Abstract
This systematic review and meta-analysis evaluated the effect of whole-body vibration training on fibromyalgia. To identify relevant studies, we conducted a comprehensive search of the PubMed, Cochrane Library, Embase, and Scopus databases from their inception through May 2, 2025. We included studies if they (1) enrolled participants with fibromyalgia; (2) treated participants by using a whole-body vibration training intervention; (3) applied exercise, standard care, or no intervention as the control treatment; and (4) included clinical outcomes such as the Fibromyalgia Impact Questionnaire, pain level, quality of life, stability index, and motor function in the related tasks. This meta-analysis included seven randomized controlled trials. The analyses demonstrated that whole-body vibration training led to statistically significant improvements in Fibromyalgia Impact Questionnaire scores (standardized mean difference [SMD]: − 0.37, 95% confidence interval [95% CI]: [− 0.73, − 0.01]), overall stability index (SMD: −0.55, 95% CI: [− 0.95, − 0.15]), and performance in the 6-minute walking test (SMD: 1.65, 95% CI: [1.11, 2.20]). The findings suggest that whole-body vibration training is a viable therapeutic option for individuals with fibromyalgia. Limitations of this study include its relatively small sample size, variations in diagnostic criteria, and the lack of standardized guidelines. To confirm the benefits of whole-body vibration training, additional large-scale, well-designed randomized controlled trials should be conducted.
Similar content being viewed by others
Introduction
Fibromyalgia is a disorder characterized by chronic widespread musculoskeletal pain, fatigue, sleep disturbance, cognitive impairment, headache, muscle stiffness, irritable bowel syndrome, depression, and decreased functional capacity. The symptoms of fibromyalgia affect quality of life1,2,3,4,5. Approximately 2–8% of the general population (up to 15% depending on the diagnostic criteria used) experience fibromyalgia1,2,3,4,5,6,7,8. Fibromyalgia also imposes a substantial socioeconomic burden due to chronic pain, functional limitations, and high rates of comorbidity, leading to increased health care utilization and reduced productivity. Annual direct medical costs average $2,274 per patient9, while indirect costs from work disability and reduced productivity are estimated at $5,000 to $10,000 per year10,11. Patients with fibromyalgia miss an average of 4 to 12.5 work weeks annually, including time lost due to absenteeism and workforce withdrawal, and often exhibit reduced work performance when present10. Treatment approaches are primarily classified as pharmacological or nonpharmacological. Nonpharmacological treatments include exercise-based interventions, education, and psychological therapies4,5,12,13. Due to limited understanding of the disease and a lack of scientific evidence, management of fibromyalgia remains a challenge1,2,12,13. According to recommendations in current guidelines, an integrative and tailored approach, incorporating the aforementioned nonpharmacological methods, should be implemented for treating fibromyalgia to enhance the quality of life among individuals with this disorder4,5,13.
Whole-body vibration (WBV) training is a form of exercise in which individuals receive vibrations generated from a specialized platform14,15. Vibration frequency, amplitude, mode, and duration of exposure influence the effects of WBV training in patients with fibromyalgia14,15. Research has demonstrated that WBV training can not only enhance muscle strength16,17,18, balance16,17,18, walking ability16,17,18, and health-related quality of life16 but also reduce musculoskeletal pain19 and the risk of falls16,17. Given these potential benefits, WBV is a promising exercise-based treatment for fibromyalgia. Several randomized controlled trials (RCTs) have assessed whether WBV training improves outcomes such as Fibromyalgia Impact Questionnaire (FIQ) scores20,21,22,23,24,25, pain levels12,13,14,15,16,17,18,19,20,21,22,23, balance indices20,22,26,27, quality of life20,22,25, and motor function21,22,25,26.
Evidence on WBV for fibromyalgia remains limited and somewhat inconsistent28,29,30,31,32. Existing reviews often include a small number of fibromyalgia-specific trials and report mixed findings across outcomes such as pain and quality of life. Moreover, WBV is frequently underrepresented among the exercise interventions studied, and the overall quality of evidence remains modest28,29,30,31,32. In response to these limitations and the growing interest in WBV, we conducted a comprehensive systematic review and meta-analysis. Our study incorporated trials not included in previous meta-analyses, addressed the underrepresentation of WBV by focusing solely on WBV-specific interventions, improved overall evidence quality through rigorous methodological standards and a comprehensive risk of bias assessment, and broadened the investigation to outcomes that had been insufficiently explored. This study aimed to evaluate the effectiveness of WBV in alleviating fibromyalgia symptoms and improving quality of life.
Methods
Study design and registration
The methodology of this study adhered to the recommendations stipulated in the Cochrane Handbook for Systematic Reviews of Interventions. This systematic review and meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines33,34. This systematic review was registered with the International Prospective Register of Systematic Reviews (registration number CRD42024610251) on November 16, 2024.
Eligibility criteria
Eligibility was assessed using the Patient (P), Intervention (I), Comparison (C), and Outcome (O) framework:
-
a.
(P) Participants with fibromyalgia diagnosed per American College of Rheumatology criteria.
(I) WBV training as the primary intervention.
-
b.
(C) Control: same exercise regimen, standard care, or no intervention.
-
c.
(O) Clinical outcomes: FIQ, pain, quality of life, stability index, or motor function scores.
The inclusion criteria for this review were as follows: studies (1) that are randomized controlled trials, including pilot or crossover designs with an adequate washout period; (2) involving participants diagnosed with fibromyalgia according to American College of Rheumatology criteria; (3) evaluating WBV training as the primary intervention compared with the same exercise regimen, standard care, or no intervention; and (4) reporting outcomes such as FIQ scores, pain, quality of life, stability index, or motor function scores.
We excluded non–peer-reviewed articles such as conference papers, letters to the editor, and study protocols, as well as crossover studies without sufficient washout periods. No language restrictions were applied.
Search strategy
Each author conducted an independent review of the literature, performed data extraction, and cross-checked the extracted data according to the PRISMA guidelines34.
A comprehensive search for relevant articles was conducted across the PubMed, Embase, Cochrane Library, and Scopus databases from inception to May 2, 2025. The search strategy involved combining the terms fibromyalgia and vibration, along with their synonyms, truncated terms, and Medical Subject Heading (MeSH) terms. The strategy is detailed in the Supplementary Appendix. RCTs were identified using the refined search function of the databases, if available. Additionally, we manually examined the reference lists of eligible articles to identify any additional relevant trials. To assess the eligibility of all identified articles, two reviewers independently screened article titles and abstracts, and any discrepancies were resolved through discussion with a third reviewer. Subsequently, the full texts of the remaining articles were thoroughly screened to determine their eligibility.
Data items
Two authors independently extracted the following data from each article: type of RCT, inclusion criteria, participant numbers, mean age, protocols utilized in different groups, follow-up duration, and outcome measures. Any discrepancies were resolved through discussion with a third reviewer. In case of unclear or missing data, we contacted the respective authors through email to obtain the necessary information.
Outcome measurements
The primary outcome was the FIQ score, which has demonstrated a reliability with Cronbach’s α ranging from 0.72 to 0.95 and moderate to strong validity across different studies35,36. Secondary outcomes included pain scores; quality of life, as assessed by the SF-36 and 15D questionnaires; balance, measured by the Stability Index using the Biodex Balance System; and motor function in task-specific evaluations. In the meta-analysis, data with the most immediate follow-up duration were pooled for analysis.
Risk-of-bias assessment
Study quality was assessed using the Physiotherapy Evidence Database (PEDro) scale37. This scale is a reliable tool for evaluating risks of bias in RCTs37. Discrepancies between the two reviewers in study quality scores were resolved through discussion with the third reviewer. Scores for items 2–11 were summed to obtain total PEDro scale scores, which range between 0 and 10. Scores of < 4, 4–5, 6–8, and 9–10 were considered poor, fair, good, and excellent, respectively. Regardless of PEDro score, all identified articles were included in this meta-analysis.
Statistical analysis
For studies employed a three-arm design comparing WBV training with another intervention and a control group, we extracted data from the WBV training and control groups to maintain consistency with the overall objective of evaluating the effectiveness of WBV in patients with fibromyalgia. In studies comparing different types of WBV platforms with a control group, we included the WBV group that demonstrated more favorable outcomes. For all included trials, we extracted post-intervention data rather than change scores, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. This approach is recommended when using standardized mean differences (SMDs), as the standard deviations for post-intervention values and change scores reflect different sources of variability and should not be combined. Using post-intervention data ensures greater consistency and statistical validity across studies33.
Statistical analyses were conducted using RevMan 5.4 software provided by the Cochrane Collaboration (https://training.cochrane.org/online-learning/core-software#RevMan). Continuous data are expressed as the changes in outcome measures from baseline. In case means and standard deviations were not provided in the studies, we contacted the respective authors through email to obtain the raw data. If means and standard deviations were unavailable, the data were estimated through the calculation of correlation coefficients following the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions as well as the approaches of Luo et al. and Wan et al.33,38,39 Statistical significance was set at P < 0.05. To objectively assess statistical heterogeneity, the I2 test was employed; I2 values of ≥ 50% indicated significant heterogeneity40. Given the diverse methodologies employed by the included articles, this meta-analysis was conducted using a random-effects model. Continuous variables are presented as standardized mean differences (SMDs) with 95% confidence intervals (CIs). Effect sizes quantify the magnitude of a difference or the strength of an association between variables, providing a standardized measure of the observed effect. Cohen’s d-based SMDs were estimated for indicating the clinical significance of relationships; SMDs of < 0.2, 0.2–0.5, 0.5–0.8, and > 0.8 indicate clinically meaningless, small, moderate, and large effects, respectively41.
Sensitivity analyses were conducted by sequentially excluding one or two studies at a time to evaluate the impact of heterogeneity on the overall effect estimates. Potential outliers, defined as studies with results that differed substantially from the majority, were assessed in accordance with the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions33. These analyses were performed to examine the robustness of the pooled results and to explore sources of heterogeneity.
Notably, a formal assessment of publication bias, such as funnel plot analysis, was not performed due to the limited number (< 10) of studies included in the review, as such methods are considered unreliable under these conditions according to the Cochrane Handbook for Systematic Reviews of Interventions.³³.
Quality of evidence
Quality of evidence and confidence in effect estimates were assessed employing the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach42. This approach involves evaluating the quality of a study by considering study design, risk of bias, inconsistency, imprecision, indirectness, and publication bias. Effect sizes and trends are also accounted for42.
Results
Study selection
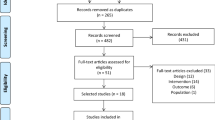
In total, 370 studies were initially identified (63 from PubMed, 157 from Embase, 104 from Scopus, and 46 from the Cochrane Library). Among these studies, 172 were duplicates and were excluded. Duplicates were identified by EndNote 2043. Subsequently, screening of titles and abstracts was conducted. Screening identified 174 studies that did not meet the inclusion criteria and were thus excluded. We then reviewed the full text of the remaining 24 studies and identified 17 that did not meet the inclusion criteria for the following reasons: 9 did not focus on whole-body vibration, 2 lacked available mean or standard deviation data, 2 involved duplicate study populations, 3 did not assess outcomes of interest, and 1 had an unsuitable experimental design. Ultimately, this study included seven trials21,22,23,24,25,26,27. The article selection process is illustrated in Fig. 1.
Study characteristics
The selected studies comprised a total of 93 participants in intervention groups and 85 participants in control groups. All the selected studies were parallel RCTs21,22,23,24,25,26,27. Regarding outcomes, five studies reported FIQ scores21,22,23,24,25, three reported pain scores21,22,23. three provided overall stability index values22,26,27, three reported anteroposterior stability index values22,26,27, three reported mediolateral stability index values22,26,27, two examined quality of life22,25, and four reported motor function21,22,25,26. Intervention duration was 12 weeks in three studies22,24,27, 8 weeks in one study26, 6 weeks in two studies21,25, and 4 weeks in one study23. Table 1 lists the characteristics of the included studies.
The vibration parameters across included studies varied in platform type, frequency, amplitude, and session duration. Adsuar et al., Mingorance et al., Olivares et al., and Sañudo et al. used the Galileo Fitness platform (Novotec Medical GmbH, Germany);22,24,25,27 Ribeiro et al. used the FitVibe® Excel Pro (GymnaUniphy, Belgium);21 Sañudo et al. (2013), the Power Plate (Power-Plate International B.V., Netherlands);26 and Alev et al. the Compex Winplate. The Galileo Fitness platform operated with oscillatory vibrations, while the FitVibe® Excel Pro and Power Plate systems utilized vertical vibrations. For the Compex Winplate, the literature does not explicitly specify whether the platform delivers vertical or oscillatory vibrations. Frequencies ranged from 12.5 to 40 Hz, with Ribeiro et al. progressively increasing frequency from 35 to 40 Hz21,23. Amplitudes ranged from 2 to 4 mm, with some studies adjusting for bilateral versus unilateral exercises25. Session durations also varied: Ribeiro et al. 3–10.6 min;21 Alev et al. 33 min;23 Olivares et al. and Adsuar et al. 8–11 min progressively over 12 weeks;24,27 while other protocols applied fixed durations of 7–10 min22,25,26. These variations highlight the absence of standardized WBV protocols, as summarized in Table 2.
Risk-of‐bias assessment
Two reviewers independently evaluated the quality of the included RCTs by using the PEDro scale37. PEDro scores for all included studies ranged between 5 and 8. On the basis of these scores, six studies were categorized as having good quality21,23,24,25,26,27, and one study was deemed to have fair quality22. Table 3 presents a summary of the results of the assessment of the risk of bias for the included studies.
FIQ scores
Five studies evaluated FIQ scores21,22,23,24,25. These studies involved 79 patients in intervention groups and 75 patients in control groups. Our analysis yielded an SMD of − 0.64 (95% CI [− 1.21, − 0.08]) and an I2 of 65%, indicating significant differences favoring the intervention group (Fig. 2A).
Forest plot for all results. (A) FIQ score, (B) pain score, (C) overall stability index, (D) mediolateral stability index, (E) anteroposterior stability index, (F) quality of life. Motor function in the related tasks, including (G) 6MWT, and (H) lower body dynamic strength. SD, standard deviation; CI, confidence interval; 6MWT, 6-minute walking test.
Pain scores
Three studies examined the pain scores21,22,23. These studies involved 47 patients in intervention groups and 45 patients in control groups. Our analysis yielded an SMD of − 0.89 (95% CI [− 1.80, 0.03]) and an I2 of 76%, indicating no significant difference between the groups (Fig. 2B).
Overall stability index
Three studies investigated overall stability index values22,26,27. These studies involved 52 patients in intervention groups and 48 patients in control groups. Our analysis yielded an SMD of − 0.55 (95% CI [− 0.95, − 0.15]) and an I2 of 0%, indicating significant differences favoring the intervention group (Fig. 2C).
Mediolateral stability index
Three studies examined mediolateral stability index values22,26,27. These studies involved 52 patients in intervention groups and 48 patients in control groups. Our analysis yielded an SMD of − 0.27 (95% CI [− 0.67, 0.12]) and an I2 of 0%, indicating no significant difference between the groups (Fig. 2D).
Anteroposterior stability index
Three studies evaluated anteroposterior stability index values22,26,27. These studies involved 52 patients in intervention groups and 48 patients in control groups. Our analysis yielded an SMD of − 0.32 (95% CI [− 0.73, 0.08]) and an I2 of 2%, indicating no significant difference between the groups (Fig. 2E).
Quality of life
Two studies evaluated quality of life22,25. These studies involved 34 patients in intervention groups and 32 patients in control groups. Our analysis yielded an SMD of 0.72 (95% CI [− 0.61, 2.04]) and an I2 of 85%, indicating no significant difference between the groups (Fig. 2F).
Motor function
Motor function was assessed in two patient subgroups (6-minute walking test [6MWT] and lower body dynamic strength subgroups). Two studies evaluated motor function by using the 6MWT21,22. These studies involved 37 patients in intervention groups and 35 patients in control groups. Our analysis yielded an SMD of 1.65 (95% CI [1.11, 2.20]) and an I2 of 0%, indicating a significant difference favoring the intervention group (Fig. 2G). Two studies assessed motor function by evaluating lower body dynamic strength (repetition of half squats in 60 s)25,26. These studies involved 28 patients in intervention groups and 22 patients in control groups. Our analysis yielded an SMD of 0.22 (95% CI [− 0.34, 0.78]) and an I2 of 0%, indicating no significant difference between the groups (Fig. 2H).
Adverse effects
Adverse effects were reported in four of the included RCTs23,24,25,26. In total, 18 participants discontinued WBV training. WBV training was discontinued for the following reasons: comorbidities (four participants)23,25,26, another treatment (one participant)23, incompatible work schedules (four participants)25,27, lack of interest (two participants)27, pain (one participant)27, inability to exercise after an injury (one participant)25, and personal reasons (five participants)26. None of the participants discontinued WBV training because of the intervention itself. This observation indicated that the intervention was generally well tolerated.
Sensitivity analysis
Outliers were removed in the sensitivity analysis. For example, after the study by Ribiero et al. was excluded from the score of FIQ results, our analysis yielded an SMD of − 0.37 (95% CI [− 0.73, − 0.01]) and an I2 of 0%, suggesting a significant difference favoring the intervention group21. Furthermore, upon the exclusion of the study by Alev et al. from the pain score results, our analysis yielded an SMD of − 1.31 (95% CI [− 1.82, − 0.79]) and an I2 of 0%, indicating a significant difference favoring the intervention group. After the removal of both these articles, lower heterogeneity was observed (I2 < 50%; Table 4).
Quality of evidence
Quality of evidence was assessed using the GRADE approach. Our analysis yielded low quality of evidence for FIQ scores, pain scores, stability index, quality of life, and motor function. Quality of evidence details are given in Table 5.
Discussion
The results of this study revealed that in contrast to the control group, WBV training led to significant improvements in FIQ scores, overall stability index, and 6MWT performance in the intervention group. In addition, we evaluated the strength of relationships between variables by calculating effect sizes. All significant results were not only statistically meaningful but also clinically significant (SMD > 0.2); small, moderate, and large effect sizes were found for FIQ scores, overall stability index, and 6MWT performance (SMD: 0.2–0.5, 0.5–0.8, and > 0.8, respectively)41. Nevertheless, WBV training did not result in significant enhancements in pain scores, quality of life, mediolateral stability index, anteroposterior stability index, or lower body dynamic strength. While our study demonstrated a significant improvement in the Overall Stability Index, the Mediolateral Stability Index and Anteroposterior Stability Index did not reach statistical significance. This seemingly paradoxical outcome may be attributed to the nature of the Overall Stability Index as a composite measure that integrates sway in both the mediolateral and anteroposterior directions44,45. As it is calculated using the root mean square of the directional indices, moderate reductions across both planes can result in a significant improvement overall, even if individual changes are not statistically significant44,45. Furthermore, the Overall Stability Index may be more sensitive to early or general improvements in postural control, whereas directional indices may require larger sample sizes or longer intervention durations to detect significant changes45. Regarding the lower dynamic strength outcomes, we closely examined the studies conducted by the same research team. According to the authors, they hypothesized that WBV training influenced the remodeling of central balance control circuits rather than directly improving muscle strength26. However, this interpretation does not fully align with current understanding of WBV mechanisms and should be noted as a point of consideration. Further investigation by future studies is warranted to clarify this discrepancy. Our results indicate that WBV training may yield clinically meaningful and significant improvements in functions related to the performance of activities of daily living in patients with fibromyalgia, particularly in improving mobility and fall risk.
A sensitivity analysis was performed by sequentially excluding one or two studies for assessing the stability and reliability of the meta-analysis results. Alev et al. assessed the outcomes of interest at 3 and 6 months after WBV training, with a focus on long-term effects, and concluded that WBV training did not significantly ameliorate pain23. Delayed evaluations at these later time points (rather than immediate assessments) may fail to capture the immediate pain-reducing effects of WBV training. The effects of WBV training tend to diminish over time22. This observation likely explains the lack of improvement in pain scores reported in their study and the variations observed in the sensitivity analysis in the current study. Regarding FIQ scores, the study by Ribeiro et al. appears as an outlier in the sensitivity analysis although the study found significant improvement remains unclear, it may be a chance difference resulting from an insufficient sample size21.
WBV training is a relatively new treatment employed to reduce pain, alleviate depression, and enhance muscle strength and balance in physiotherapy. Despite its widespread use, the exact mechanism underlying its effectiveness is not fully understood. A prevalent hypothesis is that WBV reduces pain by enhancing spinal inhibition of nociceptive input and promoting cortical reorganization21,26,46. This aligns with the gate control theory, which suggests that non-nociceptive input from large-diameter afferents can suppress pain transmission at the dorsal horn47. WBV also stimulates muscle spindle afferents, enhancing the tonic vibration reflex and modulating segmental reflex activity22,48,49. At the supraspinal level, WBV may improve sensorimotor integration, particularly in individuals with chronic pain or impaired motor control46. This reorganization of sensory and motor networks may contribute to functional improvements. Additionally, WBV may enhance neuromuscular activation and alter contractile properties through co-activation of alpha and gamma motor neurons, ultimately improving strength and balance23,48,49. These theoretical frameworks explain how WBV training contributes to symptom relief in individuals with fibromyalgia.
The price of WBV platforms used in the included studies ranges from approximately 1,500 to over 15,000 USD, depending on the model. Such costs may significantly limit accessibility in healthcare systems with constrained financial resources. To our knowledge, no studies have specifically assessed the use of WBV therapy for patients with fibromyalgia in low- and middle-income countries (LMICs). Implementing this intervention in LMICs presents several challenges, including unreliable electricity, limited infrastructure, inadequate maintenance capacity, and a shortage of trained personnel50. According to the World Health Organization, up to 75% of medical devices in LMICs remain unused due to a mismatch between device design and local contextual needs50. Further research is warranted to evaluate the cost-effectiveness of WBV therapy in low and middle income countries. Adapting intervention protocols, such as using shorter session durations, simplifying equipment design, may help improve the accessibility and sustainability of this modality.
Based on our analyses, we recommend WBV training as a short-term treatment for patients with fibromyalgia, as it improves activities of daily living, balance, and functional exercise capacity. These outcomes were measured using the FIQ, Overall Stability Index, and 6MWT, respectively. The effectiveness of WBV is likely linked to enhanced sensorimotor integration, neuromuscular activation, and improved proprioception in individuals with chronic pain. The protocol used in studies evaluating FIQ scores involved vibration frequencies of 12.5, 20, 25, 30, 35, or 40 Hz, amplitudes of 2, 3, or 4 mm, session durations of 3–10.6, 7, 8–11, 10, or 33 min, administered 2 or 3 times per week over 4, 6, or 12 weeks21,22,23,24,25. For improving balance, as assessed by the Overall Stability Index, protocols used frequencies of 12.5, 25, or 30 Hz, amplitudes of 2–3 mm, and session durations of 7, 8–11, or 10 min, delivered 3 times per week for 8 or 12 weeks22,26,27. To enhance 6MWT performance, protocols included frequencies of 25 or 35–40 Hz, amplitudes of 25 or 35–40 mm, and session durations of 3–10.6 or 7 min, conducted 3 times per week for 6 or 12 weeks21,22.
Several studies investigated the effects of WBV training on chronic musculoskeletal pain, including fibromyalgia. Dong et al. analyzed 16 RCTs focusing on pain relief in patients with chronic musculoskeletal pain28. Their findings revealed significant differences in pain relief between patients with osteoarthritis and chronic low back pain but no significant difference in pain relief between patients with other chronic musculoskeletal pain diseases. Notably, only one of the included RCTs addressed fibromyalgia; thus, Dong et al. recommended that further and more expansive research be conducted. Bidonde et al. conducted a review of seven studies for examining the effects of WBV training on fibromyalgia and found no significant differences in outcomes29. Moretti et al. conducted a review of two RCTs that evaluated the effects of WBV training on pain, fatigue, and quality of life in patients with fibromyalgia. Results for pain were inconclusive, and no significant differences were found for the other outcomes. Incidentally, evidence was rated as being of very low quality30. Similarly, Collado-Mateo et al. reviewed three RCTs and concluded that WBV training may have beneficial effects on pain, balance, quality of life, and fatigue in patients with fibromyalgia; however, their analysis was not as extensive or rigorous31. Zhang et al. conducted a systematic review and network meta-analysis of 57 RCTs that investigated the effects of different exercise interventions on pain scores, sleep quality, and depression in patients with fibromyalgia; the results revealed that WBV training exerted beneficial effects on sleep quality and depression. Notably, only three of the included RCTs involved WBV32.
Our review included studies that had not previously been included in a meta-analysis. Moreover, our review extended the investigation to include outcomes such as FIQ scores21,22,23,24,25, pain scores21,22,23, balance indices22,26,27, quality of life22,25, and motor function in the related tasks22,25,26. These outcomes were not thoroughly explored in other studies. Importantly, this study revealed significant improvements in FIQ scores, overall balance index, and 6MWT performance. Therefore, this study provides a more updated and comprehensive review of the literature, offering additional insights into the potential benefits of WBV training for patients with fibromyalgia.
This study has several key strengths. First, this is the latest systematic review and meta-analysis of RCTs to evaluate the effectiveness of WBV training in patients with fibromyalgia. Second, this study provided a thorough evaluation of the effects of WBV training across multiple domains, including FIQ scores, pain scores, stability index, quality of life, and motor function in the related tasks. Third, this study conducted an extensive search for relevant RCTs in multiple major databases without language restrictions, and broad inclusion criteria were applied. Finally, the quality of the majority of the selected RCTs was deemed good, as assessed using the PEDro scale.
This study has several limitations. First, relatively few studies were included in this systematic review. Second, considerable heterogeneity observed in some outcomes may be attributed to differences in treatment protocols, including platform types, vibration settings, training duration, timing of assessments, and evaluation tools. The use of varying fibromyalgia diagnostic criteria (ACR 1990, 2010, and 2016), which differ in their emphasis on tender points, symptom severity, and pain distribution, may have further contributed to clinical variability. These inconsistencies underscore the need for consensus-based guidelines to standardize both WBV protocols and diagnostic approaches, thereby improving comparability across studies and enhancing clinical applicability. Third, the follow-up periods in the included studies were insufficient for assessing long-term outcomes; only one study provided follow-up data up to 6 months. Fourth, in most of the included studies, blinding of participants and therapists was not feasible due to the nature of the WBV training intervention. Fifth, variations were noted in the sample sizes and the number of studies included for each outcome. Sixth, most of the included studies focused primarily on physical function, with relatively few evaluating fatigue, sleep quality, social participation, or emotional health. Only Ribeiro et al.’s study showed that WBV training significantly improved sleep quality and depression21, underscoring the need for more research on outcomes beyond physical function. Thus, caution should be exercised when interpreting the results. These limitations should be addressed in future studies. Moreover, patient-centered outcomes such as satisfaction, adherence, cost-effectiveness, and perceived benefit should be incorporated to enhance clinical relevance. Large-scale and well-designed RCTs should be conducted to provide robust evidence on the effectiveness of WBV training in patients with fibromyalgia.
Conclusion
This systematic review and meta-analysis evaluated the effects of WBV training in patients with fibromyalgia by analyzing data from several RCTs. The findings demonstrated that WBV training resulted in clinically meaningful improvements in activities of daily living, as measured by FIQ scores, as well as enhancements in balance and 6MWT performance, which may contribute to a reduced risk of falls. However, WBV training did not produce significant improvements in pain intensity, as measured by VAS scores, or in overall quality of life. These findings indicate that WBV training may be a viable therapeutic option for patients with fibromyalgia. To definitively establish the effectiveness of this intervention, larger-scale well-designed RCTs should be conducted.
Data availability
The datasets generated during or analysed during the current study are available from the corresponding author on reasonable request.
References
Siracusa, R. et al. Fibromyalgia: Pathogenesis, mechanisms, diagnosis and treatment options update. Int. J. Mol. Sci. 22 (8), 3891 (2021).
Bellato, E. et al. Fibromyalgia syndrome: Etiology, pathogenesis, diagnosis, and treatment. Pain Res. Treat. 2012, 426130 (2012).
Sumpton, J. E. & Moulin, D. E. Fibromyalgia. Handb. Clin. Neurol. 119, 513–527 (2014).
Bair, M. J. & Krebs, E. E. Fibromyalgia (Japanese version). Ann. Intern. Med. 172(5), ITC33–ITC48 (2020).
Clauw, D. J. Fibromyalgia: A clinical review. JAMA 311 (15), 1547–1555 (2014).
McBeth, J. & Jones, K. Epidemiology of chronic musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 21 (3), 403–425 (2007).
Jones, G. T. et al. The prevalence of fibromyalgia in the general population: A comparison of the American college of rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol. 67 (2), 568–575 (2015).
Neumann, L. & Buskila, D. Epidemiology of fibromyalgia. Curr. Pain Headache Rep. 7 (5), 362–368 (2003).
Wolfe, F. et al. A prospective, longitudinal, multicenter study of service utilization and costs in fibromyalgia. Arthritis Rheum. 40 (9), 1560–1570 (1997).
Penrod, J. R. et al. Health services costs and their determinants in women with fibromyalgia. J. Rheumatol. 31 (7), 1391–1398 (2004).
Robinson, R. L. et al. Depression and fibromyalgia: Treatment and cost when diagnosed separately or concurrently. J. Rheumatol. 31 (8), 1621–1629 (2004).
Martinez, J. E. & Guimarães, I. Fibromyalgia: Are there any new approaches? Best Pract. Res. Clin. Rheumatol. 38 (1), 101933 (2024).
Jones, E. A. et al. Management of fibromyalgia: An update. Biomedicines 12 (6), 1266 (2024).
Cardinale, M. & Wakeling, J. Whole body vibration exercise: Are vibrations good for you? Br. J. Sports Med. 39 (9), 585–589 (2005).
Wang, Z., Zhang, X. & Sun, M. The application of whole-body vibration training in knee osteoarthritis. Joint Bone Spine. 89 (2), 105276 (2022).
Bruyere, O. et al. Controlled whole body vibration to decrease fall risk and improve health-related quality of life of nursing home residents. Arch. Phys. Med. Rehabil. 86 (2), 303–307 (2005).
Cheung, W. H. et al. High-frequency whole-body vibration improves balancing ability in elderly women. Arch. Phys. Med. Rehabil. 88 (7), 852–857 (2007).
Kawanabe, K. et al. Effect of whole-body vibration exercise and muscle strengthening, balance, and walking exercises on walking ability in the elderly. Keio J. Med. 56 (1), 28–33 (2007).
Elfering, A. et al. Stochastic resonance whole body vibration reduces musculoskeletal pain: A randomized controlled trial. World J. Orthop. 2 (12), 116–120 (2011).
Moawd, S. A., Abdelhalim, E. H. N. & Ibrahim, A. M. A comparison between the effects of vibration exercise and needle therapy on fibromyalgia symptoms and well-being in community-dwelling older adults: A randomized control study. Geriatr. Nurs. 59, 485–490 (2024).
Ribeiro, V. G. C. et al. Efficacy of whole-body vibration training on brain-derived neurotrophic factor, clinical and functional outcomes, and quality of life in women with fibromyalgia syndrome: A randomized controlled trial. J. Healthc. Eng. 2021, 7593802 (2021).
Mingorance, J. A. et al. A comparison of the effect of two types of whole-body vibration platforms on fibromyalgia: A randomized controlled trial. Int. J. Environ. Res. Public. Health. 18 (6), 3007 (2021).
Alev, A. et al. Effects of whole body vibration therapy in pain, function, and depression of the patients with fibromyalgia. Complement. Ther. Clin. Pract. 28, 200–203 (2017).
Olivares, P. R. et al. Tilting whole body vibration improves quality of life in women with fibromyalgia: A randomized controlled trial. J. Altern. Complement. Med. 17 (8), 723–728 (2011).
Sañudo, B. et al. The effect of 6-week exercise programme and whole body vibration on strength and quality of life in women with fibromyalgia: A randomised study. Clin. Exp. Rheumatol. 28 (6 Suppl 63), S40–S45 (2010).
Sañudo, B. et al. Changes in body balance and functional performance following whole-body vibration training in patients with fibromyalgia syndrome: A randomized controlled trial. J. Rehabil Med. 45 (7), 678–684 (2013).
Adsuar, J. C. et al. Whole body vibration improves the single-leg stance static balance in women with fibromyalgia: A randomized controlled trial. J. Sports Med. Phys. Fit. 52 (1), 85–91 (2012).
Dong, Y. et al. Whole body vibration exercise for chronic musculoskeletal pain: A systematic review and meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 100 (11), 2167–2178 (2019).
Bidonde, J. et al. Whole body vibration exercise training for fibromyalgia. Cochrane Database Syst. Rev. 9 (9), CD011755 (2017).
Moretti, E. et al. Efficacy of whole-body vibration for pain, fatigue, and quality of life in women with fibromyalgia: A systematic review. Disabil. Rehabil. 40 (9), 988–996 (2018).
Collado-Mateo, D. et al. Effects of whole-body vibration therapy in patients with fibromyalgia: A systematic literature review. Evid. Based Complement. Alternat Med. 2015, 719082 (2015).
Zhang, K. D. et al. Effect of exercise interventions on health-related quality of life in patients with fibromyalgia syndrome: A systematic review and network meta-analysis. J. Pain Res. 15, 3639–3656 (2022).
Higgins, J. P. T. et al. August. Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (updated (2024).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Reviews. 10 (1), 89 (2021).
Burckhardt, C. S., Clark, S. R. & Bennett, R. M. The fibromyalgia impact questionnaire: Development and validation. J. Rheumatol. 18 (5), 728–733 (1991).
Bennett, R. M. et al. The revised fibromyalgia impact questionnaire (FIQR): Validation and psychometric properties. Arthritis Care Res. (Hoboken). 61 (11), 1600–1606 (2009).
Moseley, A. M. et al. Evidence for physiotherapy practice: A survey of the physiotherapy evidence database (PEDro). Aust J. Physiother. 48 (1), 43–49 (2002).
Luo, D. et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27 (6), 1785–1805 (2018).
Wan, X. et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135 (2014).
Melsen, W. G. et al. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 20 (2), 123–129 (2014).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences 2nd edn (Routledge, 2013).
Iorio, A. et al. Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ 350, h870 (2015).
EndNote Version EndNote 20. Clarivate; (2013).
Biodex Medical Systems. Biodex Balance System SD: Operation Manual (Biodex Medical Systems, Inc., 2010).
de Oliveira Lira, J. L. et al. Minimal detectable change for balance using the biodex balance system in patients with Parkinson disease. PMR. 12 (3), 281–287 (2020).
Gay, A. et al. Proprioceptive feedback enhancement induced by vibratory stimulation in complex regional pain syndrome type I: An open comparative pilot study in 11 patients. Joint Bone Spine. 74 (5), 461–466 (2007).
Melzack, R. & Wall, P. D. Pain mechanisms: A new theory. Science 150 (3699), 971–979 (1965).
Burke, D. et al. The responses of human muscle spindle endings to vibration during isometric contraction. J. Physiol. 261 (3), 695–711 (1976).
Burke, D. et al. The responses of human muscle spindle endings to vibration of non-contracting muscles. J. Physiol. 261 (3), 673–693 (1976).
World Health Organization. Medical Devices: Managing the Mismatch: An Outcome of the Priority Medical Devices Project (World Health Organization, 2010).
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Author information
Authors and Affiliations
Contributions
Po-Chuan Chang and Fu-An Yang conceptualized and designed the study, and drafted the manuscript. Hung-Chou Chen and Tian-Shin Yeh critically revised the manuscript for intellectual content. Yu-An Chen conducted a comprehensive search for articles that met the eligibility criteria. Po-Chuan Chang extracted the relevant data and assessed the quality of the selected trials. Yi-Tien Su and Tian-Shin Yeh provided statistical expertise, analyzed and interpreted the data, Hung-Chou Chen acted as the corresponding author and approved the final version of the manuscript. Po-Chuan Chang and Fu-An Yang contributed equally to this study. Hung-Chou Chen and Tian-Shin Yeh also contributed equally to this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chang, PC., Yang, FA., Chen, YA. et al. A systematic review and meta-analysis of randomized controlled trials evaluating the effect of whole body vibration training on fibromyalgia. Sci Rep 15, 32918 (2025). https://doi.org/10.1038/s41598-025-18282-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18282-6