Abstract
Airborne influenza infections cause significant disease in animals and people. However, there is limited information on the dynamics of viral emissions and size distribution of airborne virus-laden particles generated by infected animals. In this study, we used pigs as a model for the airborne transmission of influenza A virus (IAV) and we quantified nasal shedding, viral RNA load of airborne particles emitted from pigs experimentally infected with a swine-origin H1N1 IAV, and characterized the size distribution of the virus-laden particles generated from infected pigs over the course of infection. We found that the peak of nasal shedding and airborne IAV-laden particles across multiple size ranges took place at 2 days post inoculation (DPI), with higher viral RNA load found in larger particles. The amount of airborne IAV emitted by infected pigs over the course of infection in particles > 8 μm was significantly higher than that in particles between 0.22 and 1.7 μm. These findings help understand the risk of airborne transmission of IAV in pigs and provide information to help control airborne infections more effectively.
Similar content being viewed by others
Introduction
Airborne transmission of infectious agents has been documented for many years, and airborne pathogens are some of the most difficult to control and contain1,2,3. Airborne pathogens such as influenza virus and severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) have caused dramatic losses of human life and have led to economic and social disruptions worldwide. In livestock, airborne viruses such as porcine reproductive and respiratory syndrome virus (PRRSV), influenza A virus (IAV), foot-and-mouth disease virus (FMDV) and porcine epidemic diarrhea virus (PEDV)4,5,6,7 have had significant economic impact and have posted challenges to food security and public health.
Influenza A virus (IAV) is an important respiratory pathogen of both human and animals8. In humans, IAVs cause annual epidemics of varying severity, including mild common cold symptoms to severe lung injury with fatal outcomes9, and have been responsible for several known pandemics10,11,12. IAVs pose a constant threat to public health due to its potential to generate new reassorted IAV derived from strains of various species, such as the 2009 H1N1 pandemic strain, which was derived from avian, human and swine influenza viruses from Europe, Asia, and North America13. IAVs can be found in the air of public places such as health care centers, day-care centers, airplanes, agricultural fairs and live animal markets14,15,16,17. The concentrations of airborne IAV varies among settings and values ranging between 5,800 and 37,000 RNA copies/m3 of air17 have been reported. IAVs have been shown to transmit bidirectionally between human and swine18,19 and during outbreaks in swine, airborne IAV can be detected inside barns for approximately 20 days. Concentrations ranging from 104 to 107 RNA copies/m3 of air have been reported representing a risk to both swine and people20. In addition, IAV has also been found in aerosol samples collected in exhausted air and downwind at up to 2.1 km from infected farms5, indicating the potential for regional transmission.
When airborne viruses spread, they are typically incorporated into particles of varying composition including not only respiratory secretions but also fecal material, dust, debris, water droplets, and in animal premises, bedding and hair particles21. The size of the virus-laden particles largely determines the behavior of the particles22,23. Sufficiently large particles, tens of micrometers in diameter, may settle quickly on surfaces or mucous membranes, while smaller particles can remain in the air for sustained periods of time24. Upon being inhaled, particles of different sizes can deposit in different parts of the respiratory tract25, which may cause different adverse health effects26. Particles of different sizes may carry different viral loads, leading to different levels of infectivity22,27. Exploration of the relationship between particle size, viral load and infectivity of airborne particles is necessary to understand how the viral diseases spread and help refine the control measures.
Distribution of IAV particle size varies between studies and settings. IAV was found in particles 1–4 μm in diameter (49% of particles) and particles > 4 μm (46% of particles) in health care facilities16, and particles < 2.5 μm (64% of viral copies) when detected in public places17. SARS-CoV-2 was detected in 17.7% of submicron particles (0.18–1 μm) and 81.9% of supermicron particles (> 1 μm) collected in a health care facility and in home care environments28. A few studies have reported successful isolation and quantification of infectious SARS-CoV-2 in aerosol samples from hospital and residential settings with COVID-19 patients29,30. In addition, there is limited information on the size, viral load and infectivity of airborne virus-laden particles that infected animals generate. Zhou et al. devised a transmission chamber that separates virus-laden particles by size, and found ferret-to-ferret influenza transmission was mediated by airborne particles larger than 1.5 µm31. Alonso et al. reported that IAV was detected in all particle size ranges from 0.4 μm to > 9 μm generated by experimentally infected pigs. However, infectious viruses were only isolated from particles larger than 2.1 µm21. It remains unknown whether the viral load and size distribution of virus-laden particles changes over the course of infection, which could have implications for disease control.
Understanding the dynamics of viral emissions from infected individuals and quantifying the viral load released into the air following infection could help investigate the epidemiology of the viruses and develop more effective strategies to control them. Milton et al. found that viral copy numbers in exhaled breath of influenza patients declined rapidly with day after onset of illness. Fine particles (≤ 5 μm) contained more influenza RNA copies, while surgical masks were more effective to reduce viruses in the coarse fraction (> 5 μm)42. Gustin et al. reported that ferrets infected by highly transmissible influenza viruses exhaled more infectious virus within aerosol particles < 5 μm than ferrets infected by influenza viruses that did not readily transmit32. Pulit-Penaloza et al. found that efficient ferret-to-ferret transmission through the air is directly associated with early release in the course of infection of influenza A virus-laden particles, especially particles > 4 µm33. Quantification of airborne viruses could be considered when assessing the potential of transmission for new influenza virus strains33, as the establishment of infection in the respiratory tract of a susceptible host requires exposure to a sufficient amount of infectious viruses27. Yezli and Otter reviewed the infectious doses reported for influenza viruses determined from experimental infections of human volunteers, and suggested that the infectious dose of IAV airborne infection was several orders of magnitude lower than that of nasal infection34. Doses of 3 median tissue culture infectious dose (TCID50) of influenza virus were reported to infect 50% of the tested volunteers through small particle aerosols35. Yang et al. estimated the inhalation dose over 1 h for an adult to be 30 ± 18 TCID50, based on the average concentration of airborne IAV in public places, which should be adequate to induce infection17. In ferrets, the 50% ferret infectious dose (FID50) for aerosol delivery of human influenza virus was estimated at 1.9 PFU, while the ferrets infected by a human influenza virus were found to exhale infectious virus at rates of 1 to 4 PFU/min32. These findings highlight the importance of quantifying airborne viruses to assess the potential of influenza aerosol transmission.
In our study, we utilized connected animal isolator chambers that permitted unidirectional air airflow from the infected to the sentinel pigs without allowing direct contact among the pigs (Fig. 1). We quantified nasal shedding and viral RNA load in airborne particles emitted from pigs experimentally infected with a swine-origin H1N1 influenza A virus. We characterized the size distribution and viral RNA load of airborne virus-laden particles generated from infected pigs over the course of infection using Andersen cascade impactors (ACI), which are aerosol samplers capable of collecting airborne particles and separating them by aerodynamic size. We aimed at better understanding IAV airborne transmission in pigs and providing information to design more effective infection control measures.
Results
Quantification of virus RNA nasal shedding and airborne virus RNA-laden particles from pigs
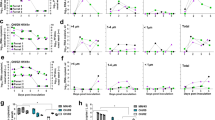
In order to quantify shedding of viral RNA in IAV-infected pigs and in aerosols collected from the environment housing the infected pigs, four tests were conducted using three animal isolators connected with air-permeable ducts (Fig. 1). In each test, four pigs inoculated with IAV were placed in two connected isolators (isolators 1 and 2), and two uninoculated sentinel pigs were placed in isolator 3, with the airflow moving unidirectionally from inoculated pigs to sentinel pigs. Mean IAV RNA concentrations in nasal swabs collected daily from inoculated pigs in each of the four tests are shown in Fig. 2. In the inoculated pigs, IAV RNA was detected in nasal swabs from 1 DPI through 6 DPI which is when the study was concluded, and the viral RNA concentrations ranged between 4.85 and 9.12 log10 RNA copies/mL. The peak nasal shedding was at 2 DPI, with a mean concentration of 8.24 log10 RNA copies/mL. The nasal shedding at 2, 3, and 4 DPI was significantly higher than at 6 DPI (p < 0.05). Sentinel pigs became infected after 1 day of exposure to the infectious aerosols (results not shown).
Influenza A virus RNA concentration in nasal swabs of inoculated pigs collected daily (a) and geometric mean concentration of all the nasal swabs collected each day from the inoculated pigs (b). Error bars represent one geometric standard deviation and statistical differences are shown as (*) p < 0.05.
Mean IAV RNA concentration in the aerosol samples collected by ACIs each day is shown in Table 1. IAV RNA was detected in the air from inoculated pigs as early as 1 DPI and daily throughout 6 DPI which is when the study was terminated. IAV RNA concentration ranged between 1.21 × 103 and 6.14 × 105 RNA copies/m3. The peak of airborne IAV RNA concentration was at 2 DPI (2.83 × 105 RNA copies/m3 on average), which was significantly higher than that at 6 DPI (p < 0.05).
Size distribution and viral RNA load of virus-laden particles over the course of IAV infection
In order to size the infectious aerosols emitted by the inoculated pigs throughout the course of the IAV infection, the concentration of airborne IAV RNA by particle size range emitted daily in each test was determined by sampling aerosols using ACIs capable of separating the virus-laden particles in nine size ranges. Results of mean IAV RNA concentrations for the four tests are shown in Table 2. Results of each duplicate sampling for each test are shown in Table S1. The mean size distribution of the four tests is illustrated in Fig. 3 while the mean size distribution with one standard deviation for each day are shown in Supplemental Fig. S1. Viral RNA was detected as early as 1 DPI throughout 6 DPI in particles of all size ranges tested (0.22 to > 8 μm) except from particles < 0.22 μm at 6 DPI.
To determine whether the release of viral RNA load in aerosol emissions over the course of infection were significantly different among particle size ranges, we plotted viral RNA concentration in the air from 0 to 6 DPI for each particle size range (Fig. 4) and compared the values of the area under the curve (AUC) (Fig. 5). The results comparing the AUC revealed that the viral RNA load in aerosol emissions from 1 DPI throughout 6 DPI in particles > 8 μm were significantly higher than those in particles 0.22–1.7 μm (p < 0.05) (Fig. 5). A downtrend of airborne viral concentration from 2 DPI to 6 DPI was observed in multiple particle size ranges (Fig. 4). No viable viruses were isolated from any of the aerosol samples collected using the ACIs in this study.
Quantification of influenza viral RNA emitted in the air by inoculated pigs over the course of infection calculated as the area under the curve (AUC) in each particle size range. Error bars represent one standard deviation. Letters on each bar are used to report results of pairwise comparisons; bars with no shared letters indicate there are significant differences (p < 0.05). The significance level was adjusted using the Bonferroni method.
Size distribution of total particles over the course of influenza infection
Total airborne particle number concentrations from inoculated pigs were measured using an optical particle counter (OPC) in Test 4. The daily size distribution of total airborne particles is shown for the course of IAV infection (Fig. 6). The shape of the particle size distribution stayed similar throughout the course of test 4, although there was variation in the number concentration (Fig. 6). Total airborne particle concentration measured each day was plotted by particle size range in Fig. 7 and summarized in Fig. S2. An increase of the number concentration was observed from 2 DPI to 5 DPI in particles across all size ranges (0.3 μm – > 10 μm). There were more particles within the first submicrometer size bin (0.3–5 μm) than in larger size bins (Fig. S2).
Discussion
Understanding the dynamics of viral emission and size distribution of airborne viral particles generated by infected animals is essential to better understand viral airborne transmission and to establish more effective disease control measures. In this study, we characterized the nasal shedding, and the concentration and size distribution of virus-laden particles and total particles generated from pigs experimentally infected with influenza over the course of infection. Influenza is an important respiratory disease that affects animals and people, and the results can be used to better quantify the risks of airborne influenza transmission in animals and people. We report that the concentration of IAV RNA in nasal shedding was highest at days 2–4 post infection, highest in aerosols at 2 DPI and that IAV RNA could be detected in multiple particle size ranges with higher viral RNA load found in larger particles over the course of IAV infection.
Robust nasal shedding and early emissions of viral RNA into the air at sufficient levels for transmission might be two indicators of highly transmissible influenza viruses33. The swine-origin H1N1 influenza virus evaluated in this study was detected in aerosols as early as 1 DPI and peaked at 2 DPI, showing its potential of high transmissibility by infecting sentinel pigs within 1 day of being exposed to infectious aerosols. The viral RNA loaded across a wide size range of particles over the course of infection indicate that the virus might be transmitted simultaneously across both short and long distances. In addition, the increase of total particle numbers across multiple size ranges over time was considered to benefit virus dissemination during infection36. This information can help predict the potential for emerging viruses to become airborne, assess the scale and losses of outbreaks, and design prevention strategies in advance, e.g. applying chemical disinfectant to the environment and fomites, or installing air filtration systems or some other aerosol biocontainment technologies.
In Alonso et al. (2015), airborne IAV emitted by experimentally infected pigs was detected in particles ranging from 0.4 to 10 µm21which is in line with our results. Alonso et al. also isolated viable IAV from particles larger than 2.1 µm21. In our study, we were not able to isolate viable viruses from any of the aerosol samples, despite the fact that infection of sentinel pigs happened shortly after IAV pig inoculation indicating that viable viruses were being transmitted through the air. The air flow rate of 850 L/min used in the isolator system resulted in very high air exchange rates of 28.5 per hour, and the ACI sampling methods might have induced a portion of virus losses and inactivation, which may have affected the isolation of viruses from aerosol samples. Thus, we focused our analysis on using RNA copies even though PCR analysis does not distinguish between viable and non-viable virus although the infectivity of the virus is the parameter of real concern. Unfortunately, inability to isolate viruses from aerosols is a common objection to aerosol studies, including the study presented here. Future studies using viability PCRs may be able to circumvent the lack of virus isolation from aerosols.
The use of an ACI for collecting aerosols enabled the separation of particles by size range. Prior research has shown that solid particles may bounce upon impact with the collection plates of the ACI and travel to a lower stage, leading to an incorrect sizing of the particles. However, a previous study37 concluded that particle bounce may not always be an issue when using the Andersen impactor to sample viral aerosols. Furthermore, traditional sticky coating materials such as grease may be virucidal or cytotoxic to cell cultures necessary to isolate the viruses. Given this information, and the fact that certain coating materials may cause cell culture toxicity and affect virus viability, ultimately, we decided not to coat the collection plates in this study.
Results from this study also support the role of the aerosol route to transmit IAV in pigs since sentinel pigs became infected within one day of inoculating the principal pigs. These results suggest that the dose required to infect pigs through aerosols may be low. Investigating the minimum infectious dose required to infect individuals through the airborne route is important in order to assess the risk of transmission and to implement proper control measures. Previous studies suggested that minimum infectious dose of IAV through nasal drops could be several orders of magnitude higher than that through aerosols37. Our study provided data on IAV RNA copies in aerosols that can be used to estimate IAV exposure levels but given that no viable virus was isolated from the air samples, the IAV infectious dose required to infect pigs still needs to be elucidated.
In summary, under the conditions of this study, the swine-origin H1N1 IAV was transmitted through aerosols and infected naïve sentinel pigs very rapidly. IAV-laden particles across a wide size range were generated and became airborne shortly after infection and as early as 1 DPI, with higher viral RNA load found in larger particles over the course of infection. Overall, these results support the role of airborne transmission of influenza and highlight that the risk of infectious aerosols may be higher shortly after the onset of the infections. These results are helpful to further understand the risk of airborne influenza in animals and people and highlight the need to continue developing effective control measures against airborne viruses.
Materials and methods
All methods were carried out in accordance with relevant guidelines and regulations and reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Experimental setup
A modular animal isolator system specially designed to house animals under BSL-2 conditions was used to investigate the airborne viral particles of IAV emitted from infected pigs (Fig. 1). The system consisted of three air-tight self-contained isolators built of stainless steel and plexiglass materials capable of operating under negative ventilation conditions. Isolators 1 and 2 were connected through a 17-inch-long modular door connector equipped with two grids that only allowed air flow. Another identical connector and a four-foot-long air-permeable tunnel were installed between isolator 2 and isolator 3. In each test, there were four pigs inoculated with IAV and then placed in isolators 1 and 2 (two pigs per isolator). In isolator 3, there were two uninoculated pigs serving as sentinels. The airflow inside the isolator system moved unidirectionally from inoculated pigs to sentinel pigs, which was generated by a blower connected to the air outlet in isolator 3. The air flow rate was set at 850 L/min (30 cfm) making the air change rate of 28.5 per hour inside the system. The incoming and exhaust air of the system was filtered using HEPA filters. A dehumidifier was connected to the entrance of the air in isolator 1 to keep the relative humidity (RH) inside the system within 40–60%. The temperature inside the system was kept at 78–80 °F by the heaters in each isolator. Feeders and waterers were available in each isolator, ensuring the pigs had free access to food and water throughout the duration of the study. Sampling ports, located near the top of each side of the isolators, were used for air sampling.
Animal infection
A total of 24 eighteen to twenty-one-day-old weaned pigs of either sex (6 pigs per test) were purchased from a closed herd negative for IAV and other diseases of importance to pigs (e.g. PRRSV, Mycoplasma hyopneumoniae). Pigs were sampled by collecting blood and nasal swabs on arrival at the isolation units and confirmed negative for IAV by RT-PCR and ELISA (Veterinary Diagnostic Laboratory, University of Minnesota). In each test, two out of six pigs (sentinels) were moved into isolator 3 at 72 h after arrival, and the other four pigs were inoculated intranasally with 2 mL (1 mL per nostril) of 105.75 TCID50/mL H1N1 influenza virus inoculum and placed into isolators 1 and 2 (two pigs per isolator). The materials and methods of virus growth for IAV are described in Supplementary Materials S1. Clinical signs were recorded for all pigs daily throughout the duration of the study. All pigs were euthanized using an overdose of a pentobarbital solution. Protocols and procedures followed throughout the study were approved by the University of Minnesota Institutional Animal Care and Use Committee (Protocol ID: 2306–41177 A), and the Institutional Biosafety Committee (Protocol ID: 2108–39310 H).
Sampling procedures
Pigs were removed from the isolator and manually restrained for daily sampling. In order to prevent cross-contamination between groups, the sentinel pigs from isolator 3 were sampled first. Then the feeder and waterer were refilled and the waste inside the isolator cleaned. After placing the sentinel pigs back into their isolator and locking the door, these procedures were repeated in the inoculated pigs. Personal protective equipment (PPE) consisting of N95 mask, disposable nitrile gloves, hair net, Tyvek® coverall, goggles, face shield and disposable plastic gown were worn by the researchers handling the pigs. Researchers changed gloves and plastic gown each time after handling pigs from the previous group and before proceeding to the next group. Tools used for feed and water were designated for each isolator and decontaminated every day. Sentinel and inoculated pigs were sampled daily by collecting nasal swabs (BBL CultureSwab™, liquid Stuart single plastic applicator; Becton, Dickinson and Com. Sparks, MD) from 0 to 6 days post IAV inoculation.
To measure the concentrations of airborne IAV and the virus-laden particle size distribution generated by inoculated pigs, aerosol samples were collected twice per day through the sampling port of isolator 2 using an Andersen cascade impactor (ACI). The ACI is an aerosol sampler capable of collecting airborne particles and separating them by aerodynamic size in up to 9 intervals, ranging from < 0.22 μm to > 8 μm, when operating at 90 L/min. Aerosol samples were collected for 30 min in duplicate each day. After collection, samples were eluted directly from each ACI collection plate using a cell scraper and 3 mL of virus transport media, composed of DMEM supplemented with 2% of 7.5% bovine serum albumin fraction V (Life Technologies, Grand Island, NY), 1.5 µg/mL trypsin-TPCK, 1X antibiotic-antimycotic (Life Technologies, Grand Island, NY) and 50 µg/mL gentamicin (Life Technologies, Grand Island, NY). The filter, which was a glass microfiber filter, on the final ACI stage was eluted with 15 mL of virus transport media. Samples from each of the ACI plates and filter were eluted and stored separately and divided into two aliquots, placed on ice and transported to the laboratory. The sizes and concentrations of the total airborne particles inside the system were measured from the sampling port of isolator 2 using an optical particle counter (AeroTrak 9306 Handheld Particle Sizer, TSI, Inc., St. Paul, MN), which reported particle number concentrations in 6 size intervals ranging from 0.3 μm to > 10 μm. Sampling took place for test 4 only.
Sample processing and testing
Nasal swabs were resuspended in 2 mL of virus transport media, vortexed for 15 s, and the suspension was divided into two aliquots. For all the samples, including aerosol samples, one aliquot was placed at 4 °C for testing within 48 h from collection and the other aliquot was stored at – 80 °C for later use.
Viral RNA was extracted from 50 µL of each sample using the MagMAX™ -96 Viral RNA Isolation Kit (Applied Biosystems by Thermo Fisher Scientific, CA) according to the manufacturer’s instructions with a semi-automatic MagMAX Express-96 Deepwell Magnetic Particle Processor (Applied Biosystems by Thermo Fisher Scientific, CA). The RNA was eluted with 50 µL of elution buffer and quantified by quantitative RT-PCR using Ag Path-ID One Step RT-PCR Kit (Applied Biosystems, Foster City, CA). For IAV RT-qPCR, primers and probe targeting the IAV matrix gene were used39. The components of the RT-qPCR reaction mixture (25 µL) and the thermocycling conditions for IAV were described in Nirmala et al.40. The absolute viral genome copy number in each sample, expressed as RNA copies/mL, was quantified using cycle threshold (Ct) values and standard curves constructed using serial ten-fold dilutions of standard curve samples specific for IAV with known concentrations. Samples with Ct value ≤ 35 were considered positive.
For the PCR positive aerosol samples, virus isolation was performed using Madin-Darby canine kidney (MDCK) cells (see Supplementary Materials S1). First, 100 µL or 200 µL of each sample were diluted to 250 µL with the appropriate virus growth media respectively, and each of the 250 µL diluted samples was inoculated into one well containing cell monolayers in a 6-well plate and incubated at 37 °C with 5% CO2 for 1 h. Then 2 mL of virus growth media was added to each cell well and incubated at 37 °C with 5% CO2. Cytopathic effects were recorded at 5 days post IAV inoculation.
Data analysis
Viral RNA load (genome copies/m3 of air) collected from each stage of the ACI was normalized by dividing by the width of the corresponding size interval of the ACI on a logarithmic scale. Total viral RNA load was obtained by adding up the viral RNA results from each of the ACI stages and the filter. Number concentration of total particles (total particles/m3 of air) measured by the optical particle counter (OPC) were normalized by dividing by the width of the corresponding size interval of the OPC on a logarithmic scale. The amount of viral RNA load for airborne particles at each size range over the course of infection was estimated by calculating the area under the time-concentration curve (AUC) using R41. To determine whether the viral concentration in nasal swabs varied significantly among different days, repeated measures analyses of variance (ANOVA) was conducted. P-values ≤ 0.05 were considered statistically significant. Multiple comparisons of means were performed according to the post-hoc paired t-test, using a Bonferroni corrected significance level. To determine whether the viral concentration of airborne particles varied significantly among different days, and whether the AUC of airborne viral RNA copies differed significantly among different size ranges, Kruskal-Wallis test was conducted, with multiple comparisons by post-hoc Dunn’s test33.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hyslop, N. S. Factors influencing the epidemiology and epizootiology of airborne diseases. J. Am. Vet. Med. Assoc. 159, 1500–1507 (1971).
Jimenez, J. L. et al. What were the historical reasons for the resistance to recognizing airborne transmission during the COVID-19 pandemic? Indoor Air. 32, e13070 (2022).
Ather, B., Mirza, T. M. & Edemekong, P. F. Airborne Precautions. StatPearls (2024). https://www.ncbi.nlm.nih.gov/books/NBK531468/
Alonso, C. et al. Evidence of infectivity of airborne Porcine epidemic diarrhea virus and detection of airborne viral RNA at long distances from infected herds. Vet. Res. 45, 73 (2014).
Corzo, C. A., Culhane, M., Dee, S., Morrison, R. B. & Torremorell, M. Airborne detection and quantification of swine influenza A virus in air samples collected inside, outside and downwind from swine barns. PLoS One. 8, 1–7 (2013).
Gloster, J. et al. Foot-and-mouth disease - Quantification and size distribution of airborne particles emitted by healthy and infected pigs. Vet. J. 174, 42–53 (2007).
Dee, S., Otake, S., Oliveira, S. & Deen, J. Evidence of long distance airborne transport of Porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae. Vet. Res. 40, 39 (2009).
Landolt, G. A. & Olsen, C. W. Up to new tricks—A review of cross-species transmission of influenza A viruses. Anim. Health Res. Rev. 8, 1–21 (2007).
Peteranderl, C., Herold, S. & Schmoldt, C. Human influenza virus infections. Semin. Respir. Crit. Care Med. 37, 487–500 (2016).
Taubenberger, J. K. & Morens, D. M. 1918 Influenza: the mother of all pandemics. Emerg. Infect. Dis. 12, 15–22 (2006).
Lindsay, M. I. J., Herrmann, E. C. J., Morrow, G. W. J. & Brown, A. L. J. Hong Kong influenza: clinical, microbiologic, and pathologic features in 127 cases. JAMA 214, 1825–1832 (1970).
Trotter, Y. J. et al. Asian influenza in the united states, 1957–1958. Am. J. Hyg. 70, 34–50 (1959).
Qu, Y. et al. Evolutionary genomics of the pandemic 2009 H1N1 influenza viruses (pH1N 1v). Virol. J. 8, 250 (2011).
Choi, M. J. et al. Live animal markets in minnesota: A potential source for emergence of novel influenza A viruses and interspecies transmission. Clin. Infect. Dis. 61, 1355–1362 (2015).
Bowman, A. S. et al. Influenza A(H3N2) virus in swine at agricultural fairs and transmission to humans, Michigan and ohio, USA, 2016. Emerg. Infect. Dis. 23, 1551–1555 (2017).
Blachere, F. M. et al. Measurement of airborne influenza virus in a hospital emergency department. Clin. Infect. Dis. 48, 438–440 (2009).
Yang, W., Elankumaran, S. & Marr, L. C. Concentrations and size distributions of airborne influenza A viruses measured indoors at a health centre, a day-care centre and on aeroplanes. J. R Soc. Interface. 8, 1176–1184 (2011).
Lopez-Moreno, G. et al. Evidence of influenza A infection and risk of transmission between pigs and farmworkers. Zoonoses Public. Health. 69, 560–571 (2022).
Nelson, M. I. & Vincent, A. L. Reverse zoonosis of influenza to swine: new perspectives on the human-animal interface. Trends Microbiol. 23, 142–153 (2015).
Neira, V. et al. Characterization of viral load, viability and persistence of influenza A virus in air and on surfaces of swine production facilities. PLoS One. 11, e0146616 (2016).
Alonso, C., Raynor, P. C., Davies, P. R. & Torremorell, M. Concentration, size distribution, and infectivity of airborne particles carrying swine viruses. PLoS One. 10, e0135675 (2015).
Zuo, Z. et al. Association of airborne virus infectivity and survivability with its carrier particle size. Aerosol Sci. Technol. 47, 373–382 (2013).
Austin, E., Brock, J. & Wissler, E. A model for deposition of stable and unstable aerosols in the human respiratory tract. Am. Ind. Hyg. Assoc. J. 40, 1055–1066 (1979).
Randall, K., Ewing, E. T., Marr, L. C., Jimenez, J. L. & Bourouiba, L. How did we get here: what are droplets and aerosols and how Far do they go? A historical perspective on the transmission of respiratory infectious diseases. Interface Focus. 11, 20210049 (2021).
Carvalho, T. C., Peters, J. I. & Williams, R. O. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 406, 1–10 (2011).
Pan, M., Lednicky, J. A. & Wu, C. Y. Collection, particle sizing and detection of airborne viruses. J. Appl. Microbiol. 127, 1596–1611 (2019).
Gralton, J., Tovey, E., McLaws, M. L. & Rawlinson, W. D. The role of particle size in aerosolised pathogen transmission: A review. J. Infect. 62, 1–13 (2011).
Cvitešić Kušan, A. et al. The size distribution of SARS-CoV-2 genetic material in airborne particles sampled in hospital and home care environments occupied by COVID-19 positive subjects. Sci. Total Environ. 892, (2023).
Vass, W. B. et al. Detection and isolation of infectious SARS-CoV-2 Omicron subvariants collected from residential settings. Aerosol Sci. Technol. 57, 1142–1153 (2023).
Lednicky, J. A. et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 100, 476–482 (2020).
Zhou, J. et al. Defining the sizes of airborne particles that mediate influenza transmission in ferrets. Proc. Natl. Acad. Sci. U S A. 115, E2386–E2392 (2018).
Gustin, K. M., Katz, J. M., Tumpey, T. M. & Maines, T. R. Comparison of the levels of infectious virus in respirable aerosols exhaled by ferrets infected with influenza viruses exhibiting diverse transmissibility phenotypes. J. Virol. 87, 7864–7873 (2013).
Pulit-Penaloza, J. A. et al. Kinetics and magnitude of viral RNA shedding as indicators for influenza A virus transmissibility in ferrets. Commun. Biol. 6, 1–10 (2023).
Yezli, S. & Otter, J. A. Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ. Virol. 3, 1–30 (2011).
Alford, R. H., Kasel, J. A., Gerone, P. J. & Knight, V. Human influenza resulting from aerosol inhalation. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. (New York N Y). 122, 800–804 (1966).
Lindsley, W. G. et al. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J. Occup. Environ. Hyg. 9, 443–449 (2012).
Wang, L. et al. Evaluation of coating materials on the characterization of size and viability of virus-laden particles collected with an Andersen cascade impactor. J. Aerosol Sci. 182, 106454 (2024).
Weber, T. P. & Stilianakis, N. I. Inactivation of influenza A viruses in the environment and modes of transmission: A critical review. J. Infect. 57, 361–373 (2008).
Slomka, M. J. et al. Real time reverse transcription (RRT)-polymerase chain reaction (PCR) methods for detection of pandemic (H1N1) 2009 influenza virus and European swine influenza A virus infections in pigs. Influenza Other Respir. Viruses. 4, 277–293 (2010).
Nirmala, J. et al. Evaluation of viral RNA extraction methods to detect Porcine reproductive and respiratory syndrome and influenza A viruses from used commercial HVAC air filters from swine farms. J. Aerosol Sci. 151, 105624 (2021).
R Core Team. R. R: A language and environment for statistical computing (2013).
Donald K., et al. Influenza Virus Aerosols in Human Exhaled Breath: Particle Size Culturability and Effect of Surgical Masks PLoS Pathogens 9(3) e1003205 https://doi.org/10.1371/journal.ppat.1003205 (2013).
Acknowledgements
Seth Reicks, Joaquin Alvarez Norambuena, Mariana Meneguzzi, Chong Li.
Author information
Authors and Affiliations
Contributions
M.T. and C.H.: idea generation, obtained funding, oversaw studies, reviewed manuscript. L.W.: performed studies, analyzed data, wrote draft manuscript, reviewed manuscript. J.M.: supported studies, analyzed data, contributed to draft and reviewed manuscript. B.O.: supported studies and reviewed manuscript. M.Y.: supported studies, provided lab support and reviewed manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, L., Morán, J., Yang, M. et al. Size distribution and viral RNA load of influenza virus-laden airborne particles emitted from pigs over the course of an H1N1 infection. Sci Rep 15, 33301 (2025). https://doi.org/10.1038/s41598-025-18467-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18467-z