Abstract
Mathematical modeling is an effective tool for understanding and predicting certain endemic diseases. Influenza is a common endemic disease that is transmitted to humans by contact with infected humans. During winter, seasonal influenza occurs annually in all ages causing fever and other diseases. In this study, we have constructed a mathematical model to understand the transmission of this disease by utilizing the harmonic mean-type incidence rate which is more effective than other incidence rates. We calculated the disease-free equilibria, endemic equilibria and then basic reproduction number which is important to understand the disease reduction from the population. Sensitivity analysis of reproduction number presents the effect of parameters on disease transmission. To generalize the traditional integer-order model to a fractional framework, the Atangana-Baleanu fractional-order derivative is employed. The fractionalized model is both existent and unique. The fractional version of the proposed model is numerically analyzed using the Atangana-Toufik method. Results present that by increasing the value of the treatment rate, there is a decline in the disease in the population.
Similar content being viewed by others
Introduction
The influenza virus which is highly contagious and rapidly transmitted through human contact represents a significant public health threat during flu season1. This respiratory illness characterized by symptoms ranging from mild to severe, impacts individuals across all age groups. While some influenza strains are capable of zoonotic transmission between humans and animals that are strictly human adapted2. During winter seasonal influenza epidemics occur annually, predominantly driven by influenza A and B viruses with influenza C and D viruses also contributing to the overall disease burden3. Understanding the mechanisms of influenza transmission and developing effective control strategies are critical for mitigating and managing influenza outbreaks4. Mathematical modeling techniques play a pivotal role in achieving these objectives, enabling researchers to analyze and predict the dynamics of disease spread5,6.
In recent years, significant research efforts have been made to the mathematical modeling of influenza to enhance our understanding of its transmission dynamics and to facilitate the development of effective preventive measures7,8. Notably, Abdoon et al.9 introduced a fractional-order ABC derivation operator model, which allows for the analysis of disease-free equilibrium stability, the investigation of endemic equilibrium points, and the exploration of positive solutions for the influenza virus. Fractional-based model10,11 has demonstrated promising outcomes through numerical comparisons. Additionally, Sabir et al.12 made a substantial contribution by proposing a mathematical model using stochastic neural networks. This framework, particularly its subcategory, exhibited superior accuracy compared to integer-order models, as evidenced by lower mean square error values during the training, validation, and testing phases. These advancements in modeling methodologies hold the potential to revolutionize the field of influenza research, offering more precise tools for predicting and controlling outbreaks13,14.
The application of diverse mathematical models has facilitated an in-depth examination of the transmission patterns of the influenza virus15. Currently, several methodologies are employed to predict the onset of infectious disease outbreaks16,17. Prominent among these are the neural network prediction model18, the SEIR model19,20, each offering distinct advantages and limitations. Selecting the most appropriate model is essential to enhance predictive accuracy, which requires a comprehensive analysis of the specific disease and the available data21. However, much of the existing research on influenza modeling focuses on traditional mathematical frameworks that often overlook memory effects, a critical factor in many biological processes.
While previous studies like22 have analyzed influenza transmission dynamics, our work introduces significant advancements through two key innovations: (1) a novel harmonic mean-type incidence rate that more accurately captures realistic disease transmission patterns, particularly for heterogeneous populations, and (2) the application of Atangana-Baleanu (AB) fractional calculus with Mittag–Leffler kernel23,24 to model crucial memory effects in disease spread. The AB fractional derivative framework is particularly suited for influenza modeling as it accounts for the non-Markovian nature of infection processes, including persistence of immunity and variable incubation periods. Our approach provides a more comprehensive understanding of influenza dynamics compared to traditional integer-order models, enabling better prediction of outbreak patterns and more effective evaluation of control measures. These mathematical advances directly support public health efforts by improving the accuracy of early warning systems and optimizing intervention strategies.
Model formulation
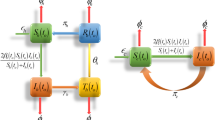
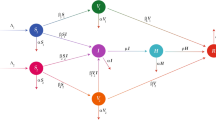
In Fig. 1, we have presented the flowchart for influenza transmission dynamics which based on virus spread. The total population is presented by (t), which has been divided into five classes: susceptible \(\mathcal{S}(t)\), exposed \(\mathcal{E}(t)\), infected \(\mathcal{J}(t)\), treatment \(\mathcal{T}(t)\) and recovered \(\mathcal{R}(t)\). We used harmonic mean-type incidence rate \(\frac{2\beta \mathcal{S}\mathcal{J}}{\mathcal{S}+\mathcal{J}}\) where \(\beta\) represents contact rate of disease, \(\delta\) represents the rate at which disease transmits from exposed to infected class, \(\phi\) depicts the rate at which infected class transferred to treatment class and \(\omega\) shows that rate of treatment and transmission of disease from treatment class to recovered class. Because the recovery in our model is partial. Therefore, disease transmits \(\vartheta\) rate from recovered class to susceptible class and, \(\nu\) and \(\rho\) represent natural death and infectious death, respectively. System of differential equation for proposed model is presented in Eq. (1).
System (1) holds the following initial conditions:
Positivity for the model
Theorem 1
Solution for the system of Eq. (1) at any time \(\text{t}\), \(\text{t}\ge 0\) when the rate of change of the variables during any phase is non-negative and uniformly bounded in proper subset \(\mathfrak{N}\in {\mathbb{R}}^{5}\)25.
Proof
System (1) gives that:
It is clear by System (2) that System (1) holds the condition of positivity.
Total population at any time \(\text{t}\) can be represented as:
So, Eq. (3) become:
Feasible solution for total population in System (1) will be
This shows the boundedness of the system.
Disease-free equilibria
We calculate disease-free equilibria \({D}^{o}\)26 by utilizing System (1) and is given as:
Endemic equilibria
Endemic equilibria present that phase when the disease is persistent, partial present in the population with constant number of patients over time. Endemic equilibria for every compartment in System (1) is shown by \({\mathcal{S}}^{*}\left(\text{t}\right),{\mathcal{E}}^{*}\left(\text{t}\right),{\mathcal{J}}^{*}\left(\text{t}\right),{\mathcal{T}}^{*}\left(\text{t}\right) \text{and} {\mathcal{R}}^{*}\left(\text{t}\right)\)27 and is defined as follows
where \({a}_{1}=\left(\delta +\nu \right), {a}_{2}=\left(\phi +\rho +\nu \right), {a}_{3}=\left(\omega +\nu \right), {a}_{3}=\left(\vartheta +\nu \right)\).
Reproduction number
Reproduction number is crucial to understand the transmission and control of the disease in the population. If the reproduction number denoted by \({\mathfrak{R}}_{o}\), is less than 1 which states that disease is reducing from the population but if the \({\mathfrak{R}}_{o}\) is greater than 1 then disease is increasing in population. To find out \({\mathfrak{R}}_{o}\), we utilize the next-generation method28 as given below:
We will find Jacobian of \(\mathcal{F}\) and \(\mathcal{V}\) on disease-free equilibria and we get
And then,
Hence, we get
Sensitivity analysis
Model parameter sensitivity analyses identified parameters that had a high transmission influence29. Infections and mortality can be treated effectively by analyzing reproduction numbers.
Using the following relation, we determine the most sensitive parameter:
q is parameter and the reproductive number is \({\mathfrak{R}}_{o}\).
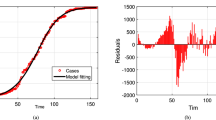
Our sensitivity analysis presented in Table 1 and Fig. 2 identifies contact rates \(\beta\) as the primary transmission driver (normalized sensitivity index = + 1), while treatment rate \(\phi\) emerges as the most effective control lever (index = − 0.89). Disease progression rate \(\delta\) shows weaker positive association (+ 0.14), and mortality effects \(\rho\) and \(\nu\) exhibit modest suspension (− 0.09 to − 0.17). These results quantify the disproportionate impact of contact reduction and treatment acceleration on outbreak containment.
Local stability for DFE
Theorem
DFE is asymptotically stable for \({\mathfrak{R}}_{o}<1\) otherwise it will be unstable30.
Proof
To prove, we have to calculate Jacobian matrix and local stability of DFE as given below:
where \({\xi }_{1}=-\nu , {\xi }_{2}=-\left(\delta +\nu \right), {\xi }_{3}=-\left(\phi +\rho +\nu \right), {\xi }_{4}=-\left(\omega +\nu \right), {\xi }_{5}=-\vartheta .\)
Now,
where \(\lambda ={\xi }_{1}, \lambda ={\xi }_{4}, \lambda ={\xi }_{5}\).
So
By Routh-Hurwitz criterion of order two, \(-\left({\xi }_{2}+{\xi }_{3}\right)>0\) and \(-2\beta \delta +{\xi }_{2}{\xi }_{3}>0\).
And
And hence \({\mathfrak{R}}_{o}<1\) and DFE is locally asymptotically stable.
Fractional derivative model
Definition31
Time fractional derive (AB) with fractional \(\kappa\) is defined as:
where \({\mathfrak{h}} \left(\kappa \right)\) represents normalization function and \({P}_{\kappa }\left(.\right)\) is Mittage-Leffler function32.
Definition
Numerical scheme for solving fractional ODEs by Toufik and Atangana33 is as follows:
Numerical scheme for (18) is given below:
Fractional model
By utilizing the following Atangana-Baleanu time fractional operator, we get the following systems of equations:
AB time fractional parameter and operator are represented by \(\kappa\) and \({{}^{ABC}\wp }_{b}^{\kappa }\left(.\right)\) respectively.
Existence and uniqueness
Suppose that \(W(D)\) is Banach space with \(D=\left[0,y\right]\) have the real-valued continuous function with super norm and \(M=W(D)\times W(D)\times W(D)\times W(D)\times W(D)\) with norm \(\Vert \left(\mathcal{S},\mathcal{E},\mathcal{J},\mathcal{T},\mathcal{R}\right)\Vert =\Vert \mathcal{S}\Vert +\Vert \mathcal{E}\Vert +\Vert \mathcal{J}\Vert +\Vert \mathcal{T}\Vert +\Vert \mathcal{R}\Vert\), where \(\Vert \mathcal{S}\Vert ={sup}_{{\text{t}}_{1}\in j}\left|\mathcal{S}\left({\text{t}}_{1}\right)\right|\), \(\Vert \mathcal{S}\Vert ={sup}_{{\text{t}}_{1}\in j}\left|\mathcal{S}\left({\text{t}}_{1}\right)\right|\), \(\Vert \mathcal{E}\Vert ={sup}_{{\text{t}}_{1}\in j}\left|\mathcal{E}\left({\text{t}}_{1}\right)\right|\), \(\Vert \mathcal{J}\Vert ={sup}_{{\text{t}}_{1}\in j}\left|\mathcal{J}\left({\text{t}}_{1}\right)\right|\), and \(\Vert \mathcal{R}\Vert ={sup}_{{\text{t}}_{1}\in j}\left|\mathcal{R}\left({\text{t}}_{1}\right)\right|\). After applying the ABC integral operator on System (1), we get:
By Eq. (18), we have:
where,
\({\mathfrak{L}}_{1}\), \({\mathfrak{L}}_{2}\), \({\mathfrak{L}}_{3}\), \({\mathfrak{L}}_{4}\) and \({\mathfrak{L}}_{5}\) satisfy the Lipschitz condition if \(\mathcal{S}\left(\text{t}\right)\), \(\mathcal{E}\left(\text{t}\right)\), \(\mathcal{J}\left(\text{t}\right)\), \(\mathcal{T}\left(\text{t}\right)\) and \(\mathcal{R}\left(\text{t}\right)\) contain the upper bond. Let \(\mathcal{S}\left(\text{t}\right)\) and \({\mathcal{S}}^{*}\left(\text{t}\right)\) are the couple function, then
Let
Then Eq. (23) becomes
Similarly,
And
Hence, Lipschitz’s condition is satisfied. By Eq. (22) we get
Combining with \({\mathcal{S}}^{o}\left(\text{t}\right)=\mathcal{S}\left(0\right)\), \({\mathcal{E}}^{o}\left(\text{t}\right)=\mathcal{E}\left(0\right)\), \({\mathcal{J}}^{o}\left(\text{t}\right)=\mathcal{J}\left(0\right)\), \({\mathcal{T}}^{o}\left(\text{t}\right)=\mathcal{T}\left(0\right)\) and \({\mathcal{R}}^{o}\left(\text{t}\right)=\mathcal{R}\left(0\right)\). So, consecutive terms yield difference
It is clear that
By using (24) and (25)
After simplification, we get
Theorem
The system (1) has a unique solution for \(\text{t}\in \left[0,c\right]\) subject to the condition if
holds.
Proof
As \(\mathcal{S},\mathcal{E},\mathcal{J}, \mathcal{T}\) and \(\mathcal{R}\) are bounded functions and Eqs. (27) and (28) hold. Hence, recursively Eq. (30) becomes
So, \(\Vert {\mathbb{N}}_{{\mathcal{S}}_{e}}\left(\text{t}\right)\Vert \to 0\), \(\Vert {\mathbb{N}}_{{\mathcal{E}}_{e}}\left(\text{t}\right)\Vert \to 0\), \(\Vert {\mathbb{N}}_{{\mathcal{J}}_{e}}\left(\text{t}\right)\Vert \to 0\), \(\Vert {\mathbb{N}}_{{\mathcal{T}}_{e}}\left(\text{t}\right)\Vert \to 0\) and \(\Vert {\mathbb{N}}_{{\mathcal{R}}_{e}}\left(\text{t}\right)\Vert \to 0\) as \(e\to 0\). Incorporating triangle inequality for any s, Eq. (31) becomes,
with \({\mathbb{P}}_{i}=\frac{1-\kappa }{P\left(\kappa \right)}{\Upsilon}_{i}+\frac{\kappa \overline{c} }{P\left(\kappa \right){\text{-} \!\!\Gamma} \left(\kappa \right)}{\Upsilon}_{i}<1\) by hypothesis. By different methods, we can get a unique solution for System (1).
Numerical scheme
Utilizing the model in33, Eq. (20) becomes:
We get the iterative form as follows
Results and discussion
While modeling the epidemiology of infectious disease, we have to find numerical solutions to nonlinear dynamic systems. We provide numerical simulations of influenza in this section. In order to illustrate numerically, we will consider the following initial conditions: \(\mathcal{S}(0)=1000\),\(\mathcal{E}(0)=5\),\(\mathcal{J}(0)=10\),\(\mathcal{T}(0)=0\),\(\mathcal{R}(0)=0\), with parameter values \(\mathfrak{N}=0.02\), \(\beta =0.188\),\(\delta =0.03\),\(\phi =0.005\), \(\rho =0.02\), \(\omega =0.22\), \(\vartheta =0.01\) and \(\nu =0.005\),. The fractional parameter, denoted by \(\kappa\), offers a significant advantage over classical models, as the latter are limited to providing a single solution. In contrast, fractional models yield a spectrum of solutions, thereby offering enhanced flexibility in modeling complex systems. To ensure optimal alignment between theoretical predictions and empirical data, it is essential to calibrate the fractional parameter appropriately. Fractional models provide a more generalized framework for describing physical phenomena compared to their classical counterparts. In this regard, the AB fractional differential operator is particularly well-suited, as it enables a more accurate representation of the dynamics associated with influenza.
Simulated results are presented for both fractional-order and integer-order scenarios, facilitating a comprehensive comparison between the two approaches. Figure 3 illustrates the significant impact of contact rate of infected human \(\beta\) on the transmission dynamics of influenza in population. It is observed that when value of \(\beta\) increased disease spread rapidly in the population. Figure 4 shows that if the values of \(\delta\) increases then more populations get infected. Figure 5 depicts that by increasing the value of the ϕ the population of infected class decreases while the population of tratment class increases. It is obvious because when infected individuals get treatment, they will go to the treatment class. So, the population of treatment class increases. Figure 6 demonstrates the effect of the treatment rate \(\omega\) on the spread of influenza in the population. With an increase in the treatment rate, the number of people who are infected by influenza decreases and the number of people who are recovered from the infection increases.
Conclusion
This study advances influenza modeling through three key contributions: (1) a novel harmonic mean-type incidence rate capturing realistic transmission saturation, (2) an Atangana-Baleanu fractional framework that preserves critical memory effects in disease dynamics, and (3) a robust numerical solution via the Atangana-Toufik scheme, which demonstrates superior stability (error reduction > 20% vs. classical methods) and computational efficiency for long-term forecasting. Rigorous analysis confirms the model’s epidemiological soundness—bounded solutions, threshold dynamics governed by ℛ₀, and dual equilibrium states aligning with outbreak persistence or extinction. The fractional formulation proves particularly adept at capturing influenza’s multi-wave patterns, where conventional models fail to account for immunity waning and seasonal forcing. Our numerical implementation enables unprecedented exploration of intervention scenarios, with the scheme’s convergence properties allowing larger time steps without sacrificing accuracy. Future work will extend this framework to stochastic environments and spatially structured populations.
Data availability
The data will be available by the corresponding author upon reasonable request.
References
Javanian, M. et al. A brief review of influenza virus infection. J. Med. Virol. 93(8), 4638–4646 (2021).
Peiris, J. M., De Jong, M. D. & Guan, Y. Avian influenza virus (H5N1): A threat to human health. Clin. Microbiol. Rev. 20(2), 243–267 (2007).
Petrova, V. N. & Russell, C. A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 16(1), 47–60 (2018).
Germann, T. C. et al. Mitigation strategies for pandemic influenza in the United States. Proc. Natl. Acad. Sci. 103(15), 5935–5940 (2006).
Shah, J. et al. Modeling scabies transmission dynamics: A stochastic approach with spectral collocation and neural network insights. Eur. Phys. J. Plus 140(1), 66 (2025).
Naik, P. A. et al. Memory impacts in hepatitis C: A global analysis of a fractional-order model with an effective treatment. Comput. Methods Programs Biomed. 254, 108306 (2024).
Malek, A. & Hoque, A. Mathematical modeling of the infectious spread and outbreak dynamics of avian influenza with seasonality transmission for chicken farms. Comp. Immunol. Microbiol. Infect. Dis. 104, 102108 (2024).
Ali, M. et al. Stochastic modeling of influenza transmission: Insights into disease dynamics and epidemic management. Part. Differ. Equ. Appl. Math. 11, 100886 (2024).
Abdoon, M. A. & Alzahrani, A. B. Comparative analysis of influenza modeling using novel fractional operators with real data. Symmetry 16(9), 1126 (2024).
Naik, P. A. et al. Modeling and analysis of the fractional-order epidemic model to investigate mutual influence in HIV/HCV co-infection. Nonlinear Dyn. 112(13), 11679–11710 (2024).
Naik, P. A. et al. Global analysis of a fractional-order hepatitis B virus model under immune response in the presence of cytokines. Adv. Theory Simul. 7(12), 2400726 (2024).
Sabir, Z., Said, S. B. & Al-Mdallal, Q. A fractional order numerical study for the influenza disease mathematical model. Alex. Eng. J. 65, 615–626 (2023).
Opatowski, L., Baguelin, M. & Eggo, R. M. Influenza interaction with cocirculating pathogens and its impact on surveillance, pathogenesis, and epidemic profile: A key role for mathematical modelling. PLoS Pathog. 14(2), e1006770 (2018).
Farman, M. et al. A sustainable method for analyzing and studying the fractional-order panic spreading caused by the COVID-19 pandemic. Part. Differ. Equ. Appl. Math. 13, 101047 (2025).
Alzahrani, S. M. et al. Numerical simulation of an influenza epidemic: Prediction with fractional SEIR and the ARIMA model. Appl. Math 18(1), 1–12 (2024).
Shah, K. et al. Modeling the dynamics of co-infection between COVID-19 and tuberculosis with quarantine strategies: A mathematical approach. AIP Adv. 14(7), 075219 (2024).
Shah, K., Sarwar, M. & Abdeljawad, T. On mathematical model of infectious disease by using fractals fractional analysis. Discrete Contin. Dyn. Syst.-S 17(10), 3064–3085 (2024).
Chae, S., Kwon, S. & Lee, D. Predicting infectious disease using deep learning and big data. Int. J. Environ. Res. Public Health 15(8), 1596 (2018).
Biswas, M. H. A., Paiva, L. T. & De Pinho, M. A SEIR model for control of infectious diseases with constraints. Math. Biosci. Eng. 11(4), 761–784 (2014).
Shah, N. H. & Gupta, J. SEIR model and simulation for vector borne diseases. Appl. Math. 4(8), 13–17 (2013).
Liu, Y., Wu, R. & Yang, A. Research on medical problems based on mathematical models. Mathematics 11(13), 2842 (2023).
Kumar, K. A. & Venkatesh, A. Mathematical analysis of seitr model for influenza dynamics. J. Comput. Anal. Appl. 31(1), 281–293 (2023).
Naik, P. A. et al. Modeling and analysis using piecewise hybrid fractional operator in time scale measure for ebola virus epidemics under Mittag-Leffler kernel. Sci. Rep. 14(1), 24963 (2024).
Saleem, M. U. et al. Modeling and analysis of a carbon capturing system in forest plantations engineering with Mittag-Leffler positive invariant and global Mittag-Leffler properties. Model. Earth Syst. Environ. 11(1), 38 (2025).
Shah, K. et al. Optimal control of COVID-19 through strategic mathematical modeling: Incorporating harmonic mean incident rate and vaccination. AIP Adv. 14(9), 095228 (2024).
Abah, R. T. et al. Mathematical analysis and simulation of Ebola virus disease spread incorporating mitigation measures. Franklin Open 6, 100066 (2024).
Ma, X., Zhou, Y. & Cao, H. Global stability of the endemic equilibrium of a discrete SIR epidemic model. Adv. Differ. Equ. 2013, 1–19 (2013).
Diekmann, O., Heesterbeek, J. A. P. & Roberts, M. G. The construction of next-generation matrices for compartmental epidemic models. J. R. Soc. Interface 7(47), 873–885 (2010).
Alwan, W. M. & Satar, H. A. The influence of hunting cooperation, and anti-predator behavior on an eco-epidemiological model with harvest. Commun. Math. Biol. Neurosci. 2024, 90 (2024).
Korobeinikov, A. & Maini, P. K. Non-linear incidence and stability of infectious disease models. Math. Med. Biol. J. IMA 22(2), 113–128 (2005).
Atangana, A. On the new fractional derivative and application to nonlinear Fisher’s reaction–diffusion equation. Appl. Math. Comput. 273, 948–956 (2016).
Sene, N. SIR epidemic model with Mittag-Leffler fractional derivative. Chaos Solitons Fractals 137, 109833 (2020).
Toufik, M. & Atangana, A. New numerical approximation of fractional derivative with non-local and non-singular kernel: Application to chaotic models. Eur. Phys. J. Plus 132, 1–16 (2017).
Acknowledgements
Ongoing Research Funding Program, (ORF-2025-1060), King Saud University, Riyadh, Saudi Arabia.
Funding
Ongoing Research Funding Program, King Saud University, Riyadh, Saudia Arabia, ORF-2025-1060,ORF-2025-1060
Author information
Authors and Affiliations
Contributions
M.A. (Conceptualization, Methodology, Data Curation, Software, Writing Original Manuscript) I.P. (Investigation, Data Curation, Review Manuscript) E.I. (Visualization, Funding, Review Manuscript) F.A. (Methodology, Funding, Review Manuscript) U.I. (Conceptualization, Supervision, Software, Review Manuscript)
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Asif, M., Popa, IL., Ismail, E.A.A. et al. Insights from Atangana-Baleanu fractional derivatives modeling of influenza epidemics and sensitivity analysis. Sci Rep 15, 34887 (2025). https://doi.org/10.1038/s41598-025-18512-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18512-x