Abstract
Chronic venous obstruction affects thousands worldwide and may lead to severe long-term complications such as post-thrombotic syndrome (PTS), marked by pain, swelling, skin changes, and venous ulcers. Endovenous stenting has become a cornerstone in restoring venous outflow, yet surveillance strategies to detect stent dysfunction early remain unstandardized. Doppler ultrasound (DUS), widely available and non-invasive, holds potential as a monitoring tool, but lacks validated markers to guide long-term follow-up. In this retrospective study of 161 patients and over 1,100 DUS assessments, we examined the predictive value of hemodynamic parameters for stent dysfunction and PTS. Stent patency was assessed using DUS or CT venography, and PTS was defined by a Villalta score ≥ 5 at last follow up. Hemodynamic parameters were compared using univariate and multivariable logistic regression models. Significant stent stenosis or occlusion (≥ 50%) was more common in younger patients (mean age 37.7 vs. 48.2 years, p = 0.003), with risk increasing by 4.4% per year decrease in age (OR = 1.044, p = 0.008). Loss of respiratory modulation was strongly associated with stent dysfunction (p < 0.001). Patients who had PTS at last follow up, had lower venous flow (297 vs. 463 mL/min, p = 0.047), reduced peak velocity (p = 0.003), and impaired respiratory modulation (p = 0.017). These findings support using DUS-derived parameters for early, non-invasive detection of stent-related complications to improve long-term patient outcomes.
Similar content being viewed by others
Introduction
Post-thrombotic syndrome (PTS) is a chronic and potentially debilitating complication of deep vein thrombosis (DVT), affecting up to 50% of patients1. Characterized by pain, swelling, skin changes, and in severe cases, venous ulcers, PTS significantly impairs quality of life and contributes to long-term healthcare burden2. As DVT remains a prevalent vascular condition worldwide, strategies to prevent and monitor PTS have become a clinical priority3.
Endovenous stenting represents a cornerstone intervention in the management of both acute and chronic ilio-femoral venous obstruction4. In acute DVT, percutaneous mechanical thrombectomy (PMT) followed by stenting can restore venous outflow and reduce the risk of PTS development5. In chronic venous obstruction (CVO) and moderate to severe PTS, endovenous recanalization and stenting alleviate symptoms such as pain, edema, and ulceration, particularly in patients who are refractory to conservative therapy6.
Despite the growing adoption of venous stenting, there remains no consensus on optimal follow-up strategies for ensuring stent patency and detecting early complications7. The 2022 guidelines of the European Society for Vascular Surgery (ESVS) recommend Doppler Ultrasound (DUS) surveillance at defined intervals post-procedure8. However, they do not establish specific diagnostic threshold for detecting stent dysfunction. This absence of standardized parameters remains a critical gap in clinical practice hinders recognition of complications such as restenosis, recurrent thrombosis, and pulmonary embolism (PE), all of which carry significant morbidity and affect long-term outcomes9,10.
DUS is widely available, non-invasive, and the most practical tool for post-stenting surveillance. Prior studies have proposed hemodynamic thresholds, including peak flow velocity > 10 cm/s combined with a flow pattern modulated by breathing or post/pre-stenotic velocity ratio > 2.5, as markers of venous outflow impairment. However, their clinical validity in detecting significant in-stent stenosis remains unconfirmed in peripheral veins11,12. Establishing standardized Doppler parameters remains a critical need to improve the consistency and effectiveness of long-term stent monitoring13.
The present study investigates the prognostic value of DUS in predicting clinical and imaging outcomes after venous stenting, with a particular focus on hemodynamic parameters. Through a systematic, intermediate-term follow up, we aim to identify reliable, non-invasive Doppler indicators for stent surveillance. Specifically, we examine the association between Doppler-derived hemodynamic parameters, the presence of in-stent stenosis, and the development or persistence of PTS following stenting.
Methods
Study design and patient selection
This was a retrospective observational study conducted at the Vascular Medicine Department of a university hospital between 2015 and 2023. The aim was to evaluate hemodynamic changes and clinical outcomes in patients undergoing venous stenting for acute DVT or chronic post-thrombotic obstruction (PTO). Data were collected from electronic medical records and DUS examinations. The primary hemodynamic parameters investigated were peak flow velocity, flow volume, flow ratio, and respiratory modulation, with details of their measurement described in the Doppler Ultrasound Investigation subsection.
Inclusion criteria included patients aged 18 and 90 years who had undergone venous PMT with stenting for acute DVT or PTO (involving at least the iliac or common femoral vein); and at least one DUS follow-up performed 6 months after the procedure. Exclusion criteria included absence of follow-up data, acute stent thrombosis documented on post-procedural day 1, or patient refusal to participate or consent to data use.
Definitions
Acute DVT was defined as a thrombotic event managed with catheter-directed thrombolysis (CDT) or percutaneous mechanical thrombectomy (PMT), followed by balloon angioplasty and stenting, performed within two weeks of symptom onset as part of the initial treatment. In contrast, post-thrombotic obstruction (PTO) referred to patients with CVO secondary to prior DVT, treated more than 6 months after symptoms onset with endovenous recanalization and stenting.
PTS was defined as a Villalta score ≥ 5 at last follow-up. Due to the retrospective nature of the study, Villalta score were not systematically recorded for all patients. Therefore, only patients with available Villalta scores were included in the PTS analysis.
Chronic venous insufficiency was classified based on the CEAP classification, including patients from C3 to C6. This ranged from edema (C3) to active venous ulcers (C6). Intermediate stages included skin changes such as pigmentation or eczema (C4), and healed venous ulcers (C5)14.
Major Thrombophilia included antithrombin deficiency15, antiphospholipid syndrome16, and selected cases of protein C or protein S deficiency17.
Recent major surgery was defined as any major surgical intervention performed within 3 months prior to the thrombotic event.
Doppler ultrasound investigation
A standardized follow-up surveillance program was implemented with DUS performed at 1, 3, 6, and 12 months post-stenting, and annually thereafter. All evaluations were conducted by vascular medicine specialists within a single department to ensure consistency and accuracy.
Ultrasound protocols were standardized to minimize measurement variability. Patients were examined in the supine position with a 30° backrest elevation and arms resting comfortably at their sides. To reduce venous flow fluctuations, patients remained at rest, refrained from speaking, and maintained regular, shallow breathing without deep inspiration.
DUS evaluations focused on key hemodynamic parameters. Peak flow velocity (in cm/s) in common femoral vein and within the stented segment. Flow volume (in mL/min) was automatically calculated by the ultrasound system using the vessel diameter and time-averaged velocity measurements, assuming a circular cross-sectional area.
Venous flow was also assessed in the external iliac vein. A flow ratio was calculated, comparing the flow in the stented external iliac vein or common femoral vein (depending on the distal end of the stent), to the flow in the contralateral native vein, providing a relative index of venous flow symmetry and quantifying the hemodynamic impact of stenting on venous return.
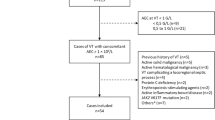
Additionally, respiratory modulation of venous flow was evaluated. Under normal conditions, peripheral veins such as the external iliac and femoral veins exhibit respirophasic flow, characterized by decreased velocity during inspiration (due to increased intra-abdominal pressure) and increased velocity during expiration. This pattern reflects unobstructed venous return. Flow was considered normal when consistent variation was observed during spontaneous breathing. Abnormal modulation was defined as either a lack of variation or variation occurring only with deep or forced inspiration, suggestive of proximal obstruction or impaired stent outflow18. Representative waveforms are provided in Fig. 1.
Doppler ultrasound flow and CT venography assessments of stent patency. Doppler Ultrasound imaging displaying normal flow synchronized with respiration. Doppler Ultrasound imaging displaying non-modulated flow. Cross-sectional CT Venography assessing stent patency and highlighting thrombus-related stenosis. Longitudinal CT Venography assessing stent patency and highlighting thrombus-related stenosis.
CT venography assessment
Pre-procedural imaging was tailored according to clinical presentation. In patients with PTO, we performed direct CT venography with contrast injected into a distal vein of the affected limb to accurately delineate venous anatomy and collateral pathways. This method enabled precise assessment of lesion length, inflow/outflow quality, and facilitated accurate stent sizing and planning. The extent of CVO was classified according to Jalaie et al.19.
In cases of acute iliofemoral DVT, patients underwent a thoraco-abdominopelvic CT scan, extending from the chest to the proximal third of the thigh, to evaluate thrombus burden, screen for embolic sources, and guide initial management. While less detailed distally, this imaging provided adequate coverage of the target segment for stent planning in acute interventions.
For follow-up at 1, 3, and 5 years, indirect CT venography via antecubital vein injection was used to assess stent patency and detect possible stent fracture (Fig. 1). Imaging was initiated 2–3 min post-injection, with scan coverage from the mid-calf to the diaphragm, ensuring visualization of both inflow and outflow zones.
Post-procedural treatment
All patients received on a standardized post-stenting regimen: combined therapeutic anticoagulation and antiplatelet therapy for the first month. Antiplatelet therapy was discontinued after 1 month, while anticoagulation was continued and reassessed at 6 months based on risk profile.
Patients with persistent risk factors, such as unprovoked or recurrent DVT, active cancer, chronic inflammatory disease, major thrombophilia or low venous inflow, continued long-term anticoagulation. This was administered either at therapeutic doses (Rivaroxaban 20 mg once daily or Apixaban 5 mg twice daily) or reduced doses (Rivaroxaban 10 mg once daily or Apixaban 2.5 mg twice daily), based on individual risk assessment.
In contrast, patients with transient risk factors, such as recent surgery (< 3 months), hormonal exposure, or prolonged immobility, who demonstrated confirmed stent patency and adequate inflow were eligible for anticoagulation discontinuation after 6 months.
Data collection
Clinical data included demographics, comorbidities, VTE risk factors, post-procedural treatment, and adherence. Hemodynamic parameters (venous peak velocity, flow, flow ratio, and respiratory modulation) were collected from DUS reports at baseline (post-procedural day 1), 6 months, 12 months, and final follow-up.
Study outcomes
The primary outcomes included stent restenosis, categorized as ≥ 50% or < 50% in-stent stenosis, the presence of PTS, and hemodynamic parameters. Stent patency was assessed using DUS or CT venography, and patients were classified according to the degree of in-stent stenosis (≥ 50% vs. <50%) at the last follow up. PTS was defined by Villalta score ≥ 5 at last follow up. Its evaluation was standardized at that time point to account for its appearance after stenting in acute DVT and its evolution in patients stented for PTO.
Hemodynamic parameters, included peak velocity (cm/s), external iliac or common femoral venous flow, external iliac or common femoral venous flow ratio, and respiratory modulation, were measured at baseline (post-procedural day1) and at follow-up intervals 6 months, 12 months, and final assessment.
Secondary outcomes included venous thromboembolic complications, treatment adherence, and the need for re-intervention. PE and in-stent occlusive thrombosis were recorded as complications in the PTS analysis, while only PE was considered in the analysis of stent patency.
Statistical analysis
All analyses were performed using IBM SPSS Statistics version 26.0. Variables were analyzed with respect to two main outcomes: in-stent stenosis and PTS. Continuous variables were compared using the Mann-Whitney U test and reported as means, median [ Q1 - Q3]. Categorical variables were compared using chi-square or Fisher’s exact, as appropriate.
To identify predictors of PTS and in-stent stenosis or occlusion, binary logistic regression models were conducted. Variables with a p-values < 0.10 in the bivariate analysis were included in the multivariable models. Odds ratios (ORs) with 95% confidence intervals were reported. A p-value < 0.05 was considered statistically significant.
Ethical considerations
This study was conducted in compliance with the Declaration of Helsinki principles and received ethics approval by GNEDS (Groupe Nantais d’Ethique et de Soins), the local ethics committee of the University Hospital of Nantes (20190606). Each patient included in this study received written information and no patient objected to the use of their data. The need for written informed consent was waived by the institutional review board due to the retrospective nature of the study in accordance with French public health code article: L 1121-1.
Results
A total of 161 patients were included in the study, 32 (19.9%) treated during acute phase of DVT and 129 (80.1%) treated for PTO, contributing over 1100 DUS assessments and enabling a comprehensive longitudinal evaluation of hemodynamic changes. Patients were categorized according to stent patency status and the presence of PTS at final follow-up.
Table 1 presents the baseline characteristics according to acute or chronic presentation. Patients treated during the acute phase were significantly younger (median age 35.5 years [27.5–49.3]) compared to those with PTO (49.0 years [34.0–60.0]; p = 0.028). The PTO group exhibited a higher proportion of female patients (62.0% vs. 40.6%; p = 0.046) and had a markedly greater prevalence of chronic venous insufficiency (32.8% vs. 6.9%; p = 0.005). Lesion extent differed significantly between groups: patients with PTO more frequently demonstrated involvement of the femoral and popliteal veins (both p < 0.001). Additionally, they required a greater number of stents (median 3.0 [2.0–4.0] vs. 2.0 [2.0–3.0]; p = 0.0002), and stent extension across the inguinal ligament was more common in the chronic group (82.0% vs. 54.2%; p = 0.043).
Stent patency and hemodynamics
At the last follow-up, 30 of 161 patients (18.6%) had ≥ 50% in-stent stenosis or complete occlusion. Table 2 summarizes baseline patient characteristics according to stent patency. Patients with ≥ 50% in-stent stenosis or complete occlusion were significantly younger (median age 37.7 years [24.5–53.0]) compared to those with < 50% stenosis (48.0 years [34.0–62.0]; p = 0.003). Pulmonary embolism was more prevalent in the ≥ 50% stenosis group (22.2%) versus 1.8% in the < 50% group (p < 0.001).
In patients with ≥ 50% stenosis or occlusion, a progressive decline in normal respiratory flow modulation was observed: from 63.2% at 6 months (p = 0.034) to 57.1% at 12 months (p < 0.001), and 62.5% at final follow-up (p < 0.001). However, no significant changes in peak velocity or venous flow were observed over time (Table 3).
Age was found to be a significant predictor of stent occlusion or stenosis. For each one-year decrease in age, the odds of developing stent occlusion or stenosis increased by 4.4% (OR = 1.044; 95% CI: 1.012–1.081; p = 0.008). This suggests that younger patients were at a higher risk of experiencing stent-related complications compared to older individuals. Other variables, including female sex, chronic venous insufficiency, femoro-popliteal vein involvement, and cessation of anticoagulation, were not found to be statistically significant predictors of stent occlusion or stenosis (Table 4).
Post-thrombotic syndrome and hemodynamics
At last follow up, 29 of 103 patients (28.2%) had PTS. Among these, 5 of 29 patients (17.2%) were from the acute DVT group (newely developed PTS), while 24 of 29 patients (82.8%) were from the PTO group (persistent PTS). Patients who developed PTS tended to be older (median age 51.5 years [37.5–69.0]; p = 0.065) and more frequently had underlying chronic venous insufficiency (30.8% vs. 14.7%; p = 0.077), although these differences did not reach statistical significance (Table 5).
Hemodynamics differences became more pronounced over time. At 6-months, peak velocity did not differ between PTS and non-PTS groups (median 29.5 cm/s [22.5–40.0] vs. 31.0 cm/s [20.0–40.0]; p = 0.39). Venous flow tended to be lower in the PTS group (median 523.0 mL/min [410.0–627.0]) versus non-PTS (median 627.0 mL/min [460.0–710.0]; p = 0.13), with similar rates of normal respiratory modulation (~ 81%). By 12 months, normal respiratory modulation persisted in 100% of non-PTS patients but significantly declined in the PTS group (82.4%; p = 0.014). At final follow-up, patients with PTS showed significantly lower peak velocity (median 29.7 cm/s [26.1–35.0] vs. 33.0 cm/s [30.0–40.0]; p = 0.003), reduced iliac venous flow (median 370.0 mL/min [261.5–580.0] vs. 462.0 mL/min [375.0–580.0]; p = 0.047), and impaired respiratory modulation (80.0% vs. 97.8%, p = 0.017) (Table 6). These differences were driven by progressive hemodynamic deterioration in the PTS group, whereas non-PTS patients maintained relatively stable parameters across follow-up.
Multivariable analysis confirmed that older age increased the odds of PTS by 12% per additional year (OR = 1.12; 95% CI: 1.02–1.23; p = 0.020), while higher venous flow was protective. For every unit increase in venous flow, the odds of developing PTS decreased by 1.1% (OR = 0.989; 95% CI: 0.980–0.998; p = 0.019) (Table 7).
Discussion
In this retrospective cohort of 161 patients undergoing iliofemoral venous stenting, 18.6% developed in-stent restenosis ≥ 50% and 28.2% had PTS at last follow-up. These findings indicate that, even after technically successful stenting, patients remain at risk for long-term anatomical and clinical complications. Hemodynamically, our analyses showed that early alterations in DUS parameters, particularly loss of respiratory modulation and reductions in venous flow, were associated with subsequent stent dysfunction and PTS. Together, these results underscore the importance of systematic DUS surveillance not only to monitor stent patency but also to identify patients at higher risk for progressive venous disease.
As shown in Table 1, patients treated for PTO had more extensive disease than those with acute DVT, including higher rates of femoro-popliteal involvement, greater number of stents, and more frequent crossing of the inguinal ligament. These anatomical and clinical differences reflect the distinct pathophysiology between acute and chronic disease. The increased number of stents in the PTO group is therefore consistent with the greater anatomical extent of disease; however, whether stent number itself has an independent impact on long-term outcomes could not be addressed in this study and warrants further investigation.
Venous stent patency is influenced by numerous factors9,20, including inflow quality, technical success of recanalization[19,221,22], underlying thrombophilia, provoking risk factors, and adherence to anticoagulation therapy23. Despite this multifactorial context, younger age emerged as a significant predictor of stent stenosis or occlusion in our study. The mean age of patients was 37.7 years, and each one-year decrease in age was associated with a 4.4% increase in the odds of developing ≥ 50% stenosis or complete occlusion (OR = 1.044). This findings aligns with previous research identifying age < 40 years as a risk factor for decreased primary patency24,25. Younger patients also required re-intervention more frequently (mean age 32 vs. 46 years; p < 0.01), consistent with prior studies on venous stenting in acute iliofemoral DVT26. Although the underlying mechanisms are not fully understood, potential contributors include a more active inflammatory response leading to neointimal hyperplasia, a higher prevalence of thrombophilic conditions, and increased mechanical stress due to physical activity.
Hemodynamically, patients with ≥ 50% stent stenosis demonstrated a progressive decline in respiratory modulation between 6 and 12 months, even when other parameters such as peak velocity and venous flow remain stable. This suggests that loss of respiratory modulation may precede detectable changes in velocity or flow volume, positioning as a sensitive marker of stent dysfunction. These findings support incorporating waveform analysis into routine stent surveillance, especially in younger, higher-risk populations.
In contrast, older age was a significant predictor of PTS, with each additional year of age increasing the odds of PTS by 12% (OR = 1.12). This finding is consistent with results from the GARFIELD-VTE registry and the European Society of Vascular Medicine position paper, which reported up to a threefold increased risk of PTS in older populations27,28. This association may reflect the cumulative impact of chronic venous stasis and age-related vascular remodeling, with together contribute to valve dysfunction, venous hypertension and progressive outflow impairment29. In contrast, higher venous flow was associated with a protective against PTS, with a 1.1% decrease in the odds of developing PTS per unit increase in flow (OR = 0.989), likely reflecting better preservation of venous hemodynamics and reduced progression toward outflow compromise30.
PTS is a dynamic condition, and its hemodynamic impact becomes more evident with serial evaluations. In our study, patients with PTS showed significantly reduced peak velocity (18.9 cm/s vs. 33.6 cm/s, p = 0.003) and iliac venous flow (297.0 mL/min vs. 462.7 mL/min, p = 0.047), along with impaired respiratory modulation (82.3% vs. 98.0%, p = 0.04), indicating progressive venous dysfunction. The decline in respiratory modulation at 12 months further reinforces its potential as an early indicator of disease onset31,32. Similarly, repeated Villalta assessments may improve PTS detection, as scores fluctuate over time and a single measurement may not fully capture disease progression33. In our study, repeated evaluations may have allowed us to identify more PTS cases, emphasizing the need for serial assessments to improve diagnostic accuracy and track disease evolution.
Strengths and limitations
This study is among the first to systematically analyze hemodynamic parameters for assessing venous stent patency and PTS progression, utilizing DUS to monitor key markers such as respiratory modulation, venous flow, and peak velocity. The large dataset and longitudinal design provide valuable real-world insights, and the identification of respiratory modulation as an early indicator of stent dysfunction offers practical utility for clinical follow-up. However, the retrospective design introduces potential biases, and the lack of invasive validation methods limits confirmation of findings. Operator variability, non-standardized criteria, and inconsistent patient follow-up may affect the reproducibility. Our cohort included both acute and chronic DVT patients, reflecting real-world practice but introducing biological heterogeneity. While acute lesions are typically inflamed and chronic ones fibrotic, this variable was recorded, analyzed, and not significantly associated with outcomes in our data. Nonetheless, differences in vein wall characteristics may impact long-term stent performance. Additionally, assessing venous flow remains challenging due to its lower velocity and variability, which are influenced by patient positioning and respiration. Most patients were enrolled before the publication of the ESVS 2022 guidelines8, which may explain deviations from current surveillance protocols and grading-based therapeutic decisions.
Currently, defining a standard for the DUS evaluation of patients with venous stents remains challenging. Patency assessment in color mode, using slow-flow modes such as Superb Microvascular Imaging (SMI), may help to evaluate the proximal positioning of the iliovertebral pinch on the left, and the respiratory modulation of flows. Distally, assessing the number of veins confluent at the common femoral level is essential for evaluating inflow quality, along with patency and the presence of fibrous strands or venous retractions. Flow rates and venous velocity peaks should be interpreted with caution, as they vary significantly depending on patient positioning and breathing patterns. Unlike arterial stenosis assessment, peak velocities in venous flow are harder to evaluate due to the weaker venous circulation, which limits acceleration at stenotic sites, especially in cases with post-thrombotic sequelae. Moving forward, improving imaging techniques and establishing clear evaluation criteria will be essential for better diagnosis, follow-up, and long-term management of venous stents.
Conclusion
This study identifies significant associations between Doppler ultrasound–derived hemodynamic parameters and the presence of venous stent dysfunction and post-thrombotic syndrome. In particular, impaired respiratory modulation, reduced peak velocity, and lower venous flow were linked to ≥ 50% stent stenosis or occlusion, suggesting their potential as non-invasive indicators for early detection.
These findings support the need for standardized Doppler criteria to improve consistency in post-stenting surveillance. Future prospective, multicenter studies are essential to validate these associations and to assess the clinical utility of incorporating hemodynamic parameters into routine follow-up protocols.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to patient confidentiality and institutional data protection policies but are available from the corresponding author on reasonable request.
References
Wang, S., Hossack, J. A. & Klibanov, A. L. From anatomy to functional and molecular biomarker imaging, and therapy: ultrasound is safe, ultrafast, portable, and inexpensive. Invest. Radiol. 55, 559–572 (2020).
van Rijn, M. J. E. & Kakkos, S. K. Early thrombus removal in iliofemoral deep vein thrombosis to prevent Post-thrombotic syndrome. Eur. J. Vasc. Endovasc. Surg. 65, 169–170 (2023).
Li, W. et al. Revisiting the open vein hypothesis to reduce the postthrombotic syndrome: implications for multidisciplinary care and research: A scientific statement from the American heart association. Circulation 151, e1051–e1071 (2025).
Shekarchian, S. et al. Editor (eds) ‘s Choice Quality of life after stenting for iliofemoral venous obstruction: A randomised controlled trial with one year follow up. Eur. J. Vasc. Endovasc. Surg. 66, 678–685 (2023).
Avgerinos, E. D., Black, S., van Rijn, M. J. & Jalaie, H. The role and principles of stenting in acute iliofemoral venous thrombosis. J. Vascular Surgery: Venous Lymphatic Disorders. 12, 101868 (2024).
Breen, K. Role of venous stenting for venous thromboembolism. Hematology 2020, 606–611 (2020).
Harth, K. C. & Kiguchi, M. M. Appropriateness of care: deep venous procedures. Semin. Vasc. Surg. 37, 156–163 (2024).
Maeseneer, M. G. D. et al. Choice—European Society for Vascular Surgery (ESVS) 2022 clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur. J. Vasc. Endovasc. Surg. 63, 184–267 (2022).
Espitia, O. et al. Predictive factors of stent patency in iliofemoral venous diseases in a multicentre cohort study. Eur. J. Vasc. Endovasc. Surg. 65, 564–572 (2023).
Zhang, L. et al. Three-Year outcomes, risk factors for restenosis after stenting for DVT combined with Iliac vein compression syndrome. Clin. Appl. Thromb. Hemost. 30, 10760296241283821 (2024).
Labropoulos, N., Borge, M., Pierce, K. & Pappas, P. J. Criteria for defining significant central vein stenosis with duplex ultrasound. J. Vasc Surg. 46, 101–107 (2007).
Sebastian, T. et al. Duplex ultrasound investigation for the detection of obstructed Iliocaval venous stents. Eur. J. Vasc. Endovasc. Surg. 60, 443–450 (2020).
Vedantham, S. et al. Delphi consensus on reporting standards in clinical studies for endovascular treatment of acute iliofemoral venous thrombosis and chronic iliofemoral venous obstruction. Circ. Cardiovasc. Interv. 16, e012894 (2023).
Lurie, F. et al. The 2020 update of the CEAP classification system and reporting standards. J. Vascular Surgery: Venous Lymphatic Disorders. 8, 342–352 (2020).
Rodgers, G. M. & Mahajerin, A. Antithrombin therapy: current state and future outlook. Clin. Appl. Thromb. Hemost. 29, 10760296231205279 (2023).
Barbhaiya, M. et al. The 2023 ACR / EULAR antiphospholipid syndrome classification criteria. Arthritis Rheumatol. 75, 1687–1702 (2023).
Padda, I. S., Patel, P. & Citla Sridhar, D. StatPearls. http://www.ncbi.nlm.nih.gov/books/NBK557814/ (StatPearls Publishing, 2025).
Kim, E. S. et al. Interpretation of peripheral arterial and venous doppler waveforms: A consensus statement from the society for vascular medicine and society for vascular ultrasound. Vasc Med. 25, 484–506 (2020).
Jalaie, H. et al. Prognostic value of a classification system for iliofemoral stenting in patients with chronic venous obstruction. Eur. J. Vasc Endovasc Surg. S1078-5884 (24), 00873–00876. https://doi.org/10.1016/j.ejvs.2024.10.002 (2024).
Sebastian, T. et al. Self-Expandable nitinol stents for the treatment of nonmalignant deep venous obstruction. Circ: Cardiovasc. Interventions. 13, e009673 (2020).
Menez, C. et al. Endovascular treatment of Post-thrombotic venous Ilio-Femoral occlusions: prognostic value of venous lesions caudal to the common femoral vein. Cardiovasc. Intervent Radiol. 42, 1117–1127 (2019).
David, A. et al. Short- and Mid-Term outcomes of endovascular stenting for the treatment of Post-Thrombotic syndrome due to iliofemoral and caval occlusive disease: A Multi-Centric study from the French society of diagnostic and interventional cardiovascular imaging (SFICV). Cardiovasc. Intervent Radiol. 45, 162–171 (2022).
Gautier, G. et al. Pharmaco-mechanical catheter-directed thrombolysis versus recanalization and stenting for post thrombotic syndrome after lower limb deep vein thrombosis: a comparative study. Quant. Imaging Med. Surg. 12, 1664–1673 (2022).
Knipp, B. S. et al. Factors associated with outcome after interventional treatment of symptomatic Iliac vein compression syndrome. J. Vasc Surg. 46, 743–749 (2007).
Neglén, P., Hollis, K. C., Olivier, J. & Raju, S. Stenting of the venous outflow in chronic venous disease: Long-term stent-related outcome, clinical, and hemodynamic result. J. Vasc. Surg. 46, 979–990 (2007).
Pouncey, A. L. et al. Risk factors and classification of reintervention following deep venous stenting for acute iliofemoral deep vein thrombosis. J. Vascular Surgery: Venous Lymphatic Disorders. 10, 1051–1058e3 (2022).
Visonà, A. et al. Post-thrombotic syndrome: A position paper from European society of vascular medicine. Vasa 50, 331–340 (2021).
Prandoni, P. et al. Incidence and predictors of post-thrombotic syndrome in patients with proximal DVT in a real-world setting: findings from the GARFIELD-VTE registry. J. Thromb. Thrombolysis. 57, 312–321 (2024).
Molnár, A. Á. et al. The aging venous system: from varicosities to vascular cognitive impairment. GeroScience 43, 2761–2784 (2021).
Farrell, J. J., Sutter, C., Tavri, S. & Patel, I. Incidence and interventions for post-thrombotic syndrome. Cardiovasc. Diagn. Ther. 6, 623–631 (2016).
Publishing, L. & CVDTeam. Role of duplex ultrasound investigation in the management of postthrombotic syndrome. Servier - Phlebolymphology. http://www.phlebolymphology.org/role-duplex-ultrasound-investigation-management-postthrombotic-syndrome/ (2016).
Gautam, G., Sebastian, T. & Klok, F. A. How to differentiate recurrent deep vein thrombosis from postthrombotic syndrome. Hamostaseologie 40, 280–291 (2020).
Cate-Hoek, T. Prevention and treatment of the post-thrombotic syndrome. Res. Pract. Thromb. Haemost. 2, 209–219 (2018).
Acknowledgements
We would like to thank Bachir Atallah for his valuable technical support in data analysis, which contributed to this study.
Author information
Authors and Affiliations
Contributions
YS, MM, LC, BE, CK, LD, ER data acquisition, YS, OE manuscript writing YS, OE, FD, BM study conception and data interpretation. All authors were involved in manuscript revision and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Skaff, Y., Douane, F., Chastaingt, L. et al. Impact of hemodynamic doppler ultrasound parameters on patency and post-thrombotic syndrome in patients with iliofemoral venous stents. Sci Rep 15, 33123 (2025). https://doi.org/10.1038/s41598-025-18327-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18327-w