inclusion complexes of Psidium gaudichaudianum leaf essential oil and β-caryophyllene on 2-hydroxypropyl-β-cyclodextrin. Molecules 28(15), 5909–5909 (2023).
Vasconcelos, L. C. et al. Phytochemical analysis and effect of the essential oil of Psidium L. species on the initial development and mitotic activity of plants. Environ. Sci. Pollut. Res. 26(25), 26216–26228 (2019).
Kumar, A., Kanwar, R. & Mehta, S. K. Development of phosphatidylcholine/tween 80 based biocompatible clove oil-in-water nanoemulsion as a green nanocarrier for controlled herbicide delivery. Environ Pollut 293, 118558 (2022).
Agnello, A. C., Huguenot, D., van Hullebusch, E. D. & Esposito, G. Phytotoxicity of citric acid and Tween® 80 for potential use as soil amendments in enhanced phytoremediation. Int. J. Phytoremediation 17(7), 669–677 (2015).
Grippa, G. A., Morozesk, M., Nati, N. & Matsumoto, S. T. Estudo genotóxico do surfactante tween 80 em Allium cepa. Rev. Bras. Toxicol. 23, 11–16 (2010).
Singh, N., Singh, H. P., Batish, D. R., Kohli, R. K. & Yadav, S. S. Chemical characterization, phytotoxic, and cytotoxic activities of essential oil of Mentha longifolia. Environ. Sci. Pollut. Res. 27(12), 13512–13523 (2020).
Lichtenthaler, H. K. & Wellburn, A. R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 11(5), 591–592 (1983).
Santos, T., Deroldo, R. & Marin-Morales, M. A. Phyto-Genotoxicity assessment of different associations between sludges from water and sewage treatment plants, before and after the bioremediation process. Environ. Sci. Pollut. Res. 29(26), 40029–40040 (2022).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. https://www.r-project.org/.
Santos, A. L. et al. Chemical characterisation of Piper amalago (Piperaceae) essential oil by comprehensive two-dimensional gas chromatography coupled with rapid-scanning quadrupole mass spectrometry (GC×GC/QMS) and their antilithiasic activity and acute toxicity. Phytochem. Anal. 29(5), 432–445 (2018).
Monteiro, L. M. et al. The cardiorenal effects of Piper amalago are mediated by the nitric oxide/cyclic guanosine monophosphate pathway and the voltage-dependent potassium channels. Pharmaceuticals 16(11), 1630–1630 (2023).
Silva, J. A., Oliveira, F. F., Guedes, E. S., Bittencourt, M. A. L. & Oliveira, R. A. Atividade antioxidante de Piper arboreum, Piper dilatatum E Piper divaricatum. Rev. Bras. Pl. Med. 16(3), 700–706 (2014).
Andrade, E. H., Alves, C. N., Guimarães, E. F., Carreira, M. M. & Maia, J. G. S. Variability in essential oil composition of Piper dilatatum L.C. rich. Biochem. Syst. Ecol. 39(4–6), 669–675 (2011).
de Lima Moreira, D. & Pereira, R. A. Chemodiversity of essential oils in Piper L. (Piperaceae) species from the Restinga of Marambaia Island, Rio de Janeiro-RJ, Brazil. Rev. Virtual Quím. 13(5), 1203–1215 (2021).
Perigo, C. V. et al. The chemical composition and antibacterial activity of eleven Piper species from distinct rainforest areas in southeastern Brazil. Ind. Crop. Prod. 94, 528–539 (2016).
Jaramillo-Colorado, B. E., Duarte-Restrepo, E. & Pino-Benítez, N. Evaluación de la actividad repelente de aceites esenciales de plantas piperáceas del departamento de chocó. Colombia. Rev. Toxicol. 32(2), 112–116 (2015).
Cysne, J. B., Canuto, K. M., Pessoa, O. D. L., Nunes, E. P. & Silveira, E. R. Leaf essential oils of four Piper species from the state of ceará—northeast of Brazil. J. Braz. Chem. Soc. 16(6b), 1378–1381 (2005).
Aswathanarayan, J. B. & Vittal, R. R. Nanoemulsions and their potential applications in food industry. Front. Sustain. Food Syst. 3, 1–21 (2019).
Ghosh, V., Mukherjee, A. & Chandrasekaran, N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochem. 20(1), 338–344 (2013).
Baboota, S., Shakeel, F., Ahuja, A., Ali, J. & Shafiq, S. Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 57(3), 315–332 (2007).
Palermo, D., Giunti, G., Laudani, F., Palmeri, V. & Campolo, O. Essential Oil-based nano-biopesticides: Formulation and bioactivity against the confused flour beetle Tribolium confusum. Sustainability 13(17), 9746 (2021).
Jaiswal, M., Dudhe, R. & Sharma, P. K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 5(2), 123–127 (2014).
Pan, X. et al. Covalent interaction between rice protein hydrolysates and chlorogenic acid: Improving the stability of oil-in-water emulsions. J. Agric. Food Chem. 67(14), 4023–4030 (2019).
Heurtault, B. Physico-chemical stability of colloidal lipid particles. Biomaterials 24(23), 4283–4300 (2003).
Mayer, S., Weiss, J. & McClements, D. J. Behavior of vitamin E acetate delivery systems under simulated gastrointestinal conditions: Lipid digestion and bioaccessibility of low-energy nanoemulsions. J. Colloid Interface Sci. 404, 215–222 (2013).
Barradas, T. N. et al. Development, characterization and evidence of anti-endometriotic activity of phytocannabinoid-rich nanoemulsions. Int. J. Pharm. 643, 123049 (2023).
Macías, F. A., Mejías, F. J. & Molinillo, J. M. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 75(9), 2413–2436 (2019).
Verdeguer, M., Sánchez-Moreiras, A. M. & Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 9(11), 1571 (2020).
Veryer, K. & Bozok, F. Chemical constituents of Dysphania botrys (L.) mosyakin and clemants essential oil: Herbicidal and antimicrobial activities. J. Essent. Oil-Bear. Plants 26(2), 411–419 (2023).
Dutra, Q. P. et al. Phytocytotoxicity of volatile constituents of essential oils from Sparattanthelium Mart. species (Hernandiaceae). Sci. Rep. 10(1), 12213 (2020).
Jiang, C. et al. Evaluation of the phytotoxic effect of the essential oil from Artemisia absinthium. Ecotoxicol. Environ. Saf. 226, 112856 (2021).
Martino, L. D., Mancini, E., de Almeida, L. F. R. & Feo, V. D. The antigerminative activity of twenty-seven monoterpenes. Molecules 15(9), 6630–6637 (2010).
Jiang, C. et al. Evaluation of the phytotoxic effect of the essential oil from Artemisia absinthium. Ecotoxicol. Environ. Saf. 226, 112856 (2021).
Vasconcelos, L. C. et al. Phytochemical screening and phytocytotoxic effects of the tropical Myrcia vittoriana (Myrtaceae). An. Acad. Bras. Cienc. 94, e20210820 (2022).
Dutra, Q. P. et al. Phytocytotoxicity of volatile constituents of essential oils from Sparattanthelium Mart. species (Hernandiaceae). Sci. Rep. 10, 1–11 (2020).
Araniti, F. et al. Loss of gravitropism in farnesene-treated arabidopsis is due to microtubule malformations related to hormonal and ROS unbalance. PLoS ONE 11(8), e0160202 (2016).
Araniti, F. et al. The allelochemical farnesene affects arabidopsis thaliana root meristem altering auxin distribution. Plant Physiol. Biochem. 121, 14–20 (2017).
Nishida, N., Tamotsu, S., Nagata, N., Saito, C. & Sakai, A. Allelopathic effects of volatile monoterpenoids produced by salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 31(5), 1187–1203 (2005).
Hazafa, A. et al. Nano-Biopesticides as an Emerging Technology for Pest Management (IntechOpen, 2021).
Salvia-Trujillo, L., Rojas-Graü, A., Soliva-Fortuny, R. & Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 43, 547–556 (2015).
Hazrati, H., Saharkhiz, M. J., Niakousari, M. & Moein, M. Natural herbicide activity of Satureja hortensis L. essential oil nanoemulsion on the seed germination and morphophysiological features of two important weed species. Ecotoxicol. Environ. Saf. 142, 423–430 (2017).
Fernandes, S. Y. et al. Pre-emergent bioherbicide potential of Schinus terebinthifolia Raddi essential oil nanoemulsion for Urochloa brizantha. Biocatal. Agric. Biotechnol. 47, 102598 (2023).
Turek, C. & Stintzing, F. C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 12(1), 40–53 (2013).
Krähmer, H. et al. What makes a molecule a pre- or a post-herbicide—How valuable are physicochemical parameters for their design?. Pest Manag. Sci. 77(11), 4863–4873 (2021).
Ootani, M. A. et al. Phytotoxic effects of essential oils in controlling weed species Digitaria horizontalis and Cenchrus echinatus. Biocatal. Agric. Biotechnol. 12, 59–65 (2017).
Dumanović, J., Nepovimova, E., Natić, M., Kuča, K. & Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 11, 552969 (2021).
Rys, M. et al. The effect of caraway oil-loaded bio-nanoemulsions on the growth and performance of barnyard grass and maize. Sci. Rep. 14(1), 1–17 (2024).
Tanaka, A. & Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant Biol. 9(3), 248–255 (2006).
Pouresmaeil, M., Nojadeh, M. S., Movafeghi, A. & Maggi, F. Exploring the bio-control efficacy of Artemisia fragrans essential oil on the perennial weed convolvulus arvensis: Inhibitory effects on the photosynthetic machinery and induction of oxidative stress. Ind. Crops Prod. 155, 112785 (2020).
Sánchez-Muñoz, B. A., Aguilar, M. I., King-Díaz, B., Rivero, J. F. & Lotina-Hennsen, B. The sesquiterpenes β-caryophyllene and caryophyllene oxide isolated from Senecio salignus act as phytogrowth and photosynthesis inhibitors. Molecules 17(2), 1437–1447 (2012).
Silveira, G. L., Lima, M. G. F., Dos Reis, G. B., Palmieri, M. J. & Andrade-Vieria, L. F. Toxic effects of environmental pollutants: Comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere 178, 359–367 (2017).
Ping, K. Y., Darah, I., Yusuf, U. K., Yeng, C. & Sasidharan, S. Genotoxicity of Euphorbia hirta: An Allium cepa assay. Molecules 17(7), 7782–7791 (2012).
Fidyt, K., Fiedorowicz, A., Strządała, L. & Szumny, A. β-Caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Med. 5(10), 3007–3017 (2016).
de Assis Alves, T. et al. Toxicity of thymol, carvacrol and their respective phenoxyacetic acids in Lactuca sativa and Sorghum bicolor. Ind. Crop. Prod. 114, 59–67 (2018).
Türkoĝlu, Ş. Determination of genotoxic effects of chlorfenvinphos and fenbuconazole in Allium cepa root cells by mitotic activity, chromosome aberration, DNA content, and comet assay. Pestic. Biochem. Physiol. 103(3), 224–230 (2012).
Tan, T. et al. Recent advances in understanding the mechanisms of elemene in reversing drug resistance in tumor cells: A review. Molecules 26(19), 5792 (2021).
Jiang, Z., Jacob, J. A., Loganathachetti, D. S., Nainangu, P. & Chen, B. β-elemene: Mechanistic studies on cancer cell interaction and its chemosensitization effect. Front. Pharmacol. 8, 105 (2017).
Pantano, G. et al. Toxicity of the sawdust used for phosphorus recovery in a eutrophic reservoir: Experiments with Lactuca sativa and Allium cepa. Environ. Sci. Pollut. Res. 28(14), 18276–18283 (2021).
Fernandes, T. C. C., Mazzeo, D. E. C. & Marin-Morales, M. A. Origin of nuclear and chromosomal alterations derived from the action of an aneugenic agent—trifluralin herbicide. Ecotoxicol. Environ. Saf. 72(6), 1680–1686 (2009).
Becaro, A. A. et al. Cytotoxic and genotoxic effects of silver nanoparticle/carboxymethyl cellulose on Allium cepa. Environ. Monit. Assess. 189(7), 1–10 (2017).
Acknowledgements
We would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (FAPES) for financial support.
Author information
Authors and Affiliations
Contributions
L.C.V., L.A.M., T.T.C., R.R.d.A.S., A.V.d.A.O. and T.V.d.O.—preparation of the material and collection of the leaves of the plant, L.C.V., L.A.M., A.d.S.B., G.S.M., J.S.d.O., K.F.d.S., M.B.S., I.S.I. and S.C.d.S.—execution of the experiments; L.C.V.—data analysis; L.C.V.—written the first draft of the manuscript; M.M.P.F., T.T.C. and T.V.d.O—idealization and supervision. And all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Vasconcelos, L.C., Mendes, L.A., dos Santos Bergamin, A. et al. Bioherbicidal and cytogenotoxic potential of nanoemulsions containing essential oils from Piper amalago and Piper dilatatum. Sci Rep 15, 32327 (2025). https://doi.org/10.1038/s41598-025-18224-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18224-2