Abstract
Microplastics (MP) are considered as a new persistent environmental pollutant. The study investigated the impact of probiotics on reproductive toxicity induced by polystyrene microplastics (PS-MP). It was observed that PS-MP administration caused dose-dependent testicular damage and negatively regulated the expression of kisspeptin and its receptors in the hypothalamus. However, supplementation of probiotics significantly mitigated oxidative stress and inflammation in cell experiments. In animal experiments, probiotics were found to improve testicular damage, decrease sperm nitric oxide, and increase blood levels of sex hormones in a dose-dependent manner. These findings suggest that probiotics may play a role in reducing testicular damage caused by PS-MP through their anti-inflammatory and antioxidant properties. This study provides valuable insights into mitigating the reproductive damage associated with microplastic exposure and offers a new approach to addressing microplastic toxicology for reproductive health.
Similar content being viewed by others
Introduction
Microplastics refer to plastic particles, fragments, or films with a diameter of less than 5 mm, which can be divided into primary and secondary plastic particles1. Primary microplastics refer to those smaller than five millimeters manufactured for direct use or as precursors of other products; secondary microplastics refer to larger plastic products that gradually form smaller particles through physical or chemical action in the environment over time2. In recent years, the total global production of microplastics has increased sharply, and the total global production has exceeded 200 million tons since 20023; the latest data have shown that the total global production in 2021 has reached 367 million tons4. These microplastics will be discharged into the ocean along with the river. According to the total plastic production along the coasts of various countries, it can be found that there is a large amount of plastic in developing countries, such as China, India, and other Southeast Asian countries5. Common types of plastic particles include high- and low-density polyethylene (polyethylene, PE), polystyrene (PS), polypropylene (polypropylene, PP), etc., which are often used as raw materials for food containers. Microplastics can remain in food6.
The sharp increase in plastic production has brought about the harm of polluting various ecological circles and finally entering the human body through the food chain. Due to its small size, MP is easily swallowed by organisms and accumulates in the body7, and through bioaccumulation, the higher the consumer in the food chain, the higher the concentration of microplastics accumulated in the body8 due to the wide spectrum of MPs sizes, including different plastic particle materials, shapes, and surfaces9. It is highly likely that MPs may be absorbed by an organism through ingestion or inhalation, gather in gastric tissue, and then enter the circulatory system, accumulating in other organs, such as the liver and testes10,11,12,13. Therefore, in recent years, the harm caused by microplastics has been the subject of active study. Revealing a growing concern for their impact on biological systems, particularly the male reproductive system.
Studies have shown that plastic particles will cause reproductive dysfunction in male mice14. The cause may be that microplastics enter the body’s blood circulation and disrupt the blood-testis barrier (BTB), which is crucial for maintaining the specialized microenvironment required for spermatogenesis. This can happen through oxidative stress, death of spermatogenic cells, swelling of the testicles, problems with androgen-producing cells, interference with gene regulation, disruption of ionic homeostasis, and damage to the vascular endothelium15, causing damage to the testicular tissue and affecting the reproductive system16; in addition, smaller microplastics can enter cells and increase oxidative stress through the production of reactive oxygen species (ROS)17. This oxidative burden can cause lipid peroxidation, adversely affecting sperm morphology and function, and leading to decreased sperm quality and testosterone production. The activation of stress-response pathways such as p38 and JNK MAPK has also been implicated in polystyrene microplastic-induced testicular oxidative stress18. Additionally, exposure to microplastics has been shown to decrease the activity of vital antioxidant enzymes in both blood and testicular tissues19.
Beyond oxidative stress, MPs promote the activation of NF-κB and the secretion of inflammatory cytokines, such as TNF-α, contributing to a state of chronic inflammation within the body. This inflammatory response can directly and indirectly inhibit spermatogenesis, leading to reduced semen quality and promoting cell apoptosis in both testicular tissue and sperm cells20,21. Such mechanisms of toxicity, involving oxidative stress, inflammation, and cellular apoptosis, are well-established in the context of various environmental toxicants impacting male reproduction.
The hypothalamic-pituitary–gonadal axis (HPG axis) mainly regulates the reproductive endocrine system, in which the hypothalamus contains the important gene kisspeptin and its receptor GPR54 (G protein coupled receptor 54), which regulates the release of Gonadotropin Releasing Hormone (GnRH) to stimulate the Anterior Pituitary producing follicle-stimulating hormone (FSH) and luteinizing hormone (LH). The male LH hormone will act on the interstitial cells of the testes and can generate testosterone. Testosterone and FSH will work together in Sertoli cells of sperm testes to promote the production and maturation22. Previous studies have found that inhibiting the action of kisspeptin in the context of inflammation will indirectly lead to impaired sperm maturation23. Exposure to polystyrene microplastics has been shown to downregulate the expression of kisspeptin and its receptor GPR54 in the hypothalamus, potentially disrupting this delicate endocrine balance.
While the detrimental effects of microplastics on male reproductive health are increasingly recognized, research exploring effective therapeutic strategies to mitigate this damage remains limited. This gap highlights the urgent need for interventions that can counteract MP-induced toxicity. Probiotics, known for their anti-inflammatory and antioxidant properties, as well as their ability to modulate the gut microbiota, which influences the HPG axis and testicular function, represent a promising, yet underexplored, therapeutic avenue.
Summarizing the above, after understanding the male reproductive dysfunction that microplastics may cause, we will further explore whether probiotic supplementation can improve the possibility24,25,26,27. Lactococcus lactis has been confirmed to negatively regulate TLR-4, expression of TLR-4, and achieve anti-inflammatory effects28; Lactobacillus rhamnosus has the function of stabilizing the intestinal mucosal barrier29; Lactobacillus plantarum has anti-inflammatory and immunomodulatory activities30,31. Therefore, this study first explored the antioxidant and anti-inflammatory effects of postbiotics on the cytotoxicity of PS-MP through cultured probiotic metabolites, and observed whether probiotics can improve the damage to the reproductive system in animal models.
Materials and methods
Microplastics
The polystyrene microplastics (PS-MP) used in this experiment were purchased from Yao-Hong Biotechnology Co., Ltd. (New Taipei, Taiwan). The size is 0.4–0.6 µm, the concentration is 5% w/v, and the number of particles is 1.14 × 1012 particles/mL. Before starting the experiment, PS-MP was formulated at the desired concentration, and the particles were sonicated for 30 min.
Probiotics
The probiotics used in this experiment were purchased from Bened Biomedical Co., Ltd. (Taipei City, Taiwan), including Lactococcus lactis, Lacticaseibacillus rhamnosus, and Lactiplantibacillus plantarum. (1 × 1011 CFU per pack). Calculate the total number of bacteria that must be fed to the mice in one day and dissolve the required probiotic powder in an appropriate amount of deionized water at room temperature to obtain a probiotic solution for animal experiments. Referring to the method of Vale and Mayer with modifications32, an appropriate amount of probiotic powder was cultured in a sterilized MRS broth medium, and after culture for 1 to 2 days at 37 °C in an anaerobic environment, calculated 108 viable bacteria with a hemocytometer, absorb the culture medium with this number of bacteria and add sterile 0.1% protein gluten dilution to 300 ml, then filter with 0.22 μm membrane to filter out bacteria to obtain probiotic metabolites for cell experiments (postbiotics, Pb).
Cells and animals
The doses of polystyrene microplastics (PS-MP) used in this study (1 mg/kg, 5 mg/kg, and 10 mg/kg b.w.) were selected based on33 and 108 CFU / day probiotics, CFU/day probiotic group, and the same number of probiotics were fed, respectively, under 10 mg/kg B.W.. PS-MP-induced injury34. These concentrations were chosen to encompass a range from environmentally relevant exposure levels to higher doses sufficient to elicit measurable physiological changes.
Sacrifice after 7 weeks to explore the degree of damage to the reproductive system caused by PS-MP. Mouse macrophage: The RAW 264.7 cell line was provided by the American Type Culture Collection (ATCC, Rockville, MD, USA). Seven-week-old male rats of the Sprague–Dawley strain (SD) were purchased from BioLasco Taiwan Co., Ltd. (Yilan, Taiwan). The rats were reared at the Terrestrial Animal Experiment Center of the University in a stainless steel rat cage, the temperature was controlled at 23 ± 1 °C, the humidity was 40–60%, and the light–dark cycle was 12 h. Both feed and water were supplied without restriction, and experiments were carried out after one week of domestication.
Experimental process
Forty-eight male SD rats were acclimated for one week and randomly divided into 6 groups at 8 weeks of age, with 8 rats in each group, namely, the control group, low, medium, and high PS-MP dose groups, the experimental probiotic group, and the probiotic control group. Three PS-MP concentrations were established at 1 mg/kg, 5 mg/kg, and 10 mg/kg B.W.. These concentrations were referenced and modified from33. In the experimental group, in addition to feeding 10 mg/kg of PS-MP, 108 bacterial counts of probiotics were administered; for the probiotics (10^8 CFU/day), the chosen dosage was determined based on34. This approach allowed us to evaluate potential dose-dependent effects of PS-MP and the therapeutic efficacy of probiotics. Syringes and centrifuge tubes were wetted with heparin (Heparin, 500 IU/ml) in advance, and blood was drawn from the abdominal portal vein. The blood obtained was placed in a 15 ml centrifuge tube, centrifuged at 1570 × g for 15 min at 4 °C using a low-speed centrifuge (Kubota 3500, Kubota, Tokyo, Japan), and the supernatant was collected as plasma for subsequent analysis. At the time of sacrifice by using CO2, the organs were removed and weighed, and one of the hypothalamus was immediately stored at − 80 °C for analysis, and the other testes were stored in 10% formalin for tissue sectioning. Sperm analysis was performed on the day of sacrifice. All experimental protocols were meticulously reviewed and approved by the National Taiwan Ocean University Institutional Animal Care and Use Committee (Approval number: 109-027). All methods were carried out in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and relevant institutional guidelines and regulations. Furthermore, all experimental methods are reported in adherence to the ARRIVE guidelines, ensuring transparency and reproducibility.
Toxicity of PS-MP and postbiotics on cells and anti-inflammatory and antioxidant effects of postbiotics on cells
First, the PS-MP cell viability test and postbiotics were performed on RAW 264.7 cells by the MTT method35. After determining the highest harmless concentration of postbiotics in PS-MP-induced toxicity, relative experiments were performed using 2,7-dichlorofluorescein diacetate (DCFH-DA) (Sigma, USA) to detect intracellular ROS production. And NO production was carried out using the Greiss method36.
Testicular damage analysis and histopathological analysis
After taking the testis soaked in 10% formalin, first use a scalpel to remove the head and tail and cut several testicular tissue slices with a thickness of about 0.5 cm from the rest, and the thickness should not exceed the embedding box. The group was marked on the lid, then soaked in formalin, and then sent to RaPid Science Co., Ltd.. (Taichung, Taiwan) at room temperature, outsourced to make testicular H&E-stained sections. After obtaining the testicular tissue section, image processing software (ImageJ Version 1.51) was used to determine the diameter of seminiferous tubules, the thickness of the epithelium, the area of seminiferous, and the area of seminiferous lumen/seminiferous tubules (%) to evaluate the influence of testicular seminiferous tubules on PS-MP. LDH ELISA kits (Randox, Crumlin, UK) were used to measure lactate dehydrogenase activity in rat testicular tissue to assess the degree of PS-MP injury in testicular tissue.
Sperm count and motility analysis
Sperm preparation and analysis were performed as described by the previous method. Briefly, the epididymides of the rat were dissected, removed, and the caudal epididymis was minced in 5 mL of prewarmed RPMI medium to allow spermatozoa to leave the epididymal tubules and incubated for 30 min at 37 °C. The percentage of motile spermatozoa and abnormalities were recorded under a light microscope and counted by using a hemocytometer37.
Oxidative stress and antioxidant enzymes
For MDA quantification,100 µL of plasma or tissue homogenate was put into a light-proof centrifuge tube and add 200 µL of reaction reagent (15%, w/v trichloroacetic acid in 0.25 N HCl and 0.375%, w/v thiobarbituric acid in 0.25 N HCl) Mix well, then heat at 100 °C for 15 min and then cool down, then add 300 µL of n-butanol and mix vigorously, then centrifuge at 1500 × g for 10 min and take the upper layer of liquid, and finally use an Multiskan GO analyzer (Thermo Fisher Scientific,Finland) to measure Absorbance value at wavelength 532 nm, and compare with standard (1, 1, 3, 3-tetramethoxypropane) to convert sample MDA concentration (nmol/mL)38. The activities of glutathione peroxidase (GPx) were measured by RANSEL kits (Randox Laboratories, Crumlin, U.K.). 5 µL of testicular homogenate was added to 250 µL of reaction reagent. Finally, 10 µL of glutathione reductase was added. Multiskan GO analyzer (Thermo Fisher Scientific,Finland) was used to measure the absorbance at a wavelength of 505 nm in 2 and 3 min, respectively, and compare the standard curve to convert the GPx activity (U/ml)39.
Inflammatory response
Take 100 µL of plasma, tissue homogenate and sperm, and adjust the sperm count between each group to 106 sperm/ml, take 100 µL of nitrite (standard) of each concentration and each sample in 96 wells, add 50 µL of 0.1% SUL solution (0.1% SUL in 2.5% phosphoric acid) and 0.1% 50 µL NED solution (NED in ddH2O) were mixed evenly, incubated in the dark for 10 min, and absorbance at 540 nm was measured with an Multiskan GO analyzer (Thermo Fisher Scientific,Finland) and converted according to the standard curve NO concentration40. The TNF-α concentration of the samples was determined using TNF-α ELISA kits (eBioscience, Inc., USA). Add 100 µL of plasma, testicular homogenate, and standard, and 50 µL biotinylated anti-TNF-µL HRP and react at room temperature for 30 min. After washing three times, add µL TMB substrate and react in the dark for 20 min at room temperature. Finally, add µL, stop reagent, measure the absorbance at a wavelength of 450 nm with an Multiskan GO analyzer (Thermo Fisher Scientific, Finland), and convert the concentration to the standard curve41.
Endocrine reproductive system
Using ELISA kits (MyBioSource, San Diego, California, USA) to measure the concentration of rat H-P-G axis, including Kisspeptin, GPR54, FSH, and LH in plasma, and testosterone concentration. Add 100 µL of plasma homogenate and standard to 96 wells, react at 37 °C for 2 h, remove the supernatant, add 100 µL biotin antibody and react at 37 °C for 1 h, wash three times, and add 100 µL HRP-avidin was reacted at 37 °C for 1 h, washed five times, and then added 90 µL of TMB and incubated in the dark for 30 min. Finally, 50 µL of stop solution was added, and the absorbance at 450 nm was measured with a Multiskan GO analyzer (Thermo Fisher Scientific, Finland) and compared with the standard curve. Converted kiss1 content42.
Statistical analysis
The experimental results are expressed as the mean ± standard deviation (Mean ± S.E.M). Data statistical analysis was performed with GraphPad Prism 5 (GraphPad Software Inc.; San Diego, CA, USA) and SPSS 20.0 (SPSS Inc.; Chicago, IL, USA), and the univariate difference analysis was performed for specific group comparisons. Statistical differences were analyzed by one-way analysis of variance (one-way ANOVA), and then by multiple comparison method (Duncan’s test) for post hoc comparison. When the P-value was less than 0.05, it was a statistically significant difference.
Results
Antioxidant and anti-inflammatory capacity of postbiotics in LC-540 and RAW 264.7 cells
The results of the MTT of PS-MP-induced toxicity at the lowest detrimental concentration have shown that when the concentration of Pb is administered at 12.5%, the cell viability is approximately 86%. Based on this, relative ROS content and NO release were determined at 10% and at half-diluted concentrations. The results demonstrated that Pb concentrations of 2.5%, 5%, and 10% all significantly reduced the oxidative stress in cells caused by PS-MP. Specifically, the highest effective concentration of 10% could reduce oxidative stress to levels comparable to those of the control group, highlighting its potent antioxidant capacity in vitro. In terms of NO release, although individual concentrations did not achieve statistical significance in reduction, a consistent trend towards slight reduction by Pb was observed across all tested concentrations (Fig. 1), suggesting a modest anti-inflammatory effect at the cellular level.
Effects of Pb on PS-MP induced ROS production for 24 h by ROS assay and induced nitric oxide production for 24 h by NO assay in RAW 264.7 cells. Cells were adjusted to 106 cells/mL were treated for 24 h with Pb and PS-MP (100 μg/mL). Control was treated with medium only. Results are show by mean ± S.E.M. (n = 3). *P < 0.05 versus only PS-MP, **P < 0.01 versus only PS-MP.
Body weight and changes in organ coefficient after exposure to PS- MPS
After 7 weeks of PS-MP and probiotic exposure, no significant changes in overall body weight were observed across the groups. As shown in Fig. 2, the body weight of the rats in each group increased steadily, with no rapid fluctuations, and weekly weight measurements showed no significant differences between groups.. However, organ weight ratios in PS-MP-exposed rats revealed specific alterations, particularly a significant decrease in testicular weight ratios within the high-dose PS-MP group compared to the control group. Crucially, this decline was significantly improved following probiotic administration, suggesting a protective effect on testicular mass. Conversely, no significant changes were noted in the epididymal weight ratio (Table 1).
Body weight of rats between 7 to 15 weeks-old. Sample was intragastrically administered at 8 weeks-old. Date are shown as the mean ± S.E.M. (n = 8 rats/group). Con: control; PS-MP1: 1 mg/kg Polystyrene microplastic (PS-MP) per day; PS-MP5: 5 mg/kg PS-MP per day; PS-MP10:10 mg/kg PS-MP per day; PS-MP10 + Pro: 10 mg/kg PS-MP + 108 number of probiotics per day; Pro: 108 number of probiotics.
Testicular damage analysis
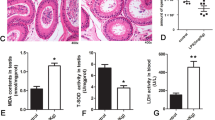
Following histopathological analysis of rat testicular tissue via H&E staining, significant morphological alterations were observed across the PS-MP-exposed groups. In the control group, seminiferous tubules were closely arranged, the wall was thicker, the interstitium had no obvious cavity, and the blood testicular barrier remained intact;
Conversely, the PS-MP1 group exhibited partial interstitial cavitation and bent/deformed seminiferous tubules, which progressively worsened in the PS-MP5 group with more extensive interstitial cavitation and numerous damaged seminiferous tubules. The PS-MP10 group showed the most severe pathology, characterized by severe deformation, damage, interstitial cavitation, and enlarged seminiferous tubule lumens.
Crucially, the PS-MP10 + PRO group, despite retaining some damage and interstitial cavitation, demonstrated significantly reduced severity compared to the PS-MP10 group, indicating a protective effect of probiotics. The Pro group’s testicular morphology was comparable to the Con group, with closely arranged seminiferous tubules, intact walls, absence of interstitial voids, and normal lumen area. These findings collectively indicate that probiotic supplementation can significantly ameliorate PS-MP-induced testicular damage in the seminiferous tubules (Fig. 3).
After 49 days of feeding with different doses of microplastic and probiotics, the changes of seminiferous tubules in rats were observed by hematoxylin and eosin (H&E) staining. Con: control; MP-PS1: 1 mg/kg Polystyrene microplastic (MP-PS) per day; MP-PS5: 5 mg/kg MP-PS per day; MP-PS10:10 mg/kg MP-PS per day; MP-PS10 + Pro: 10 mg/kg MP-PS + 108 number of probiotics per day; Pro: 108 number of probiotics.
To quantify these morphological changes, ImageJ software was used to further analyze the diameter of the seminiferous tubules, the thickness of the epithelium, the area of the seminiferous tubules, and the ratio of the lumen to the seminiferous tubules. The results showed that the diameter of the seminiferous tubules in the PS-MP group was significantly longer than in the Con group. Concurrently, the epithelial thickness was significantly thinner and the lumen area significantly larger than controls, leading to a higher lumen-to-seminiferous tubule area ratio, further confirming that PS-MP exposure induces testicular damage, and that probiotics can improve these histomorphometric parameters in male rats (Table 2).
Consistent with the observed histopathological damage, lactate dehydrogenase (LDH) activity in testicular tissue was measured as a biomarker of cellular injury43. Although there was no significant difference between the groups, the high-dose PS-MP was still the highest, approximately 417 U/L, reinforcing that PS-MP indeed causes testicular damage in a dose-dependent manner.,significantly, probiotic administration was associated with a reduction in LDH activity, thereby confirming its ability to mitigate PS-MP-induced testicular injury (Fig. 4).
Testis lactate dehydrogenase (LDH) production in rats fed different doses of MP-PS and probiotics after 49 days. Date are shownas the mean ± S.E.M. (n = 7 rats/group). Con: control; MP-PS1: 1 mg/kg Polystyrene microplastic (MP-PS) per day; MP-PS5: 5 mg/kg MP-PS per day; MP-PS10:10 mg/kg MP-PS per day; MP-PS10 + Pro: 10 mg/kg MP-PS + 108 number of probiotics per day; Pro: 108 number of probiotics. The values with different letters represent significantly different (p < 0.05) as analyzed by Duncan’s multiple range test.
Sperm appearance, number, motility rate, and abnormal rate
Microscopic examination revealed a higher incidence of sperm abnormalities in the PS-MP groups, including abnormal aggregation, decapitation, abnormal head shape, tail docking, and curling, indicative of substantial damage. Fortunately, after 7 weeks of probiotic supplementation, sperm morphology showed a clear tendency towards normalcy (Fig. 5), suggesting a protective effect.
Morphology of sperm of rats fed microplastic and probiotics after 49 days. Light microscopy of sperm in each group. To quantify each sperms are 106. Con: control; MP-PS1: 1 mg/kg Polystyrene microplastic (PS-MP) per day; PS-MP5: 5 mg/kg PS-MP per day; PS-MP10:10 mg/kg PS-MP per day; PS-MP10 + Pro: 10 mg/kg PS-MP + 108 number of probiotics per day; Pro: 108 number of probiotics.
The sperm parameters of each group were further observed, and the results showed that the total number of sperm in the PS-MP group at medium and high doses was significantly lower than that of the Con group. The mobility rate was lower in the PS-MP group than in the Con group. The medium and high doses were significantly higher than those in the Con group; these findings clearly demonstrate that PS-MP exposure adversely impacts spermatozoa quality and morphology. However, after probiotic administration, although the sperm motility rate cannot be significantly improved, it can significantly improve sperm count and abnormal ratio. The results above demonstrate that probiotics can enhance sperm quality and morphology. However, despite not achieving a statistically significant improvement in sperm motility, probiotic administration significantly improved both sperm count and reduced the abnormal sperm ratio, underscoring its ability to enhance overall sperm quality and morphology (Fig. 6).
Sperm (A) count, (B) mobility and (C) abnormality in rats fed different concentration polystyrene microplastic and probiotics after 7 weeks. Sperms were adjusted to 106 cells/mL. Data are shown as the mean ± S.E.M. (n = 7 rats/group). Con: control; PS-MP1:1 mg/kg Polystyrene microplastic (PS-MP) per day; PS-MP5: 5 mg/kg PS-MP per day; PS-MP10:10 mg/kg PS-MP per day; PS-MP10 + Pro: 10 mg/kg MP-PS + 108 number of probiotics per day; Pro: 108 number of probiotics. The values with different letters (a–d) represent significantly different (p < 0.05) as analyzed by Duncan’s multiple range test. *P < 0.05 versus control group.
Oxidative stress and antioxidant enzymes
Given that oxidative stress is a key mechanism of reproductive toxicity, we measured malondialdehyde (MDA) content to assess the extent of lipid peroxidation, as polyunsaturated fatty acids are highly susceptible to free radical attack, leading to MDA production18. The findings revealed that high-dose PS-MP resulted in the highest MDA content, particularly in sperm, where the levels were significantly elevated compared to other groups. This indicates severe lipid peroxidation and oxidative damage following PS-MP exposure.
Crucially, administration of probiotics via tube feeding significantly improved MDA levels, especially in sperm, with the probiotic-only group showing lower MDA content in sperm and plasma than even the control group, directly indicating a reduction in lipid peroxidation (Fig. 7). Furthermore, tube feeding of probiotics resulted in increased GPx activity in both plasma and testicular tissue, thereby mitigating the oxidative stress induced by PS-MP (Fig. 8) thereby directly mitigating the oxidative stress induced by PS-MP. These results conclusively confirm that probiotics can effectively reduce MDA content and significantly enhance GPx antioxidant enzyme activity in vivo, underscoring their potential therapeutic benefits in counteracting oxidative stress-induced reproductive harm.
Effects of different doses of PS-MP and probiotics on malondialdehyde (MDA) levels of (A) sperm, (B) plasma and (C) testis in rats after 7 weeks. Sperms were adjusted to 106 cells/mL. Data are shown as the mean ± S.E.M. (n = 7 rats/group). Con: control; PS-MP1:1 mg/kg Polystyrene microplastic (PS-MP) per day; PS-MP5: 5 mg/kg PS-MP per day; PS-MP10:10 mg/kg PS-MP per day; PS-MP10 + Pro: 10 mg/kg PS-MP + 108 number of probiotics per day; Pro: 108 number of probiotics. The values with different letters (a–c) represent significantly different (p < 0.05) as analyzed by Duncan’s multiple range test. *P < 0.05 versus control group.
Effects of different doses of PS-MP and probiotics on Glutathione peroxidase (GPx) levels of (A) plasma, (B) testis in rats after 7 weeks. Data are shown as the mean ± S.E.M. (n = 7 rats/group). Con: control; PS-MP1:1 mg/kg Polystyrene microplastic (PS-MP) per day; PS-MP5: 5 mg/kg PS-MP per day; PS-MP10:10 mg/kg PS-MP per day; PS-MP10 + Pro: 10 mg/kg PS-MP + 108 number of probiotics per day; Pro: 108 number of probiotics. The values with different letters represent significantly different (p < 0.05) as analyzed by Duncan’s multiple range test.
Effects of PS-MP and probiotics on the inflammatory response in rats
Beyond oxidative stress, we investigated the inflammatory response, recognizing that tumor necrosis factor-alpha (TNF-α), a key proinflammatory cytokine, is implicated in both local and systemic inflammation, driving the generation of inflammatory mediators like nitric oxide (NO) and reactive oxygen species (ROS), which detrimentally affect spermatogenesis, sperm membranes, and semen quality44. Analysis of testicular and plasma TNF-α levels revealed a notable increase in the PS-MP group, particularly at higher doses, signifying a significant difference from the control group. While probiotics showed potential in mitigating TNF-α expression (Fig. 9), the extent of reduction was not statistically significant. Nevertheless, these results clearly underscore a heightened inflammatory response in rats following PS-MP exposure, with probiotics demonstrating a modest yet positive ameliorative effect. Furthermore, investigation into nitric oxide (NO) levels was critical, as excessive NO production due to inflammation is known to damage testicular tissue and cells45. Notably, the concentration of NO in testicular tissue was significantly elevated in the high-dose PS-MP group, with a slight increase observed in plasma. However, a significant finding was that probiotic administration led to a marked improvement in testicular no concentration and a reduction in sperm no concentration. These findings emphasize the potential of probiotics in effectively mitigating the inflammatory and harmful effects associated with PS-MP exposure (Fig. 10), complementing their antioxidant actions.
Effects of different doses of PS-MP and probiotics on TNF-α production of (A) testis and (B) plasma in rats after 7 weeks. Data are shown as the mean ± S.E.M. (n = 7 rats/group). Con: control; PS-MP1:1 mg/kg Polystyrene microplastic (PS-MP) per day; PS-MP5: 5 mg/kg PS-MP per day; PS-MP10:10 mg/kg PS-MP per day; PS-MP10 + Pro: 10 mg/kg PS-MP + 108 number of probiotics per day; Pro: 108 number of probiotics. The values with different letters (a–c) represent significantly different (p < 0.05) as analyzed by Duncan’s multiple range test. *P < 0.05 vsersu control group.
Effects of different doses of PS-MP and probiotics on NO concentration of (A) plasma, (B) testis ans (C) sperm in rats after 7 weeks. Sperms were adjusted to 106 cells/mL. Data are shown as the mean ± S.E.M. (n = 7 rats/group). Con: control; PS-MP1:1 mg/kg Polystyrene microplastic (PS-MP) per day; PS-MP5: 5 mg/kg PS-MP per day; PS-MP10:10 mg/kg PS-MP per day; PS-MP10 + Pro: 10 mg/kg MP-PS + 108 number of probiotics per day; Pro: 108 number of probiotics. The values with different letters (a–c) represent significantly different (p < 0.05) as analyzed by Duncan’s multiple range test.
Effects of PS-MP and probiotics on the reproductive endocrine system of rats
Expression of Kisspeptin and its GPR54 receptor
To further understand the mechanisms underlying PS-MP-induced reproductive harm, we quantitatively analyzed key components of the hypothalamic-pituitary–gonadal (HPG) axis, given that imbalances in this axis can lead to hypogonadism in both rodents and humans46. Specifically, kisspeptin levels in plasma, hypothalamus, and GPR54 in the hypothalamus and testes were analyzed quantitatively. The results showed that there were no significant differences in kisspeptin in the plasma of PS-MP fed by the tube at each concentration. However, it could still be seen that its concentration was slightly lower than that of the control group. The tenfold dose of PS-MP had the lowest expression, which was 254 pg/ml. Encouragingly, probiotic administration increased plasma kisspeptin to approximately 350 pg/ml, comparable to the control group. However, the expression level of Kisspeptin in the hypothalamus showed that after administration of PS-MP, its expression level was significantly lower than that of the Con group, and after administration of probiotics, it could still could not recover to the same level as the Con group (Fig. 11). The expression of GPR54 in the hypothalamus decreased by tube feeding PS-MP at various concentrations, but there were no significant differences in each group, and probiotics could not improve the expression of GPR54 in the hypothalamus; however, in testicular tissue, GPR54 expression was significantly lower in the PS-MP groups compared to the control, with the high dose showing the most pronounced decrease. While probiotic administration did not lead to a statistically significant improvement in testicular GPR54, a slight increase in its expression was observed (Fig. 12).
Effect of MP-PS and probiotics on (A) plasma, (B) hypothalamus Kiss1 in rats after 7 weeks. Date are shownas the mean ± S.E.M. (n = 6 rats/group). Con: control; MP-PS1: 1 mg/kg Polystyrene microplastic (MP-PS) per day; MP-PS5: 5 mg/kg MP-PS per day; MP-PS10:10 mg/kg MP-PS per day; MP-PS10 + Pro: 10 mg/kg MP-PS + 108 number of probiotics per day; Pro: 108 number of probiotics. The values with different letters (a, b) represent significantly different (p < 0.05) as analyzed by Duncan’s multiple range test.
Effect of PS-MP and probiotics on (A) hypothalamus and (B) Testis GPR54 in rats after 7 weeks. Date are shownas the mean ± S.E.M. (n = 5 rats/group). Con: control; MP-PS1: 1 mg/kg Polystyrene microplastic (MP-PS) per day; MP-PS5: 5 mg/kg MP-PS per day; MP-PS10:10 mg/kg MP-PS per day; MP-PS10 + Pro: 10 mg/kg MP-PS + 108 number of probiotics per day; Pro: 108 number of probiotics. The values with different letters represent significantly different (p < 0.05) as analyzed by Duncan’s multiple range test.
Plasma sex hormones and testicular testosterone concentrations
Considering that PS-MP exposure downregulates kisspeptin and GPR54 in the hypothalamus, potentially disrupting the endocrine axis47, we subsequently measured the concentrations of key androgens in rat plasma and testicular tissue, including luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone. The results showed that both FSH and LH levels were lower than those of the Con group after exposure to PS-MP, with FSH levels being more significantly reduced. This likely contributed to the observed reduction in sperm quality.
Significantly, probiotic administration improved plasma FSH concentration, although LH levels remained lower (Fig. 13). Regarding testosterone, there was no significant difference in testosterone concentrations in plasma and testicular tissue; however, the high-dose PS-MP group had the lowest testosterone concentrations. Nevertheless, probiotic administration led to improved testosterone concentrations in both plasma and testicular tissue, confirming its ability to help restore some sex hormone concentrations to near-normal levels in PS-MP-exposed rats
Expression of (A) Follicle Stimulating Hormone (FSH) (B) Luteinizing Hormone (LH) in plasma of rats fed different dose of MP-PS and probiotics after 7 weeks. Date are shownas the mean ± S.E.M. (n = 7 rats/group). Con: control; MP-PS1: 1 mg/kg Polystyrene microplastic (MP-PS) per day; MP-PS5: 5 mg/kg MP-PS per day; MP-PS10:10 mg/kg MP-PS per day; MP-PS10 + Pro: 10 mg/kg MP-PS + 108 number of probiotics per day; Pro: 108 number of probiotics. The values with different letters (a–c) represent significantly different (p < 0.05) as analyzed by Duncan’s multiple range test.Expression of (C) testosterone in plasma (D) testis tissue testosterone of rats fed different dose of MP-PS and probiotics after 7 weeks. Date are shownas the mean ± S.E.M. (n = 7 rats/group). Con: control; MP-PS1: 1 mg/kg Polystyrene microplastic (MP-PS) per day; MP-PS5: 5 mg/kg MP-PS per day; MP-PS10:10 mg/kg MP-PS per day; MP-PS10 + Pro: 10 mg/kg MP-PS + 108 number of probiotics per day; Pro: 108 number of probiotics. The values with different letters (a–c) represent significantly different (p < 0.05) as analyzed by Duncan’s multiple range test.
Discussion
In recent years, there has been a gradual increase in research on plastic particles, largely due to the accelerated deterioration of land pollution caused by human activities48. While previous studies have demonstrated the harmful effects of plastic microparticles on the reproductive systems of male mammals, there is a lack of research on mitigating this damage14,17,33. This study aims to address this gap by conducting tube feeding experiments with low, medium, and high concentrations of plastic microparticles (PS-MP) and exploring the potential improvement effect of probiotics at high concentrations. Additionally, a separate probiotics group will be fed to investigate their potential role in enhancing reproductive performance. It is essential to note that the concentrations of PS-MP used in this experiment may not accurately reflect the actual exposure conditions, but were selected from a precautionary perspective to assess risk at high concentrations. Furthermore, this study acknowledges the various environmental exposure routes, such as inhalation and local exposure, and their potential implications.
Previous studies have demonstrated that polystyrene microplastics can induce testicular oxidative stress and activate the p38 and JNK MAPK signaling pathways, leading to decreased sperm quality and testosterone production in mice, as well as oxidative stress. It can cause lipid peroxidation, which adversely affects sperm morphology and function49. In addition, exposure to microplastics also reduces the activity of antioxidant enzymes in the blood and testes50. Our findings are fully consistent with these reports, providing further in vivo validation of PS-MP’s detrimental impacts on oxidative homeostasis. The results of this experiment showed that after PS-MP tube feeding, sperm NBT and MDA increased, especially in the PS-MP10 group, which was significantly increased compared to the control group. Although not statistically significant across all groups, blood and testicular tissue GPx activities consistently showed lower activity after PS-MP administration. Crucially, our study further demonstrates that probiotic administration effectively counteracts these effects. Probiotics significantly reduced oxidative stress and MDA caused by PS-MP, and increased the activity of antioxidant enzymes in both blood and testicular tissue, highlighting their potent antioxidant properties in vivo.
Building upon the link between oxidative stress and inflammation. Smaller plastic particles enter cells and increase oxidative stress through ROS production17, promoting the activation of NF-κB. The secretion of inflammatory cytokines18, such as TNF-α, is considered a proinflammatory factor in local and systemic inflammation. It can directly or indirectly inhibit spermatogenesis, decreasing semen quality44,51. The results showed that after exposure to PS-MP, the content of TNF-α in the testes and plasma increased, especially in the tenfold dose of the PS-MP group, which was significantly increased compared to the Con group. While probiotic administration resulted in a slight decrease of TNF-α in testicular tissue, a significant difference was not observed in plasma. Further supporting an anti-inflammatory role, probiotics also significantly reduced NO levels in both the testes and sperm, consistent with studies by31.
Collectively, these results indicate that while 10 mg/kg PS-MP exposure increases inflammatory factors in plasma and testicular tissue, probiotics can effectively mitigate the concentration of these inflammatory factors, thereby improving the overall reproductive system.
Beyond direct cellular and tissue damage, our study investigated the impact of PS-MP on the critical hypothalamic-pituitary–gonadal (HPG) axis, given that disrupted kisspeptin/GPR54 signaling is known to lead to hypogonadism in humans and rodents46. The results showed that both the plasma and hypothalamus had the lowest kisspeptin content in the PS-MP10 group, with a significant difference compared to the Con group. Furthermore, the GPR54 content in the hypothalamus and testicular tissue was also the lowest in the PS-MP10 group. These findings underscore a significant disruption of the HPG axis at its core regulatory levels by PS-MP exposure. In response to probiotic administration, the content of kisspeptin in plasma and GPR54 in testicular tissue could be increased, suggesting a partial restoration of HPG axis components, but it did not affect kisspeptin and GPR54 in the hypothalamus.
Consistent with the alterations observed in the hypothalamic and testicular HPG axis components, our measurement showed that the concentration of FSH in plasma decreased significantly after exposure to PS-MP; LH decreased slightly, while LH regulated testosterone, so it can be seen that the results of testosterone concentration in plasma and testicular tissue. This reduction in FSH likely contributed to the observed impaired sperm quality. Fortunately, probiotic administration led to an increase in plasma FSH concentration, although it did not significantly affect LH levels (Fig. 13). While no statistically significant differences were observed in testosterone concentrations in plasma and testicular tissue, the high-dose PS-MP group consistently exhibited the lowest testosterone levels. Crucially, probiotic administration resulted in improved testosterone concentrations in both plasma and testicular tissue. This is a vital finding, confirming that probiotics can help restore the concentration of key sex hormones to near-normal levels in rats exposed to PS-MP. The observed positive effect of probiotics on testosterone, despite not significantly affecting LH, suggests a potential mechanism involving gut microbiota modulation.
Specific bacteria in the steroid hormone gut can promote the metabolism by stimulating different enzymes52, such as Clostridium scindens in the gut, which can be the cholesterol side-chain dissociation enzyme of cholesterol that converts glucocorticoid into androgen testosterone53; however, studies have also pointed out that the gut microbiota is involved in regulating the permeability of the blood testicular barrier and regulating testicular endocrine function54, for instance, short-chain fatty acids (SCFAs) produced by gut bacteria, such as butyrate, propionate, and acetate, are known to exert anti-inflammatory effects and influence hormone metabolism, potentially contributing to the restoration of hormonal balance observed in our study55,56 .These mechanisms provide a plausible explanation for how probiotics can improve FSH and testosterone concentrations in rats, thereby contributing to the repair of the endocrine reproductive system.
Our research confirms that rats exposed to PS-MP have detrimental effects on the reproductive system. These include damage to the testicular tissue, increased oxidative stress, and disruption of the endocrine reproductive system. Probiotic supplementation shows promise in addressing the reproductive damage caused by exposure to PS-MP, with potential implications for spermatogenesis outcomes.
Conclusions
In summary, this comprehensive study systematically investigated the detrimental effects of polystyrene microplastics (PS-MP) on male reproductive health in rats and, crucially, the potential therapeutic role of probiotic intervention. Our findings clearly demonstrate that PS-MP exposure induces significant testicular damage, characterized by altered testicular morphology, decreased sperm count and motility, and increased sperm abnormalities. Mechanistically, this toxicity was linked to a prominent increase in oxidative stress (evidenced by elevated MDA and reduced GPX activity) and the induction of inflammation (increased TNF-α and NO levels). Furthermore, PS-MP negatively impacted the reproductive endocrine axis, leading to downregulation of hypothalamic kisspeptin and GPR54, and alterations in plasma FSH and LH concentrations, ultimately compromising testosterone levels.
Significantly, probiotic supplementation effectively mitigated these adverse effects. Probiotics strongly restored antioxidant capacity and reduced inflammatory markers in vivo, leading to marked improvements in testicular histomorphology and sperm quality parameters. Moreover, probiotics partially reversed the endocrine disruptions caused by PS-MP, particularly by improving plasma FSH and testosterone levels. The significance of this study is multifold. It not only reinforces the growing concern regarding environmental microplastic pollution and its adverse impact on male fertility but also provides compelling in vivo evidence for the therapeutic potential of probiotics as a novel strategy to alleviate such reproductive harm. This research offers critical insights into the mechanisms of both PS-MP toxicity and probiotic-mediated protection, highlighting the interplay between oxidative stress, inflammation, and the HPG axis. Our findings are particularly noteworthy for their translational potential, suggesting a promise for dietary interventions in mitigating environmental toxin-induced reproductive disorders.
Looking ahead, future research should explore the long-term effects of both PS-MP exposure and probiotic intervention, investigate different types and sizes of microplastics, and elucidate the precise mechanisms through which probiotics modulate the gut-gonad axis. Further studies should also consider clinical trials to assess the applicability of these findings to human male reproductive health. Ultimately, this work paves the way for the development of novel nutritional strategies to combat environmental pollutant-induced fertility issues.
Data availability
The data from this study will be made available upon request. For data access inquiries, please contact the corresponding author, Deng-Fwu Hwang (dfhwang@mail.ntou.edu.tw).
References
Thompson, R. C. et al. Lost at sea: where is all the plastic?. Science 304, 838–838 (2004).
Arthur, C., Baker, J. E. & Bamford, H. A. Proceedings of the international research workshop on the occurrence, effects, and fate of microplastic marine Debris, September 9–11, 2008, University of Washington Tacoma, Tacoma, WA, USA. (2009).
Alimba, C. G. & Faggio, C. Microplastics in the marine environment: current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 68, 61–74 (2019).
PlasticsEurope. Plastics – the Facts 2021. An analysis of European plastics production, demand and waste data, <https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021> (2021).
Meijer, L. J., van Emmerik, T., van der Ent, R., Schmidt, C. & Lebreton, L. More than 1000 rivers account for 80% of global riverine plastic emissions into the ocean. Science Advances 7, eaaz5803 (2021).
Smith, M., Love, D. C., Rochman, C. M. & Neff, R. A. Microplastics in seafood and the implications for human health. Current Environ. Health Rep. 5, 375–386 (2018).
Teuten, E. L. et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philosophical Trans. Royal Soc. B: Biol. Sci. 364, 2027–2045 (2009).
Setälä, O., Fleming-Lehtinen, V. & Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 185, 77–83 (2014).
Izuddin, W. I., Humam, A. M., Loh, T. C., Foo, H. L. & Samsudin, A. A. Dietary postbiotic lactobacillus plantarum improves serum and ruminal antioxidant activity and upregulates hepatic antioxidant enzymes and ruminal barrier function in post-weaning lambs. Antioxidants (Basel) https://doi.org/10.3390/antiox9030250 (2020).
Yang, D. et al. Microplastic pollution in table salts from China. Environ. Sci. Technol. 49, 13622–13627 (2015).
Abbasi, A., Aghebati-Maleki, A., Yousefi, M. & Aghebati-Maleki, L. Probiotic intervention as a potential therapeutic for managing gestational disorders and improving pregnancy outcomes. J. Reprod. Immunol. 143, 103244 (2021).
Amato-Lourenço, L. F. et al. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 416, 126124. https://doi.org/10.1016/j.jhazmat.2021.126124 (2021).
Kosuth, M., Mason, S. A. & Wattenberg, E. V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 13, e0194970 (2018).
Hou, B., Wang, F., Liu, T. & Wang, Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 405, 124028 (2021).
Bhardwaj, J. K., Siwach, A., Sachdeva, D. & Sachdeva, S. N. Revisiting cadmium-induced toxicity in the male reproductive system: an update. Arch. Toxicol. 98, 3619–3639. https://doi.org/10.1007/s00204-024-03871-7 (2024).
Jin, H. et al. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 401, 123430 (2021).
Xie, X. et al. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 190, 110133 (2020).
Lu, Y. & Wahl, L. M. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-κB activity in lipopolysaccharide-activated human primary monocytes. J. Immunol. 175, 5423–5429 (2005).
Ijaz, M. U. et al. Dose-dependent effect of polystyrene microplastics on the testicular tissues of the male sprague dawley rats. Dose Response 19, 15593258211019882. https://doi.org/10.1177/15593258211019882 (2021).
Ramalho-Santos, J., Amaral, S. & Oliveira, P. J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr. Diabetes Rev. 4, 46–54 (2008).
Bhardwaj, J. K., Panchal, H. & Saraf, P. Cadmium as a testicular toxicant: A review. J. Appl. Toxicol. 41, 105–117. https://doi.org/10.1002/jat.4055 (2021).
Gurung, P. & Jialal, I. Physiology, male reproductive system. StatPearls [Internet] (2020).
George, J. T., Millar, R. P. & Anderson, R. A. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology 91, 302–307 (2010).
Wang, J. et al. Meta-analysis of the effects of microplastic on fish: Insights into growth, survival, reproduction, oxidative stress, and gut microbiota diversity. Water Res. 267, 122493. https://doi.org/10.1016/j.watres.2024.122493 (2024).
Pan, I. & Umapathy, S. Probiotics an emerging therapeutic approach towards gut-brain-axis oriented chronic health issues induced by microplastics: A comprehensive review. Heliyon https://doi.org/10.1016/j.heliyon.2024.e32004 (2024).
Hunt, K. et al. Exposure to microplastics and human reproductive outcomes: A systematic review. BJOG: An Int. J. Obstetrics Gynaecol. https://doi.org/10.1111/1471-0528.17756 (2024).
Yang, S. et al. Reproductive toxicity of micro- and nanoplastics. Environ. Int. 177, 108002. https://doi.org/10.1016/j.envint.2023.108002 (2023).
Mei, H.-C. et al. Immunomodulatory activity of Lactococcus lactis A17 from Taiwan fermented cabbage in OVA-sensitized BALB/c mice. Evidence-Based Complementary and Alternative Medicine 2013 (2013).
Yan, F. et al. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132, 562–575 (2007).
Sun, Q. et al. L. plantarum, L. fermentum, and B. breve Beads Modified the Intestinal Microbiota and Alleviated the Inflammatory Response in High-Fat Diet–Fed Mice. Probiotics and antimicrobial proteins 12, 535–544 (2020).
Helli, B., Kavianpour, M., Ghaedi, E., Dadfar, M. & Haghighian, H. K. Probiotic effects on sperm parameters, oxidative stress index, inflammatory factors and sex hormones in infertile men. Human Fertility, 1–9 (2020).
Vale, G. C. & Mayer, M. P. A. Effect of probiotic Lactobacillus rhamnosus by-products on gingival epithelial cells challenged with Porphyromonas gingivalis. Arch. Oral Biol. 128, 105174 (2021).
Amereh, F., Babaei, M., Eslami, A., Fazelipour, S. & Rafiee, M. The emerging risk of exposure to nano (micro) plastics on endocrine disturbance and reproductive toxicity: from a hypothetical scenario to a global public health challenge. Environ. Pollut. 261, 114158 (2020).
Li, X. et al. Lactobacillus plantarum and Lactobacillus fermentum alone or in combination regulate intestinal flora composition and systemic immunity to alleviate obesity syndrome in high-fat diet rat. Int. J. Food Sci. Technol. 53, 137–146 (2018).
Chen, J. et al. Cellular absorption of polystyrene nanoplastics with different surface functionalization and the toxicity to RAW264. 7 macrophage cells. Ecotoxicol. Environ. Saf. 252, 114574 (2023).
Robertson, C. Comparative methodology for analyzing nitric oxide production in RAW 264.7 Murine macrophages in response to lipopolysaccharide treatment. (2019).
Collodel, G. et al. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 31, 239–246 (2011).
Tang, X. et al. Polystyrene nanoplastics exacerbated lipopolysaccharide-induced necroptosis and inflammation via the ROS/MAPK pathway in mice spleen. Environ. Toxicol. 37, 2552–2565 (2022).
Mohamed, N. A., Ahmed, O. M., Hozayen, W. G. & Ahmed, M. A. Ameliorative effects of bee pollen and date palm pollen on the glycemic state and male sexual dysfunctions in streptozotocin-Induced diabetic wistar rats. Biomed. Pharmacother. 97, 9–18 (2018).
Sun, J., Zhang, X., Broderick, M. & Fein, H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors 3, 276–284 (2003).
Dhindsa, S. et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care 39, 82–91 (2016).
Parks, G. S., Wang, L., Wang, Z. & Civelli, O. Identification of neuropeptide receptors expressed by melanin-concentrating hormone neurons. J. Comparative Neurol. 522, 3817–3833 (2014).
Farhana, A. & Lappin, S. L. in StatPearls [internet] (StatPearls Publishing, 2023).
Fraczek, M. & Kurpisz, M. Inflammatory mediators exert toxic effects of oxidative stress on human spermatozoa. J. Androl. 28, 325–333 (2007).
Han, X.-X. et al. Protective effects of astragalin on spermatogenesis in streptozotocin-induced diabetes in male mice by improving antioxidant activity and inhibiting inflammation. Biomed. Pharmacother. 110, 561–570 (2019).
Novaira, H. J. et al. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol. Endocrinol. 28, 225–238 (2014).
Graceli, J. B. et al. The impact of endocrine-disrupting chemical exposure in the mammalian hypothalamic-pituitary axis. Mol. Cell. Endocrinol. 518, 110997. https://doi.org/10.1016/j.mce.2020.110997 (2020).
Horton, A. A., Walton, A., Spurgeon, D. J., Lahive, E. & Svendsen, C. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 586, 127–141 (2017).
Dutta, S., Henkel, R., Sengupta, P. & Agarwal, A. in Male infertility 337–345 (Springer, 2020).
Ijaz, M. U. et al. Dose-dependent effect of polystyrene microplastics on the testicular tissues of the male Sprague Dawley rats. Dose-Response 19, 15593258211019882 (2021).
Bhardwaj, J. & Rathee, V. Toxicological impact of nanoparticles on reproductive system: A review. Toxicol Int 30, 605–628 (2023).
Hussain, T. et al. Relationship between gut microbiota and host-metabolism: Emphasis on hormones related to reproductive function. Animal Nutrition (2021).
Ridlon, J. M. et al. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res. 54, 2437–2449 (2013).
Al-Asmakh, M. et al. The gut microbiota and developmental programming of the testis in mice. PLoS ONE 9, e103809 (2014).
Facchin, S. et al. Short-chain fatty acids and human health: From metabolic pathways to current therapeutic implications. Life 14, 559 (2024).
Fusco, W. et al. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients https://doi.org/10.3390/nu15092211 (2023).
Acknowledgements
This work was financially supported by the Center of Excellence for Oceans, National Taiwan Ocean University, from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan, MOST 110-2320-B-019-006
Funding
This research did not receive specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
ZLK and DFH conceived and designed the research; YYH and QTT analyzed the data and performed the research; YYH, QTT, and JF wrote the original draft. ZLK, DFH, and RFS conceptualized, supervised, and reviewed the manuscript. ZLK, DFH, SM, and FK. edited and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Animal and human rights
Ethics statement: The authors followed the Institutional Animal Care and Use Committee, College of Life Science, National Taiwan Ocean University’s evaluated and approved ethics committee (IUCUC approved number 110028, 13 July 2021). The study protocol for using laboratory animals for this study followed Directive 2010/63/EU guidelines. No humans were used in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hwang, YY., Tsen, QT., Felim, J. et al. Probiotics as a therapeutic approach to alleviate reproductive harm from polystyrene microplastics in male rats. Sci Rep 15, 34783 (2025). https://doi.org/10.1038/s41598-025-18550-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18550-5