Abstract
To determine therapeutic effect of Qishen Huoxue Granule (QHG) on the myocardial injury in sepsis and whether it is through the inhibition of excessive autophagy by using network pharmacological analysis and in vitro.120 SPF male Wistar rats were divided into 6 groups. Firstly, the function of the heart were evaluated through echocardiography, and pathological changes of myocardial tissue was observed by Hematoxylin-eosin (H&E) and Transmission electron microscopy (TEM). Subsequently, enzyme-linked immunosorbent assay (ELISA) was employed to measure the levels of myocardial injury markers (cTnT and BNP) and inflammatory cytokines (TNF-α and IL-1β). Finally, the expression of proteins associated with the MasR/PI3K-AKT-mTOR pathway and autophagy-related proteins was evaluated using immunohistochemistry, immunofluorescence, RT-qPCR, and Western blot analysis.The results demonstrated that QHG significantly improved systolic function in rats, reduced levels of myocardial injury markers and inflammatory cytokines, and downregulated autophagy-related genes (ATG5, LC3, and Beclin1). However, when QHG was combined with inhibitors of the MasR/PI3K-AKT-mTOR pathway, the mRNA and protein expression levels of pathway components were reduced. Concurrently, the mRNA and protein expressions of autophagy indicators were upregulated, counteracting the inhibitory effect of QHG on excessive autophagy.QHG ameliorated the myocardial injury in sepsis rats by inhibiting the excessive autophagy in myocardial cells via activating the MasR/PI3K-AKT-mTOR pathway.
Similar content being viewed by others
Introduction
Sepsis is the imbalance of body response to infection, life-threatening organ dysfunction syndrome1. It has a high incidence, complex disease, rapid progression, poor prognosis, and a mortality rate of 30–70%, seriously affecting the safety and quality of life of patients2. Eighteen million cases of sepsis are diagnosed worldwide every year, with 70% of deaths attributed to organ failure3. Myocardial injury is one of the main complications of sepsis, closely related to the poor prognosis of patients4. Approximately 50% of sepsis patients have varying degrees of myocardial injury, with a mortality rate of up to 70% ~ 90%5. Therefore, the diagnosis and treatment of sepsis myocardial injury (SMI) has become a tricky research topic both domestically and internationally.
Autophagy is one of the pivotal ways to maintain internal environmental homeostasis. As reported, cells provide raw materials for their normal survival and metabolism through phagocytosis, wrapping and degradation of their own cytoplasmic proteins or organelles6. Autophagy in cardiomyocytes is one of the pathophysiological processes of SMI7. At the basal level, autophagy rapidly upregulates when the nutrient supply fails to meet the cellular needs8. Energy metabolism and substance reuse are maintained by autophagy, so moderate autophagy can protect against cardiac hemodynamics and neurohormonal stress9. However, excessive autophagy leads to excessive self-digestion and degradation of basic cellular components, which may lead to cell death. Therefore, inhibiting excessive autophagy may become a new idea to improve sepsis myocardial injury10.
The phosphatidylinositol 3 kinase/protein kinase B/mechanistic target of rapamycin (PI3K-AKT-mTOR) pathway, as a classic autophagy inhibition signaling pathway, runs through the entire process of sepsis occurrence and development, and is of great significance in regulating cellular autophagy levels11. Recent studies indicate that PI3K-AKT-mTOR signaling pathway is a downstream signaling pathway of ACE2-Ang(1–7)-MasR axis, and its activation inhibits autophagy12.
Traditional Chinese medicine (TCM) has been applied in the treatment of acute myocardial infarction (AMI) and prevention of heart failure (HF) for thousands of years, and an increasing number of herbal formulae have been proven to be effective13. Qishen Huoxue granule (QHG), a TCM prescription, is mainly made from Astragali Radix (named in Chinese: Huangqi), Salvia Miltiorrhizae Radix (named in Chinese: Danshen), Angelica sinensis Radix (named in Chinese: Danggui), Paeoniae Rubra Radix (named in Chinese: Chishao), Chuanxiong Rhizoma (named in Chinese: Chuanqiong) and Carthami Flos (named in Chinese: Honghua). It is reported that it can promote blood circulation and remove blood stasis14, among them, Astragali Radix and Salvia Miltiorrhizae Radix are the prescription, which can play the role of nourishing blood, replenishing qi and removing blood stasis15. In the preliminary study of UPLC/Q-TOF-MS, QHG contains saponins and flavonoids such as Ligustrazine, Geniposide, Paeoniflori, Chlorogenic acid, Aucubin, Hydroxysafflor yellow A, Cryptotanshinone, Geniposidic acid, Ligustilide, Tanshinone IIA. Geniposide protects rats from lipopolysaccharide-induced acute lung injury by regulating autophagy16. Tanshinone IIA alleviated intestinal injury induced by LPS in mice by inhibiting autophagy17, thus indicating that saponins and flavonoids could ameliorate organ dysfunction in sepsis by regulating autophagy. In addition, adjuvant treatment of QHG can reduce cTnT and NT-proBNP levels, AngII and ET-1 levels, shorten ICU stay, and reduce 28-day mortality in patients with sepsis and myocardial injury18, but its role in improving myocardial injury needs to further study. In present study, we investigated the effect of QHG on the activation of MasR/PI3KAKT-mTOR pathway and its inhibition of excessive autophagy, providing a theoretical basis for the treatment of Chinese medicine with blood circulation and blood stasis in septic myocardial injury.
Methods
Network pharmacology analysis
The six Chinese herbs of QHG compound were searched in TCMSP database (http://lsp.nwu.edu.cn/tcmspsearch.php) database. According to the principle of pharmacokinetics and the recommendation of the platform, OB ≥ 20% and DL ≥ 0.10 were limited to the obtained chemical composition as the active ingredient of QHG. The GeneCards (https://www.genecards.org/) database was used to predict the relevant disease targets, the keyword is “Sepsis cardiomyopathy”, and the Relevance score ≥ 5 was selected to obtain the disease targets. Septic heart samples (n = 20) and 11 non-failing (NF) human heart samples were obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo/) GSE79962. The linear model R package for microarray analysis (limma, version 3.50.0) was used to compare the variability of mRNA expression levels between disease and normal samples, with screening criteria set at an absolute log2FC value > 0.5 and a P value < 0.05. The above obtained drug targets, disease targets, and differential genes were crossed by the R package (VennDiagram, version 1.7.1). Then we obtained the protein-protein interaction (PPI) information through STRING (https://string-db.org/). The common target was entered into STRING11.0, and the minimum required interaction score was set to the highest confidence level (0.900). A PPI network map for the common targets was drawn using Cytoscpace3.7. The size and color of the nodes are adjusted according to the degree in the graph.
Finally, the enrichment analysis based on GO and KEGG was performed using the R package (clusterProfiler, version 4.2.1). Biological processes and pathways that met p.adjust < 0.05 and count > 2 were selected, and the top 20 were selected according to the ascending corrected P values, and the enrichment results were visualized by drawing bar charts and bubble plots using the enrichplot package (version 1.14.1).
Preparation of QHG extract
Six Chinese herbs were purchased in Beijing Kangrentang Pharmaceutical Company. Qishen Huoxue Granule (formerly known as Liquid 912, composed of Astragali Radix 60 g, Salvia Miltiorrhizae Radix 30 g, Carthami Flos 10 g, Angelica sinensis Radix 6 g, Paeoniae Rubra Radix 10 g, Chuanxiong Rhizoma 6 g) is an institutionally approved preparation by the Beijing Municipal Drug Administration (Approval Number: 050901) (Table 1). The batch numbers for the herbal ingredients are as follows: Astragali Radix (YBZ-PFKL-2021056), Salvia Miltiorrhizae Radix (YBZ-PFKL-2021035), Carthami Flos (YBZ-PFKL-2022022), Angelica sinensis Radix (YBZ-PFKL-2021037), and Paeoniae Rubra Radix (YBZ-PFKL-2021026), Chuanxiong Rhizoma (YBZ-PFKL-2021029). The decoction process for QHG was as follows: the mixed herbs were immersed in ten times their weight of ultrapure water for 1 h, followed by decoction for 60 min for two more times. The resulting liquid was then dried to form granules.
Animal model of sepsis
Cecal ligation and puncture (CLP)-induced sepsis was performed as previously described19,20. In brief, rats were anesthetized with intraperitoneal injection of pentobarbital sodium (50 mg/kg). A median incision (3 cm) was made in the abdomen, we used a 3.0 sterile silk thread for annular ligation at 1/3 from the root of the cecum to the distal cecum, The ligation site was paired through the intestinal wall twice with an 18-gauge needle. Then, a small amount of stool was extracted from the puncture hole, the cecum was returned back to the abdominal cavity, and sutures were used to close the peritoneum and skin. Rats in the Sham group performed the same procedure as above except without ligation and perforated cecum. After model establishment, all animals immediately received a subcutaneous injection of saline for resuscitation (40 mL/kg). Finally, the rats were returned to the home cage for normal rearing, closely monitor and record the rat’s postoperative performance: rats appear drowsiness, depression, slow reaction, vertical hair and other symptoms indicate the success of the mold.
Animal grouping and dosing regimen
The rats were housed in a carefully regulated environment, with temperatures kept within a stable range of 20 to 26℃ and humidity maintained between 40 and 70%. They were subjected to a consistent 12-hour light and 12-hour dark cycle, and had free and unlimited access to both food and water for one week. 120 male Wistar rats aged from 6 to 8 weeks and weighed from 280 to 320 g were used for the experiment were randomly divided into six groups: (1) Sham (n = 20); (2) CLP group (sepsis, n = 20); (3) CLP + QHG group (n = 20): 1 week after gavage, CLP established a rat sepsis model; (4) CLP + QHG + A779 group (n = 20): QHG (25.4 g/kg/day)21 1 week after gavage, CLP established a rat sepsis model, and MasR antagonist A779 (10ug/kg)22 was injected by tail vein; (5) CLP + QHG + Wortmannin group (n = 20), QHG (25.4 g/kg/day) 1 week after gavage, CLP established a rat sepsis model, and the PI3K antagonist Wortmannin (1 mg/kg)23 was injected into the tail vein; (6) CLP + QHG + Rapamycin group (n = 20), QHG (25.4 g/kg/day) 1 week after gavage, CLP established a rat sepsis model, and the mTOR antagonist Rapamycin (2 mg/kg)24 was injected into the tail vein. Both the Sham and CLP groups were infused with saline, and the Sham and CLP groups were received normal saline daily (4 mL/kg). After the drug administration experiment, we administered pentobarbital sodium (50 mg/kg) to the experimental rats to ensure deep anesthesia (without pain reflex). Subsequently, the abdominal aorta was exposed through abdominal surgery, and blood was collected by puncture until the animal had cardiac arrest (approximately 5–10 mL/200 g in rats). Check breathing and heartbeat, and if necessary, administer anesthesia for execution. After confirming death, immediately conduct tissue sampling.
Echocardiography
At 18 h after model operation, rats cardiac ultrasound was performed using VisualSonics Vevo 2100 color ultrasound diagnostic instrument, and the long axis section of the parasternal left ventricular was taken for M-type ultrasound. Then, we continuously measured LV end systolic diameter (LVIDs), LV end diastolic diameter (LVIDd), LV end-systolic volume (LVESV), LV end-diastolic volume (LVEDV), LV end systolic anterior wall thickness (LVAWs), left ventricular end diastolic anterior wall thickness (LVAWd), LV end systolic posterior wall thickness (LVPWs), end diastolic posterior wall thickness (LVPWd). The data of three cardiac cycle measurements were selected and averaged to calculate: LVEF=[(LVEDV-LVESV)/LVEDV]×100%, LVFS=[(LVIDd-LVIDs)/LVIDd] × 100%, and the differences were compared between the groups for evaluating the left ventricular systolic function of rats.
Enzyme-linked immunosorbent assay (ELISA)
The serum was collected and supernatants were obtained by centrifugation at 4℃ for 10 min. Then, according to the instructions of ELISA kits, ELISA detected the rats cTnT (CSB-E16443r, Cusabio, USA), BNP (CSB-E08594r, Cusabio, USA), TNF-α (BMS625, Thermo, USA), IL-1β (RTA 00, R & D, USA).
Hematoxylin-eosin (H&E) staining
Heart tissues were collected, and fixed in 4% paraformaldehyde for 24 h, and then embedded in paraffin. Paraffin-embedded specimens were sectioned into 5 μm sections using a microtome. Tissue sections were processed according to standard procedures, stained with H&E and then dried with neutral resin. Finally, the histological changes were observed with a light microscope with 200 amplification (IX51, Olympus, Japan).
Transmission electron microscopy (TEM)
To observe the mitochondrial morphology of cardiomyocytes, fresh myocardium was rapidly fixed with 4◦C EM fixative (2.5% glutaraldehyde) for 2 to 4 h. Cardiac tissue was washed with 0.1 M PBS for 15 min and hearts were fixed with 2% osmium acid for 1 h at room temperature. After gradient dehydration, tissues were embedded in sections and then stained with 3% urranium acetate-lead citrate for 15 min. Finally, image acquisition and analysis were performed using a transmission electron microscope (H-7650, HITACHI, Japan).
Immunohistochemical staining
The slices were sequentially dewaxed with xylene. Gradient ethanol was sliced three times for 3 min each time. Washed the slices and treated with citric acid repair solution for 20 min. The cooled slices were sealed at room temperature in goat serum for 5 min. Afterwards, PBS was rinsed three times, and rabbit anti LC3B (1:200, Abcam, ab192890) was uniformly added to the surface of the tissue. A sealing film was added and incubated overnight at 4℃. Take out the slices, clean them, and add SABC complex dropwise. Incubate in a 37℃ incubator for 20 min. Remove excess liquid from the slice, add DAB chromogenic agent, and stain again with hematoxylin. Seal with neutral adhesive and observe the color results under a microscope.
Immunofluorescence
Paraffin sections of myocardial tissue were dewaxed with gradient alcohol to water. Place myocardial slices into citric acid repair solution for high-pressure repair and wait for 5 min to repair after opening the air valve. The cooled slices were incubated at room temperature in 3% hydrogen peroxide. The incubated slices were washed three times with distilled water and PBS, and then incubated at room temperature with 5% BSA sealing solution for 30 min. Toss off BSA (do not wash) and add an anti LC3B (1:200, Abcam, ab192890) working solution overnight at 4℃. After cleaning the slices, add fluorescent secondary antibody (1:1000, CST, #7074) dropwise and incubate at 37℃ for 30 min. Then add DAPI nuclear staining solution dropwise and incubate at room temperature for 10 min. Finally, the water-soluble sealing agent was used to seal the film, and the results were observed and photographed using a fluorescence microscope (BX53, Olympus, Japan).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
According to the manufacturer’s instructions, total cellular RNA was isolated using the Trizol reagent (Thermo, USA). Complementary DNA was reverse transcribed with the PrimeScript-RT reagent kit (Vazyme Biotech Co., Ltd, China). The SYBR Premix Ex Taq kit (Vazyme Biotech Co.,Ltd, China) was used to detect the mRNA levels of GAPDH, ATG 5, Beclin1, LC3, MasR, PI3K, AKT and mTOR. Gene sequences were retrieved from Genbank and primers were designed using Premier 5.0 software and synthesized by Beijing Ruiboko Biotechnology Co., Ltd. as shown in the Table 2 below. Each gene was repeatedly detected three times on three independent samples, and then the relative expression of the target genes were calculated using 2−ΔΔCt method.
Western blot analysis
Total protein samples were extracted using protein extraction kit following the manufacturer’s instructions. BCA Assay kit (Thermo, USA) was used to measure the concentration of extracted protein. Protein specimens were separated by sulphate polyacrylamide gel electrophoresis (PAGE) and then blotted onto polyvinylidene fluoride (PVDF) membranes. The proteins on the blot were assessed using rabbit-anti-mouse primary antibodies against β-acting, ATG5, Beclin1, LC3, MasR, p-PI3K, PI3K, p-AKT, AKT, p-mTOR and mTOR (Abcam, UK). HRP-conjugated goat anti-mouse antibody (Beijing Innokai Technology Co., Ltd, China) were used to incubate with the PVDF membranes for 2 h in room temperature. Blots were visualized using the electrochemiluminescence method. The density of protein bands was quantified with the Image J software.
Statistical analysis
The data were processed by SPSS 20.0 software, and the results were expressed as the mean ± SEM. All data were tested for normality and homogeneity of variance before statistics. Comparisons between groups were analyzed by one-way ANOVA, and post hoc LSD test was used for further pairwise comparison. The t-test was used for comparison between the two groups statistical significance was indicated as P < 0.05.
Results
Differential gene expression and network pharmacology analysis
A total of 1188 differentially expressed mRNAs were identified between disease-contrast normal specimens, thereinto, with 621 mRNAs upregulated and 567 mRNAs downregulated in disease. A volcano plot was drawn using the R package (ggplot2, version 3.3.5) to show the differential expression of the genes (shown in Fig. 1A). The R package (pheatmap, version 1.0.12) package was used to map the expression of differential genes in normal samples and disease samples (Fig. 1B). We first found 189 ingredients from TCMSP database, 125 from Angelica, 202 from Salvia miltiorrhiza, 119 from red peony, 189 from safflower and 87 from Astragalus. Meanwhile, 231 active components and 289 targets were selected according to OB 30% and DL 0.18. In the GeneCards database, 1551 targets were predicted to obtain 353 septic cardiomyopathy targets according to “Relevance score ≥ 5”.
Differential gene expression and network pharmacology analysis. (A) Volcano plot of mRNA differential analysis between disease and normal samples. (B) Differential mRNA heatmap. (C) Intersection of drug targets, disease targets and differential genes. (D) Protein interaction (PPI). (E) GO enrichment bar plots for key genes; (F) KEGG enrichment of key genes.
The intersection of drug targets, disease targets, differential genes, and the results are shown in the Venn diagram, 18 key target genes were obtained: CHRM2, EDN1, SERPINE1, PPARA, ICAM1, TP53, INSR, STAT3, EDNRA, MYC, VCAM1, CXCL8, CAV1, GJA1, SELE, CCL2, SPP1, PYGM (no interaction with other proteins). Figure 1C, D.
Each of the 18 core target genes were co-enriched to 955 GO processes, among them, 930 are biological processes (BP), 5 are cellular components (CC), and 19 are molecular functions (MF). The main enrichment was for GO terms such as hypoxia response, response to reduced oxygen levels, and LPS response (Fig. 1E). KEGG was enriched to 108 pathways, mainly to: diabetic complications AGE-RAGE signaling pathway, lipid and atherosclerosis signaling pathway, PI3K-AKT-mTOR signaling pathway and other KEGG pathways (Fig. 1F).
QHG prolonged the survival in sepsis rats
The composition of traditional Chinese medicine includes Astragali Radix, Salvia Miltiorrhizae Radix, Angelica sinensis Radix, Paeoniae Rubra Radix, Chuanxiong Rhizoma, and Carthami Flos (Fig. 2A). A detailed flowchart of the in vivo assays was shown in Fig. 2B. The survival time of each group was recorded and the survival rate was calculated until 24 h after molding (Fig. 2C). Survival analysis showed that the 24 h survival rate of the CLP group of rats was 30%. Compared with the rats in the CLP group, the 24 h survival rate was increased to 80% in the CLP + QHG group. Compared with the CLP + QHG group, the 24 h survival rate decreased to 60% in the CLP + QHG + A779 group; The 24 h survival rate declined to nearly 60% in the CLP + QHG + Wortmannin group, while approaching 65% in the CLP + QHG + Rapamycin group.
QHG prolonged the survival in sepsis rats. (A) The 6 essential drug components of QHG: Radix Astragali, Radix Angelica sinensis, Radix Paeoniae Rubra, Ligusticum chuanxiong hort, Radix salvia miltiorrhizae and Carthami flos. (B) Group groups and dosing procedures. (C) The survival curves of the rats in each group. Data are presented as mean ± SEM. *P < 0.05, ns: no significant difference.
QHG can improve myocardial function in sepsis
Body surface ultrasound detected the LVIDs, LVIDd, LVEDs, LVEDd, LVAWs, LVAWd, LVPWs, and LVPWd. LVEF and LVFS were calculated to evaluate left ventricular contraction in septic rats (Fig. 3A). Representative echocardiogram is shown in Fig. 3B, and LVEF and LVFS are shown in Fig. 3C. LVEF and LVFS were normal in the Sham group. Compared with the Sham group, the CLP group had a compensatory enhanced cardiac systolic function upon stimulation of sepsis, showing a significant increase in LVEF and LVFS Fig. 3B,C (P < 0.05). LVEF and LVFS were significantly lower in the CLP + QHG group compared with the CLP group (P < 0.05). However, pharmacological inhibition of these pathways using A779, Wortmannin, and Rapamycin abolished QHG’s beneficial effects, reaffirming the pathway’s role in QHG-mediated myocardial protection.
QHG can improve myocardial function (n = 3). (A) Detection of the cardiac indicators: LVIDs, LVIDd, LVESV, LVEDV, LVAWs, LVAWd, LVPWs, LVPWd. (B) Representative echocardiographic images. (C) Quantification of LVEF, LVFS via echocardiography. Data are presented as mean ± SEM. *P < 0.05, ns: no significant difference.
QHG ameliorates the myocardial pathological changes in sepsis
Under the light microscope, it can be seen that myocardial tissue structure of the Sham group rats is intact, with orderly arrangement of myocardial cells, no obvious swelling, deformation or necrosis, and no rupture of myocardial fibers. In the CLP group, myocardial cell rupture and necrosis increased, interstitial vascular dilation was significant, vascular wall thickening was observed, and myocardial fibers were broken. The CLP + QHG group showed mild swelling of myocardial cells, congestion and dilation of myocardial interstitium, and no significant myocardial cell shrinkage or nuclear membrane rupture. The myocardial fibers in the CLP + QHG + A779 group, CLP + QHG + Wortmannin group and CLP + QHG + Rapamycin group were unevenly arranged, with nuclear pyknosis, deformation, or disappearance, and significant myocardial interstitial edema (Fig. 4A).
QHG ameliorates the myocardial pathological changes in sepsis (n = 3). (A) Histological analysis of heart via H&E staining (400×). (B) Cardiac ultrastructural analysis of heart via TEM (80kv×25000). Red arrows indicate disrupted myofibrillar architecture and disorganized cardiomyocyte structure, representing characteristic sarcomeric damage; Green arrows denote mitochondrial swelling with disorganized or indistinct cristae. Image acquired using a HITACHI H-7650 Transmission Electron Microscope.
Transmission electron microscopy revealed distinct ultrastructural differences among the experimental groups. In the Sham group, cardiomyocytes exhibited well-organized architecture with intact cellular structures, characterized by closely packed and regularly arranged mitochondrial cristae without evidence of mitochondrial swelling. In contrast, the CLP group displayed marked morphological alterations, including irregular myocyte thickness, pronounced mitochondrial swelling, structural disruption, and disorganized or indistinct mitochondrial cristae. The CLP + QHG group demonstrated significant preservation of cellular architecture, with cardiomyocytes maintaining orderly arrangement and mitochondria showing relatively intact membranes and clear structural organization. However, in the CLP + QHG + A779, CLP + QHG + Wortmannin, and CLP + QHG + Rapamycin groups, partial disruption of cellular organization was observed, manifested as disordered myocyte arrangement, variable thickness, mitochondrial swelling, and fragmented or indistinct cristae (Fig. 4B).
QHG improves myocardial markers and serum inflammatory factors in sepsis
As shown in Fig. 5A, the cTnT and BNP were significantly increased in the CLP group as compared to the Sham group (P < 0.05). Compared with the CLP + QHG group, the cTnT and BNP of CLP + QHG + A779, CLP + QHG + Wortmannin and CLP + QHG + Rapamycin were significantly increased (P < 0.05).
QHG improves myocardial markers and serum inflammatory factors in sepsis, but this effect can be reversed by MasR/PI3K-AKT-mTOR pathway inhibitors (n = 3). (A) The levels of serum myocardial markers cTnT and BNP. (B) The levels of serum inflammatory factors TNF-α and IL-1β. Data are presented as mean ± SEM. *P < 0.05, ns: no significant difference.
As illustrated in Fig. 5B, the CLP group exhibited markedly elevated serum levels of TNF-α and IL-1β compared to the Sham group (P < 0.05). QHG treatment effectively attenuated this inflammatory response, as evidenced by significantly reduced TNF-α and IL-1β levels in the CLP + QHG group relative to the CLP group (P < 0.05). However, there was no significant difference in serum inflammatory factors TNF-α and IL-1β between CLP + QHG + A779 group compared with CLP + QHG group. However, the CLP + QHG + Wortmannin group exhibited slightly elevated serum levels of IL-1β, whereas TNF-α levels compared to the CLP + QHG group were not significantly different. Notably, Rapamycin treatment (CLP + QHG + Rapamycin group) significantly reversed the anti-inflammatory effects of QHG, leading to substantial increases in serum TNF-α and IL-1β levels compared to the CLP + QHG group (P < 0.05).
QHG inhibits excessive autophagy in septic myocardial tissue
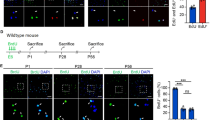
Immunohistochemical analysis revealed significant alterations in autophagy-related protein expression (Fig. 6A). Compared with the Sham group, the CLP group exhibited a marked increase in LC3 protein expression (P < 0.05). QHG treatment effectively downregulated LC3 expression (P < 0.05), whereas pharmacological inhibition of the MasR/PI3K-AKT-mTOR pathway reversed this effect, leading to significant upregulation of LC3 expression (P < 0.05). This trend is also observed in immunofluorescence (Fig. 6D).
QHG can promote autophagy in septic myocardial tissue, but this effect can be reversed by MasR/PI3K-AKT-mTOR pathway inhibitors (n = 3). (A) Representative images of immunohistochemistry for LC3. (B) Relative expression levels of the autophagy-related mRNA (ATG5、Beclin1 and LC3), (C) Representative images of Western blot for ATG5, Beclin1 and LC3II/I. (D) Representative images of immunofluorescence for LC3. Data are presented as mean ± SEM. *P < 0.05, ns: no significant difference.
The expression levels of autophagy markers (ATG5, Beclin1, and LC3) were quantitatively assessed at both mRNA and protein levels using RT-qPCR and Western Blot analyses, respectively (Fig. 6B and C). The uncut original image of Western Blot experiment is included in Supplementary Images S1 and S2.
While the Sham group maintained moderate autophagy activity, the CLP group exhibited significant upregulation of autophagy-related markers, with ATG5, Beclin1, and LC3 mRNA levels showing substantial elevation (P < 0.05). QHG treatment effectively attenuated this response, significantly reducing the expression of ATG5, Beclin1, and LC3 (P < 0.05). These findings demonstrate that QHG downregulates key autophagy indicators, providing compelling evidence for its role in suppressing excessive autophagy in septic rats. In addition, pharmacological inhibition of the MasR/PI3K-AKT-mTOR pathway reversed these effects, as evidenced by the increased mRNA and protein expression of ATG5 and Beclin1, indicating that the pathway inhibition counteracted QHG’s suppressive effect on autophagy.
QHG activates MasR/PI3K-AKT-mTOR pathway
We have performed molecular docking analyses to explore the binding affinity of key bioactive compounds in QHG (e.g., Tanshinone IIA, Geniposide, Hydroxysafflor yellow A) with core targets of the MasR/PI3K-AKT-mTOR pathway (MasR, PI3K, AKT, and mTOR). These results are now included as Supplementary Figure S3.
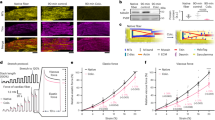
The expression profiles of key components in the MasR/PI3K-AKT-mTOR signaling pathway, including MasR, PI3K, AKT, and mTOR, were systematically evaluated at both mRNA and protein levels using RT-qPCR and Western Blot analyses (Fig. 7A-I). Quantitative analysis revealed significant downregulation of MasR, PI3K, AKT, and mTOR mRNA expression in the CLP group compared to Sham controls (P < 0.05), demonstrating substantial inhibition of this signaling pathway in septic myocardial tissue. QHG treatment effectively reversed this suppression, as evidenced by significantly elevated mRNA levels of MasR, PI3K, AKT, and mTOR in the CLP + QHG group relative to the CLP group (P < 0.05), indicating pathway activation.
QHG can improve the septic myocardium through activating the MasR/PI3K-AKT-mTOR pathway (n = 3). (A-D) Relative expression levels of the autophagy-related mRNA (MasR, p-PI3K/PI3K, p-AKT/AKT, and p-mTOR/mTOR). (E-I) Representative images of Western blot for MasR, p-PI3K/PI3K, p-AKT/AKT, and p-mTOR/mTOR. Data are presented as mean ± SEM. *P < 0.05, ns: no significant difference.
However, pharmacological inhibition of the MasR/PI3K-AKT-mTOR pathway attenuated these effects, resulting in decreased mRNA expression of pathway components and reduced protein phosphorylation ratios (p-PI3K/PI3K, p-AKT/AKT, and p-mTOR/mTOR) (P < 0.05). These collective findings demonstrate that the cardioprotective effects of QHG against excessive autophagy are mediated through activation of the MasR/PI3K-AKT-mTOR signaling pathway, as pathway inhibition effectively counteracts these protective mechanisms.
Discussion
Sepsis often leads to myocardial dysfunction, which is known as septic cardiomyopathy, is an important component of sepsis-induced multiorgan failure and is closely associated with poor prognosis of sepsis25. The pathophysiology of septic cardiomyopathy is complex, involving the release of circulating myocardial injury markers, downregulation of autophagic activity and adrenergic pathway2. However, therapeutic strategies specifically targeting excessive cardiomyocyte autophagy during septic conditions remain scarce26. Therefore, exploring novel treatments from traditional Chinese medicine (TCM), such as Qishen Huoxue Granule (QHG), to regulate myocardial autophagy levels represents a promising approach.
Autophagy is a highly regulated overall degradation mechanism for the clearance of long-lived proteins and the recycling of cytoplasmic contents27. However, the role of autophagy in cardiovascular homeostasis has been controversial. In recent years, numerous studies have shown that autophagy plays a “double-edged sword” role in maintaining cardiovascular homeostasis28. Physiological autophagy maintains cellular homeostasis29, whereas excessive autophagy triggers detrimental cardiomyocyte self-degradation30,31. Our study demonstrates that CLP-induced sepsis32 triggers pathological hyperactivation of autophagy, characterized by upregulated ATG5, Beclin1, and LC3II/I ratios and autophagosome accumulation. This excessive self-digestion aligns with previous reports linking uncontrolled autophagy to mitochondrial depletion and contractile impairment in septic hearts33,34,35.Importantly, QHG effectively reduced these autophagy markers, suggesting that modulating autophagy within an optimal range may be beneficial for myocardial protection in sepsis.
As for the identification of action targets, network pharmacology was used in the study to predict the possible targets and mechanism of QHG in improving myocardial injury in sepsis. The result of GO and KEGG enrichment analysis showed that the PI3K/AKT pathway was the most significantly enriched pathway associated with autophagy in SMI. It has also been shown that activation of the Ang(1–7)/Mas receptor (MasR) axis can counteract angiotensin II (AngII)-mediated cardiovascular disease36, while the downstream effectors of Ang(1–7)/MasR in ventricular cardiomyocyte37 and endothelial are the PI3K/AKT signaling pathway, it not only plays a central role in the regulation of growth, cell proliferation, cell survival, metabolism and other cellular processes38, but also plays an important role in cellular autophagy in sepsis. Moreover, mTOR is the most important downstream pathway of the PI3K/AKT pathway, and mTOR phosphorylation initiates from the activation of the PI3K/AKT pathway39, which is also considered as one of the key steps of autophagy inhibition40. Combined with the above network pharmacology and literature, we show that MasR/PI3K-AKT-mTOR pathway may be a potential signaling pathway target for QHG to inhibit excessive autophagy in cardiomyocytes of septic rats.
Then, the core targets in the MasR/PI3K-AKT-mTOR signaling pathway were tested.
Crucially, QHG not only upregulated MasR expression but also enhanced phosphorylation of PI3K, AKT, and mTOR. Meanwhile, QHG improved compensatory left ventricular contractility, attenuated myocardial edema and reduced serum myocardial injury markers, demonstrating its cardioprotective efficacy.
To further validate the potential drug therapeutic targets of QHG, the rats were injected with MasR inhibitor A779, PI3K inhibitor Wortmannin, and mTOR inhibitor Rapamycin in the tail vein. In rats treated with A779, the mRNA and protein expression levels of the MasR/PI3K-AKT-mTOR signaling pathway were significantly reduced, indicating that A779 effectively blocked the activity of this pathway. Meanwhile, the mRNA and protein expression of the autophagy indicators ATG5, Beclin1, and LC3 were increased, suggesting that the inhibitory effect of QHG on excessive autophagy was counteracted. In addition, echocardiographic results revealed a decline in the compensatory contraction capacity of the left ventricle in the QHG + A779 group. Light microscopy and transmission electron microscopy (TEM) further demonstrated swelling of myocardial tissue, while serum levels of myocardial injury markers were significantly elevated. These findings suggest that the MasR inhibitor A779 reversed the protective effects of QHG in septic rats. This observation aligns with the findings of Lin et al., who demonstrated that MasR agonism suppresses autophagy41. The PI3K inhibitor Wortmannin and the mTOR inhibitor Rapamycin similarly reversed the effects of QHG but exerted distinct impacts on autophagy markers. Notably, Rapamycin, which is known to promote autophagosome-lysosome fusion and enhance autophagic flux, paradoxically reduced the LC3II/I ratio due to the accelerated degradation of autophagic substrates. This phenomenon has been documented in the literature. As highlighted by Klionsky et al. in the Autophagy Guidelines, enhanced autophagic flux can result in reduced LC3-II levels, particularly when the fusion and degradation of autophagosomes and lysosomes are accelerated42. Additionally, studies have demonstrated that in rapamycin-treated cells, despite an increase in autophagosome formation, the overall LC3-II levels may decline due to the upregulation of autophagic flux43.
This study has revealed that QHG exerts its protective effects against excessive autophagy in SMI by activating the MasR/PI3K-AKT-mTOR pathway, thereby offering a novel therapeutic direction for sepsis-associated myocardial dysfunction. However, several limitations of the study should be acknowledged. First, autophagy dynamics at multiple time points post-CLP remain undefined due to animal welfare constraints. Future studies should adopt improved septic models or survival-enhancing interventions (e.g., antibiotics and fluid resuscitation) to longitudinally assess autophagy fluctuations. Additionally, while our study utilized LC3-II/I ratios and Beclin1/ATG5 expression to infer autophagy inhibition, these markers alone do not distinguish between reduced autophagosome formation and impaired autophagic degradation. Future investigations should incorporate autophagic flux assays, such as bafilomycin A1 treatment44 or p62/SQSTM1 degradation analysis45, to clarify the mechanistic specificity of QHG’s effects on autophagy regulation. Second, the complexity of MasR/PI3K-AKT-mTOR signaling warrants conditional knockout models to dissect molecular regulatory nuances precisely. Third, translating these findings to clinical practice requires dose-response and pharmacokinetic studies of QHG constituents, paving the way toward clinically viable therapeutic strategies. While Sham + inhibitor controls were not included, the selected inhibitor doses are documented to be well-tolerated in non-septic models. Our data demonstrate that the inhibitors primarily counteract QHG’s cardioprotection rather than independently aggravate injury. Future studies will formally assess inhibitor effects in non-septic conditions.
Conclusions
The research process of exploring the potential mechanism of QHG treatment for SMI is shown in Fig. 8. The study demonstrates that QHG ameliorates myocardial injury in sepsis-induced rats by inhibiting excessive autophagy in myocardial cells through the activation of the MasR/PI3K-AKT-mTOR pathway. This finding highlights a potential therapeutic mechanism for sepsis-related myocardial dysfunction, as excessive autophagy is known to contribute to cellular damage and organ failure in sepsis. By targeting this pathway, QHG appears to protect myocardial cells from injury, offering a promising avenue for further research and potential clinical applications.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
Abbreviations
- QHG:
-
Qishen Huoxue Granule
- TCM:
-
Traditional chinese medicine
- AMI:
-
Acute myocardial infarction
- HF:
-
Heart failure
- NF:
-
Non-failing
- PPI:
-
Protein-protein interaction
- LVIDs:
-
LV end systolic diameter
- LVIDd:
-
LV end diastolic diameter
- LVESV:
-
LV end-systolic volume
- LVEDV:
-
LV end-diastolic volume
- LVAWs:
-
LV end systolic anterior wall thickness
- LVAWd:
-
Left ventricular end diastolic anterior wall thickness
- LVPWs:
-
LV end systolic posterior wall thickness
- H&E:
-
Hematoxylin and eosin
- PAGE:
-
Polyacrylamide gel electrophoresis
- PVDF:
-
Polyvinylidene fluoride
References
Liu, D. et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Military Med. Res. 9, 56 (2022).
Hollenberg, S. M. & Singer, M. Pathophysiology of sepsis-induced cardiomyopathy. Nat. reviews Cardiol. 18, 424–34 (2021).
Potz, B. A., Sellke, F. W. & Abid, M. R. Endothelial ROS and impaired myocardial oxygen consumption in Sepsis-induced cardiac dysfunction. J. Intensive Crit. Care 2. (2016).
Bi, C. F., Liu, J., Yang, L. S. & Zhang, J. F. Research progress on the mechanism of sepsis induced myocardial injury. J. Inflamm. Res. 15, 4275–4290 (2022).
Xie, Y., Zhang, J., Jin, W., Tian, R. & Wang, R. Role of Thrombospondin–1 in sepsis–induced myocardial injury. Mol. Med. Rep. 24 (2021).
Rangel, M., Kong, J., Bhatt, V., Khayati, K. & Guo, J. Y. Autophagy and tumorigenesis. FEBS J. 289, 7177–7198 (2022).
Wu, B. et al. Luteolin attenuates sepsis–induced myocardial injury by enhancing autophagy in mice. Int. J. Mol. Med. 45, 1477–1487 (2020).
Liu, C. et al. Myocardial injury: where inflammation and autophagy Meet. Burns Trauma. 11, tkac062 (2023).
Zilinyi, R. et al. The cardioprotective effect of metformin in doxorubicin-induced cardiotoxicity: The role of autophagy. Molecules (Basel, Switzerland) 23 (2018).
Wu, S. et al. Trimetazidine protects against myocardial ischemia/reperfusion injury by inhibiting excessive autophagy. J. Mol. Med. 96, 791–806 (2018).
Sun, Y., Cai, Y. & Zang, Q. S. Cardiac autophagy in sepsis. Cells 8. (2019).
Xie, T. et al. Lipopolysaccharide promotes lung fibroblast proliferation through autophagy Inhibition via activation of the PI3K-Akt-mTOR pathway. Lab. Invest. 99, 625–633 (2019).
Li, X. et al. A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of the effects of Qili Qiangxin capsules in patients with chronic heart failure. J. Am. Coll. Cardiol. 62, 1065–1072 (2013).
Su, Y. L., Wang, H. & Zhang, S. W. [Effect of Qishen Huoxue granule in treating severe sepsis]. Zhongguo Zhong Xi Yi Jie he Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chinese. J. Integr. Traditional Western Med. 28, 209–211 (2008).
Wang, G. et al. Qishen Huoxue granule on the myocardium of septic rats study of the protective effect. Zhongguo J. Mod. Med. 2006, 3404–3408 (2006).
Zhang, Z. et al. Genipin protects rats against lipopolysaccharide-induced acute lung injury by reinforcing autophagy. Int. Immunopharmacol. 72, 21–30 (2019).
Yang, X. J. et al. Tanshinone IIA sodium sulfonate attenuates LPS-Induced intestinal injury in mice. Gastroenterol. Res. Pract. 2018, 9867150 (2018).
Zhang, M., Duan, M. & Li, A. Randomized controlled clinical study of Qishen Huoxue granule in treating septic myocardial injury. Beijing J. Chin. Med. 36, 785–788 (2017).
Rittirsch, D., Huber-Lang, M. S., Flierl, M. A. & Ward, P. A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36 (2009).
Sen, P. et al. Wnt/β-Catenin antagonist pyrvinium exerts cardioprotective effects in polymicrobial sepsis model by attenuating calcium dyshomeostasis and mitochondrial dysfunction. Cardiovasc. Toxicol. 21, 517–532 (2021).
Tang, H., Wang, Z. & Wang, G. X. Qishen Huoxue granules drug containing serum improves sepsis induced myocardial cell damage by inhibiting excessive autophagy. Chin. J. Physicians Chin. 23 (10), 1466–1471 (2021).
Dias-Peixoto, M. F. et al. Molecular mechanisms involved in the angiotensin-(1–7)/Mas signaling pathway in cardiomyocytes. Hypertension 52 (3), 542–548 (2008).
Liu, F. C., Tsai, Y. F. & Yu, H. P. Sirtinol attenuates trauma hemorrhage-induced hepatic injury through Akt-dependent pathway in rats. J. Trauma. Acute Care Surg. 74 (4), 1027–1032 (2013).
Yu, S. Y., Liu, L., Li, P. & Li, J. Rapamycin inhibits the mTOR/p70S6K pathway and attenuates cardiac fibrosis in adriamycin-induced dilated cardiomyopathy. Thorac. Cardiovasc. Surg. 61 (3), 223–228 (2013).
Sivapathan, S., Gehardy, B., Pathan, F., Orde, S. & Negishi, K. A review of current landscape and definition of septic cardiomyopathy. Eur. Heart J. 44, ehad6551107 (2023).
Cui, Y., Li, Y., Meng, S., Song, Y. & Xie, K. Molecular hydrogen attenuates sepsis-induced cardiomyopathy in mice by promoting autophagy. BMC Anesthesiol. 24 (1), 72. https://doi.org/10.1186/s12871-024-02462-4 (2024). Published 2024 Feb 23.
Qiu, P., Liu, Y. & Zhang, J. Review: the role and mechanisms of macrophage autophagy in sepsis. Inflammation 42, 6–19 (2019).
Choi, Y., Bowman, J. W. & Jung, J. U. Autophagy during viral infection - a double-edged sword. Nat. Rev. Microbiol. 16, 341–354 (2018).
Ali, N. H. et al. Autophagy and autophagy signaling in epilepsy: possible role of autophagy activator. Mol. Med. (Cambridge Mass). 29, 142 (2023).
Al-Kuraishy, H. M., Al-Gareeb, A. I., Saad, H. M. & Batiha, G. E. Long-term use of Metformin and alzheimer’s disease: beneficial or detrimental effects. Inflammopharmacology 31, 1107–1115 (2023).
Madonna, R. et al. Empagliflozin inhibits excessive autophagy through the AMPK/GSK3β signalling pathway in diabetic cardiomyopathy. Cardiovascular. Res. 119, 1175–1189 (2023).
Qin, S. et al. ANXA1sp attenuates sepsis-induced myocardial injury by promoting mitochondrial biosynthesis and inhibiting oxidative stress and autophagy via SIRT3 upregulation. Kaohsiung J. Med. Sci. 40, 35–45 (2024).
Bi, C. F. et al. Xuebijing injection protects against sepsis-induced myocardial injury by regulating apoptosis and autophagy via mediation of PI3K/AKT/mTOR signaling pathway in rats. Aging 15, 4374–4390 (2023).
Sang, Z., Zhang, P., Wei, Y. & Dong, S. miR-214-3p attenuates Sepsis-Induced myocardial dysfunction in mice by inhibiting autophagy through pten/akt/mtor pathway. Biomed. Res. Int. 2020, 1409038 (2020).
Patel, V. B. et al. Antagonism of angiotensin 1–7 prevents the therapeutic effects of Recombinant human ACE2. J. Mol. Med. 93, 1003–1013 (2015).
Yang, Y. Y. et al. Protective effect of angiotensin-(1–7) against hyperglycaemia-induced injury in H9c2 cardiomyoblast cells via the PI3K̸Akt signaling pathway. Int. J. Mol. Med. 41, 1283–1292 (2018).
Sobrino, A. et al. Mas receptor is involved in the estrogen-receptor induced nitric oxide-dependent vasorelaxation. Biochem. Pharmacol. 129, 67–72 (2017).
Yu, J. S. & Cui, W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Dev. (Cambridge England). 143, 3050–3060 (2016).
Gao, Y., Zhang, Y. & Fan, Y. Eupafolin ameliorates lipopolysaccharide-induced cardiomyocyte autophagy via PI3K/AKT/mTOR signaling pathway. Iran. J. Basic. Med. Sci. 22, 1340–1346 (2019).
Zhu, H. et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J. Clin. Investig. 117, 1782–1793 (2007).
Lin, L. et al. Mas receptor mediates cardioprotection of angiotensin-(1–7) against angiotensin II-induced cardiomyocyte autophagy and cardiac remodelling through Inhibition of oxidative stress. J. Cell. Mol. Med. 20, 48–57 (2016).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) published correction appears in autophagy. 12 (2), 443. (2016).
Takahashi, W. et al. Kinetics and protective role of autophagy in a mouse cecal ligation and puncture-induced sepsis. Crit. Care. 17 (4), R160 (2013).
Wang, X. et al. Icariin alleviates ferroptosis-related atherosclerosis by promoting autophagy in xo-LDL-induced vascular endothelial cell injury and atherosclerotic mice. Phytother Res. 37 (9), 3951–3963 (2023).
Jiang, P. & Mizushima, N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 75, 13–18 (2015).
Funding
This study was supported by grants from National Natural Science Foundation of China (No. 82374069, 81773931). High-level Public Health Technical Personnel Project (Subject Leader-03-15).
Author information
Authors and Affiliations
Contributions
Guoxing Wang and Miaorong Xie performed experimental design and supervision. Yufan Du and Jie Yang carried out the studies and drafted the manuscript. Tingjie Liu performed the statistical analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
I confirm that all methods were performed in accordance with the relevant guidelines. This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study has been approved by the Medical Ethics Committee of Beijing Friendship Hospital Affiliated to Capital Medical University (No. 20-1012). This study is reported in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Du, Y., Yang, J., Liu, T. et al. The therapeutic effect of Qishen Huoxue Granule on myocardial injury in sepsis rats and its underlying mechanism via suppressing excessive autophagy. Sci Rep 15, 32248 (2025). https://doi.org/10.1038/s41598-025-18229-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18229-x