Abstract
Background
Changes in platelet and white blood cell (WBC) counts during CRRT could identify patients at risk for adverse outcomes. We examined the association of change in platelet and WBC from pre-CRRT to during-CRRT with hospital mortality.
Methods
This multicenter, retrospective cohort study included 1,413 adults with AKI requiring CRRT at two tertiary medical centers (2011–2021). Platelet and WBC change from pre- to during CRRT were assessed as a percentage and categorized by standard deviation (SD). Multivariable LASSO regression and interaction analyses investigated associations with hospital mortality.
Results
A > 1 SD platelet count drop during CRRT was independently associated with hospital mortality (aOR: 1.82, 95% CI: 1.06, 3.13). A > 1 SD WBC count increase during CRRT did not significantly increase mortality (aOR 1.41, 95% CI: 0.88, 2.29). Interaction analyses revealed four sub-groups associated with increased mortality: pre-CRRT low platelet count that remained low, pre-CRRT normal platelet count that decreased, pre-CRRT elevated WBC count that remained high, and normal or elevated pre-CRRT WBC count that increased.
Interpretation
In critically ill adults, a drop in platelets after CRRT initiation was associated with increased hospital mortality. Monitoring platelet and WBC during CRRT in reference to pre-CRRT levels could help identify high-risk patients.
Similar content being viewed by others
Background
Critically ill adult patients with acute kidney injury (AKI) may require extracorporeal organ support, which includes continuous renal replacement therapy (CRRT). This vulnerable patient population has high morbidity and mortality1,2,3. Among different strategies to improve the care of these patients, accurate and actionable risk classification and sub-phenotyping to support more patient-specific interventions have gained recognition in critical care4,5,6. In this context, time-varying data during the provision of CRRT may be informative for clinical sub-phenotyping, particularly if linked to pathobiological pathways that could guide specific interventions7.
Platelet, white blood cell (WBC), and WBC subtype counts are essential indicators of hemostasis and immunoinflammatory responses8,9,10,11,12. In this context, some studies have examined the role of platelet and WBC parameters as predictors of mortality and renal function impairment in diverse critically ill populations, including those receiving CRRT11,12,13,14,15,16,17,18,19. Nevertheless, many of these studies have used static time points to assess platelet and WBC counts during CRRT treatment14,15,17,18,19,20,21. This approach may overlook the dynamic changes in these parameters as acute illness evolves. Notably, little is known about the association of change in platelet and WBC counts, specifically from pre- to during CRRT, with relevant clinical outcomes, such as hospital mortality.
The main objective of this study was to examine the association of change in platelet and WBC counts from pre-CRRT to during CRRT with hospital mortality in critically ill adults with AKI requiring CRRT. We hypothesized that a drop in platelet count and a rise in WBCs potentially signal dire underlying pathophysiology and hence associated with increased hospital mortality in this vulnerable patient population.
Methods
Study design and participants
This multicenter retrospective cohort study included critically ill adults with AKI who required CRRT during their intensive care unit stay at the University of Kentucky (UKY) Albert B. Chandler Hospital and the University of Texas Southwestern (UTSW) Medical Center between 2011 and 2021. Specifically, these two institutions used continuous veno-venous hemodiafiltration as the CRRT modality of choice and primarily regional citrate anticoagulation with acid citrate dextrose solution. HF1400® (UKY) and M100/M150® filters (UTSW) were used during the study period. Patients with coronavirus disease 2019 (COVID-19), end-stage renal disease or prior kidney transplant were excluded.
The study was approved, and the need to obtain informed consent was waived by the University of Kentucky and the University of Texas Southwestern Institutional Review Boards (IRBs 43159 and STU112015-069, respectively). All research in the study was performed in accordance with relevant guidelines/regulations and in accordance with the Declaration of Helsinki22.
Data extraction
Data were collected through automatic digital extraction from electronic health records, and ~ 10% of data were manually validated by individual record review. Patient demographics, comorbidities, laboratory tests, acute illness parameters, and final vital status were collected. The comorbidities were assessed by the Charlson Comorbidity Index23 and the severity of acute illness by the Sequential Organ Failure Assessment (SOFA) score24 on ICU admission and CRRT initiation days.
Study predictor variables
The predictor (independent) variables were percentage changes in platelet and WBC counts before and during CRRT. We calculated the average counts of platelets and WBCs during two specific timeframes on a per patient basis: up to 7 days preceding the initiation of CRRT and up to 7 days post-initiation of CRRT. The ‘up to 7 days’ period included all available laboratory values within those windows, without requiring the full 7-day data. The frequency of observations during the referred period is depicted in e-Figure 1. The percentage change was determined by dividing the difference in means between the two timeframes (delta) by the pre-CRRT mean. Next, we categorized the changes in platelet and WBC counts based on their deviation from the mean, using standard deviation (SD) as a benchmark: changes less than one SD (< 1 SD), within one SD (± 1 SD), and greater than one SD (> 1 SD) from the mean.
Study outcomes
The primary outcome was hospital mortality, defined as death recorded at the time of hospital discharge.
Statistical analyses
Means, SDs, medians, and interquartile ranges were used for describing continuous variables and counts and percentages for categorical variables. Baseline characteristics were stratified and compared by survival status. The comparisons were made using a t-test or Mann-Whitney U test for continuous variables and chi-squared test for categorical variables. Linear associations between platelet/WBC count change and hospital mortality were assessed with box-plot figures.
To address multicollinearity among ICU covariates and reduce the risk of overfitting in a high-dimensional dataset, we applied Least Absolute Shrinkage and Selection Operator (LASSO) regression for the primary analysis. This approach promotes model parsimony and enhances interpretability by shrinking less informative coefficients toward zero25. Multivariable LASSO regression analyses were performed using the percentage difference between means of platelets and WBCs before and during CRRT as the predictor variables and hospital mortality as the outcome of interest. The following candidate covariates were included based on clinical relevance and availability across both study sites: age, sex, race, study site, weight, Charlson Comorbidity Index (as a measure of comorbidity burden), SOFA score at ICU admission, SOFA score at CRRT initiation (to assess illness severity), mechanical ventilation status, baseline serum creatinine (as a proxy for kidney function), hospital length of stay, ICU length of stay, and CRRT duration. From this list, the following covariates were retained in the final multivariable models: age, Charlson Comorbidity Index, baseline serum creatinine (closest value 7-365 days prior to index admission with missing values back-calculated by resolving the estimated glomerular filtration rate [eGFR] EPI 26 to 75 ml/min/1.73m2), SOFA score at ICU admission and SOFA score at CRRT initiation.
Statistical analyses were performed using R version 4, and p < 0.05 was considered statistically significant.
Interaction analyses
Given that thrombocytopenia and leukocytosis before CRRT initiation might also be associated with hospital mortality, as supported by prior literature14,16,17,19,21,27,28, we used a combination of novel clustering exploratory tools and clinical insight to identify the 4-way interaction between platelet and WBC counts before CRRT and drop in platelets and rise in WBCs during CRRT.
Sensitivity analyses
Sensitivity analyses were conducted to assess the robustness of the primary findings by1 using a shorter 3-day window before and after CRRT initiation to calculate changes in platelet and WBC counts, and2 including patients with COVID-19 who were excluded from the primary analysis.
Results
Clinical characteristics
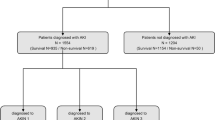
A total of 1413 critically ill adults with AKI on CRRT comprised the study cohort (Fig. 1). Among these, 753 (53.2%) died during the index hospitalization. Table 1 describes the cohort’s baseline and clinical characteristics, stratified by hospital mortality status. Supplemental e-Table1 describes baseline and clinical characteristics data stratified by study location, while e-Table 2 presents non-parametric measurements. Patients who died were similar to those who survived regarding sex, race, study location, and CRRT duration. In contrast, patients who died were older and had higher Charlson Comorbidity Index, weight, SOFA score, and more frequent use of mechanical ventilation (Table 1).
Study outcomes
Non-survivors had lower mean platelets and higher mean WBCs before and during CRRT than patients who survived (Table 1). The mean platelet count dropped 14% from pre-CRRT to during CRRT in the whole cohort (10% in those who survived versus 17.5% in patients who died, p = 0.003). The mean WBC count increased by 38.2% from pre-CRRT to during CRRT in the whole cohort (30.3% in those who survived versus 44.8% in patients who died, p = 0.005) (Table 1). Similar findings were observed in patient subgroups stratified by study location (e--Table1).
Adjusted models demonstrated a significant association between platelet drop from before to during CRRT and hospital mortality (Table 2). Patients with > 1 SD (62%) drop in platelets from before to during CRRT had an 82% increased risk of hospital mortality (adjusted odds ratio [aOR] 1.82, 95% confidence interval [CI] 1.06 to 3.13, p = 0.031). Every drop of 10% in platelets from before to during CRRT increased the adjusted odds of hospital mortality by 3% (aOR 1.03, CI 1.004–1.06, p < 0.05) (Table 2).
Adjusted models showed no independent association between > 1 SD rise in WBCs from before to during CRRT and hospital mortality (aOR 1.55, 95% CI 0.66 to 3.76, p = 0.162) (Table 3).
Figure 2 represents the adjusted odds for mortality according to the changes in platelets and WBCs from before to during CRRT, stratified by SD groups.
Change in platelets and WBC from pre-CRRT to during CRRT and in-hospital mortality. SD: Standard Deviation groups were stratified based on change in platelets/WBC after CRRT initiation= (mean of platelets/WBC after CRRT-mean of platelets/WBC before CRRT)/(mean of platelets/WBC before CRRT). A positive value indicated a rise in platelets/WBC after CRRT initiation; a negative value indicated a drop in platelets/WBC after CRRT initiation. The figure describes adjusted odds for mortality with patients with a platelet count and WBC change after CRRT initiation, stratifying by changing in platelets/ WBC after CRRT initiation SD groups. The middle lines of the boxes represent the median odds of mortality from adjusted models. Odds of mortality were adjusted for age, Charlson Comorbidity Index, baseline serum creatinine, SOFA score at ICU admission, and SOFA score at CRRT initiation.
While adjusting for the covariates used in the multivariable models, an interaction analysis revealed a statistically significant four-way interaction between four continuous variables (p < 0.001), i.e., platelet and WBC counts before CRRT, drop in platelets during CRRT, and rise in WBCs during CRRT. Due to the challenging nature of interpreting multi-way continuous variable interactions, we first categorized each continuous variables by < 1 SD, within 1 SD, or > 1 SD. The most frequently identified subgroups based on platelet and WBC changes from pre-CRRT to during CRRT also had the highest odds of hospital mortality in this cohort. This multi-pronged assessment identified four independent high-risk clinical subgroups: (1) Pre-CRRT low platelet count that remained low during CRRT (while pre-CRRT WBC count and rise in WBC count during CRRT is within 1 SD), (2) Pre-CRRT normal platelet count with a drop of > 1 SD during CRRT (while pre-CRRT WBC count and rise in WBC count during CRRT is within 1 SD), (3) Pre-CRRT elevated WBC count that remained high during CRRT (while pre-CRRT platelet count and drop in platelet count during CRRT is within 1 SD), and (4) Normal or elevated pre-CRRT WBC count that increased to > 1 SD during CRRT (while pre-CRRT platelet count and drop in platelet count during CRRT is within 1 SD) (Table 4). Supplemental e-Figs. 1 and e and 2 provide a graphic visualization of the patterns of WBCs and platelets for these four groups. Supplemental e-Figs. 3 and e and 4 describe density of measurements of platelets and WBC pre- and during CRRT.
We conducted sensitivity analyses using a shorter 3-day window to assess whether early changes in platelet and WBC counts after CRRT initiation could help identify high-risk patients. Although trends remained similar, the associations were weaker and not statistically significant. In a separate sensitivity analysis including patients with COVID-19, the associations also remained directionally consistent but not statistically significant. These patients were excluded from the primary analysis because COVID-19 is known to cause distinct immune and hematologic abnormalities, including thrombocytopenia, which could confound the relationship between platelet or WBC changes and hospital mortality29.
Discussion
Our study demonstrated that in critically ill adults with AKI requiring CRRT, a drop in platelets after CRRT initiation was independently associated with hospital mortality. We also showed that the combination of a drop in platelets and an increase in WBC from pre-CRRT to during CRRT could assist with identification of high-risk groups. Our study is unique as it evaluated the relationship between mortality and changes in platelets and WBCs over an extended period (up to 7 days before and after CRRT initiation) in critically ill patients with AKI requiring CRRT. The evaluation of change in platelet and leukocyte counts up to 7 days of CRRT was determined as the mean time on CRRT in this multicenter cohort was 7.6 days.
During CRRT, a drop in platelets can occur due to platelet activation and consumption caused by activation of the coagulation system and contact with extracorporeal surfaces. Additionally, the hemofilter may contribute to either destruction or retention of platelets during passage, which appeared attenuated by higher blood flows30. Thrombocytopenia, commonly defined as a platelet count below 150 × 109/L, increases the risk of bleeding and can indicate inflammation, poor tissue integrity, and impaired immune response16,27,31. A drop in platelet count before or during CRRT has also been associated with an increased risk of secondary infection20. In our analysis, patients with severely low pre-CRRT platelet count (< 55 × 109/L), which remained low during CRRT (± 1 SD), had an increased adjusted odds of hospital mortality by 40%. Similarly, in patients with relatively normal platelet count (> 55 × 109/L), a drop in platelets > 62% (> 1 SD) during CRRT increased the adjusted odds of hospital mortality by 60% (Table 4, e-Figure 1).
We conducted sensitivity analyses using a shorter 3-day window, instead of the 7-day period used in the primary analysis, to evaluate whether early changes in platelet and WBC counts after CRRT initiation could help identify high-risk patients. In clinical practice, prognosis may already be evident after seven days of CRRT, making later changes less useful for early risk classification. Additionally, some patients in our cohort received CRRT for only a few days, introducing variability. Although the results from the 3-day window showed similar directional trends, the associations were weaker and not statistically significant, suggesting that the longer 7-day window may better capture meaningful changes in platelet and WBC counts during CRRT.
Previous studies have found that a drop in platelet count after CRRT initiation was associated with mortality at various time points14,15,17,21. A decrease in platelet count (mean decrease of 33% from pre-CRRT baseline) within 48 h following CRRT initiation was independently associated with increased 60-day mortality (OR = 1.51, 95% CI = 1.12–2.02)17. Another study showed increasd 90-day mortality in patients with ≥ 50% drop in platelet count from the first measurement at CRRT start to within three days of CRRT initiation (59.0% vs. 35.0%, p = 0.012)14. One similar study found that a decline of ≥ 60% in platelets from the first measurement from CRRT start to within four days of CRRT initiation was independently associated with 90-day mortality (hazard ratio = 1.93, 95% CI = 1.23–3.05)21. While the association between platelet drop after CRRT initiation and mortality has been consistently established, using measurements in an extended period pre-CRRT and during CRRT is unique to our study.
Instead of relying on a single reference measurement and the lowest value following 2–4 days of CRRT14,17,21, we utilized multiple measurements of platelets and WBCs up to 7 days before (mean [SD], 4.0 [2.4] days) and 7 days after (mean [SD], 6.1 [1.4] days) CRRT initiation to calculate the drop in platelets. This strategy offers several advantages: it captures overall trends, reduces the influence of isolated outlier values, and accommodates variability in the timing and frequency of lab draws, which is common in critically ill populations. However, this approach also has limitations. Averaging measurements over a longer period may dilute sharp, clinically significant changes—such as an abrupt drop in platelet count—that the nadir method is designed to capture. As a result, the sensitivity of our model to detect short-term but important prognostic changes may be reduced. By contrast, nadir or peak-based methods may be more responsive to rapid shifts but are also more vulnerable to measurement timing and outlier influence. Our findings suggest that the 7-day averaging approach provides a more stable and comprehensive picture of platelet and WBC dynamics, but future studies could explore combining both approaches to optimize clinical prediction and subgroup identification.
During CRRT, alterations in WBC counts may occur due to several underlying factors, including inflammatory response, infections, activation of blood components during the CRRT process, medications, and pre-existing medical conditions13,32. Both leukopenia and leukocytosis are associated with AKI13,33 and mortality risk in critically ill patients13. Additionally, lymphopenia is linked to mortality and AKI risk in ICU patients with sepsis13. Our study found a non-significant association between the rise of WBCs from pre- to during CRRT and hospital mortality (Table 3). Although we did not include information regarding subtypes of WBCs, we identified that patients with severely elevated pre-CRRT WBCs (> 26.86 × 109/L), which remained high during CRRT (± 1 SD), had an increased adjusted odds of hospital mortality by 88%. Similarly, in patients with relatively normal pre-CRRT WBCs (2.05 to 26.86 × 109/L), a rise in WBCs during CRRT > 135.45% (> 1 SD) increased the adjusted odds of hospital mortality by 73% (Table 4 and e-Figure 2).
Some limitations of this study should be noted. First, this observational study is subject to selection and indication biases related to study populations and CRRT practices specific to the two academic study sites. Second, the study lacked key baseline ICU-level clinical details—such as admission source, primary diagnosis, and use of additional critical care interventions (e.g., vasopressors)—because these variables were not routinely or consistently documented in the electronic health record systems across the two participating hospitals during the study period. This limitation reduces our ability to fully characterize cohort heterogeneity and adjust for potential confounders related to illness severity and ICU context. Although we accounted for several relevant factors, including demographics, comorbidities, and illness acuity in our multivariable models, residual confounding from unmeasured variables may still be present. Third, we did not have information regarding potential causes for changes in platelets or WBCs, such as heparin-induced thrombocytopenia, infections, specific anticoagulation use for CRRT, blood product transfusions, or administration of steroids or other immunosuppressive medications. Therefore, this study cannot establish a causal relationship between platelet and WBC changes from pre-CRRT to during CRRT and mortality. Additionally, we did not include baseline platelet and WBC counts in the multivariable model, despite their known prognostic value, because our primary focus was on percentage change, which better captures dynamic trends during CRRT. Including both baseline values and their corresponding change measures could introduce multicollinearity due to their mathematical dependency; therefore, we prioritized the change variables to preserve model stability and interpretability. Finally, the utilized platelet and WBC exploratory cutoffs, determined based on SDs, are not intended to serve as clinical cutoffs. Rather, their purpose is to inform future research to assess the specificity and utility of these cutoffs in clinical sub-phenotyping.
Our study has several strengths that needs to be highlighted. First, we used a large sample of adult patients from two large academic institutions. Second, we carefully evaluated the change in platelet and WBC counts from pre- to during CRRT utilizing multiple measures up to 7 days before CRRT initiation and up to 7 days during CRRT. This differentiates our study from prior studies that used single time points subject to day-to-day laboratory and clinical variation. Third, we used multivariable regression and interaction terms to identify high-risk subgroups of patients with increased adjusted odds of mortality based on pre-CRRT and change during CRRT of platelet and WBC counts. This reflects a step towards more precise use of dynamic data for clinical sub-phenotyping, although further validation is needed.
Conclusions
In conclusion, a drop in platelets from up to 7 days before to 7 days after CRRT initiation was independently associated with hospital mortality in critically ill adults with AKI requiring CRRT. The dynamic monitoring of platelets and leukocyte counts during CRRT in reference to pre-CRRT levels could assist with identification of high-risk groups. Further, the functional aspect of platelets and leukocytes in patients exposed to CRRT should be investigated.
Data availability
Data utilized in this study is available upon reasonable request via contact with the corresponding author.
Abbreviations
- AKI:
-
acute kidney injury
- aOR:
-
adjusted odds ratio
- CI:
-
confidence interval
- CRRT:
-
continuous renal replacement therapy
- ICU:
-
intensive care unit
- SD:
-
standard deviation
- SOFA:
-
Sequential Organ Failure Assessment
- WBC:
-
white blood cell
References
Hoste, E. A. et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 41 (8), 1411–1423 (2015).
Sileanu, F. E. et al. AKI in low-risk versus high-risk patients in intensive care. Clin. J. Am. Soc. Nephrol. 10 (2), 187–196 (2015).
Brochard, L. et al. An Official ATS/ERS/ESICM/SCCM/SRLF Statement: Prevention and Management of Acute Renal Failure in the ICU Patient: an international consensus conference in intensive care medicine. Am J Respir Crit Care Med. ;181(10):1128-55. (2010).
Joannes-Boyau, O., Velly, L. & Ichai, C. Optimizing continuous renal replacement therapy in the ICU: A team strategy. Curr. Opin. Crit. Care. 24 (6), 476–482 (2018).
Ostermann, M. et al. Patient selection and timing of continuous renal replacement therapy. Blood Purif. 42 (3), 224–237 (2016).
Karkar, A. & Ronco, C. Prescription of CRRT: A pathway to optimize therapy. Ann. Intensive Care. 10 (1), 32 (2020).
Teixeira, J. P., Neyra, J. A. & Tolwani, A. Continuous KRT: A contemporary review. Clin. J. Am. Soc. Nephrol. (2022).
Proctor, M. J. et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow inflammation outcome study. Eur. J. Cancer. 47 (17), 2633–2641 (2011).
Smith, R. A. et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am. J. Surg. 197 (4), 466–472 (2009).
Azab, B., Shah, N., Akerman, M. & McGinn, J. T. Jr Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J. Thromb. Thrombolysis. 34 (3), 326–334 (2012).
Gameiro, J., Fonseca, J. A., Jorge, S., Gouveia, J. & Lopes, J. A. Neutrophil, lymphocyte and platelet ratio as a predictor of mortality in septic-acute kidney injury patients. Nefrologia (Engl Ed). 40 (4), 461–468 (2020).
Gameiro, J. et al. Neutrophil, lymphocyte and platelet ratio as a predictor of postoperative acute kidney injury in major abdominal surgery. BMC Nephrol. 19 (1), 320 (2018).
Han, S. S. et al. U-shape relationship of white blood cells with acute kidney injury and mortality in critically ill patients. Tohoku J. Exp. Med. 232 (3), 177–185 (2014).
Wu, B. et al. Decreased platelet count in patients receiving continuous veno-venous hemofiltration: A single-center retrospective study. PLoS One. 9 (5), e97286 (2014).
Guru, P. K., Singh, T. D., Akhoundi, A. & Kashani, K. B. Association of thrombocytopenia and mortality in critically ill patients on continuous renal replacement therapy. Nephron 133 (3), 175–182 (2016).
Droege, C. A., Ernst, N. E., Messinger, N. J., Burns, A. M. & Mueller, E. W. Evaluation of thrombocytopenia in critically ill patients receiving continuous renal replacement therapy. Ann. Pharmacother. 52 (12), 1204–1210 (2018).
Griffin, B. R. et al. Effects of baseline thrombocytopenia and platelet decrease following renal replacement therapy initiation in patients with severe acute kidney injury. Crit. Care Med. 47 (4), e325–e31 (2019).
Griffin, B. R. et al. Platelet decreases following continuous renal replacement therapy initiation as a novel risk factor for renal nonrecovery. Blood Purif. 51 (7), 559–566 (2022).
Jeon, Y. H., Jeon, Y., Jung, H-Y., Choi, J-Y. & Park, S-H. U-Shaped association between platelet-to-lymphocyte ratio and in-hospital mortality in critically ill patients with acute kidney injury requiring continuous renal replacement therapy: A retrospective observational cohort study. PREPRINT (Version 1). (2022).
Griffin, B. R. et al. The association of platelet decrease following continuous renal replacement therapy initiation and increased rates of secondary infections. Crit. Care Med. 49 (2), e130–e9 (2021).
Lin, J. et al. SOFA coagulation score and changes in platelet counts in severe acute kidney injury: analysis from the randomized evaluation of normal versus augmented level (RENAL) study. Nephrol. (Carlton). 24 (5), 518–525 (2019).
World Medical Association Declaration. Of helsinki: Ethical principles for medical research involving human subjects. Jama 310 (20), 2191–2194 (2013).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40 (5), 373–383 (1987).
Vincent, J. L. et al. The SOFA (Sepsis-related organ failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-Related problems of the European society of intensive care medicine. Intensive Care Med. 22 (7), 707–710 (1996).
Tibshirani, R. Regression shrinkage and selection via the lasso: a retrospective. J. Royal Stat. Society: Ser. B (Statistical Methodology). 73 (3), 273–282 (2011).
Inker, L. A. et al. New Creatinine- and Cystatin C-Based equations to estimate GFR without race. N Engl. J. Med. 385 (19), 1737–1749 (2021).
Dewitte, A. et al. Blood platelets and sepsis pathophysiology: A new therapeutic prospect in critically [corrected] ill patients? Ann. Intensive Care. 7 (1), 115 (2017).
Erlinger, T. P. et al. Leukocytosis, hypoalbuminemia, and the risk for chronic kidney disease in US adults. Am. J. Kidney Dis. 42 (2), 256–263 (2003).
Terpos, E. et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 95 (7), 834–847 (2020).
Mulder, J., Tan, H. K., Bellomo, R. & Silvester, W. Platelet loss across the hemofilter during continuous hemofiltration. Int. J. Artif. Organs. 26 (10), 906–912 (2003).
Zarychanski, R. & Houston, D. S. Assessing thrombocytopenia in the intensive care unit: the past, present, and future. Hematol. Am. Soc. Hematol. Educ. Program. 2017 (1), 660–666 (2017).
Fried, L. et al. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J. Am. Soc. Nephrol. 15 (12), 3184–3191 (2004).
Bagshaw, S. M., George, C., Bellomo, R. & Committee, A. D. M. Early acute kidney injury and sepsis: a multicentre evaluation. Crit. Care. 12 (2), R47 (2008).
Acknowledgements
JWL and JAN conceived the study design. JWL, LJL and VOS managed data extraction, transformation, and quality control. JWL performed statistical analyses. LNB, CGB, ACO, and JAN drafted the manuscript. OWM, RDT, AJT and JAN provided resources for the conduction of the study. All authors revised the manuscript, incorporated feedback, and approved the final version. JAN is supported by grants from NIH (R01DK128208, R01DK133539, U01DK12998, and U54DK137307). OWM is supported by grants from NIH (R01DK081423, R01 DK115703, R01 DK091392, and R01 DK092461). Guarantor: Javier A. Neyra is the guarantor of the content of the manuscript including the data and analysis. Notation of previous abstract publication/presentation: Poster presentation at American Society of Nephrology Kidney Week. November 3, 2023. Philadelphia, Pennsylvania.
Funding
This work was funded in part by NIH R01DK133539. JAN is supported by grants from NIH (R01DK128208, R01DK133539, U01DK12998, and U54DK137307). OWM is supported by grants from NIH (R01DK081423, R01 DK115703, R01 DK091392, and R01 DK092461). All other authors report no conflict of interest.
Author information
Authors and Affiliations
Contributions
JWL and JAN conceived the study design. JWL, LJL and VOS managed data extraction, transformation, and quality control. JWL performed statistical analyses. LNB, CGB, ACO, and JAN drafted the manuscript. OWM, RDT, AJT and JAN provided resources for the conduction of the study. All authors revised the manuscript, incorporated feedback, and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the University of Kentucky and the University of Texas Southwestern Institutional Review Boards (IRBs 43159 and STU112015-069, respectively). A waiver of informed consent was obtained given the retrospective nature of the investigation.
Consent for publication
This manuscript has not been published elsewhere and is not under consideration by any other journal. All authors have approved the manuscript and agree with its submission to Journal of Intensive Care.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bui, L.N., Lambert, J.W., Braun, C.G. et al. Change in platelet and leukocyte counts and hospital mortality in adults with acute kidney injury receiving continuous renal replacement therapy. Sci Rep 15, 33553 (2025). https://doi.org/10.1038/s41598-025-18452-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18452-6