Abstract
Microplastics (MPs) and invasive species are two of the most pressing threats to freshwater ecosystems, yet their interactions remain underexplored. This study presents the first comparative analysis of MP uptake among three coexisting invasive crayfish species (Faxonius limosus, Pacifastacus leniusculus, and Procambarus clarkii) from Lake Maggiore, a large subalpine lake subjected to intense anthropogenic pressure. A total of 90 individuals were analyzed for biometric traits and MP occurrence in intestinal content, with species identification confirmed via molecular analysis. No significant interspecific differences or correlations with biometric traits were found, though F. limosus showed the highest average concentration. Most MPs were < 1 mm polyester or polyacrylate fibers, suggesting a dominant domestic source. Additionally, this work provides the first evidence of MP uptake in F. limosus, filling a key knowledge gap. Beyond documenting MP ingestion, our findings support the potential of invasive crayfish as agents of MP removal, suggesting an ecological role with important implications for environmental monitoring and ecosystem management. These results also highlight the need for further research on trophic transfer and organ-level accumulation of MPs, especially in widely distributed invasive species that are increasingly relevant for environmental risk assessment.
Similar content being viewed by others
Introduction
Freshwater ecosystems, though covering a small portion of Earth’s surface, are highly biodiverse and provide vital ecological, economic, and cultural services1. However, they face increasing threats that jeopardize their biodiversity and functions2. Pollution from agricultural runoff, untreated sewage, industrial effluents, and plastic waste severely degrades freshwater quality and habitats3,4. Additionally, the introduction of invasive species, whether intentional or accidental, exacerbates these pressures by displacing native biota, altering trophic interactions, and facilitating the spread of pathogens5.
Plastic production has surged in recent years, driven by increasing global demand across various industries, including packaging, construction, and consumer goods6. In 2023 alone, global plastic production was estimated to exceed 413.8 million metric tons (Mt), reflecting a persistent upward trend7. Plastics, according to their sizes, are classified in macroplastics (> 5 mm), large microplastics (lMPs, 5 –1 mm), microplastics (MPs, 1 mm–1 μm) and nanoplastics (1 μm-100 nm)8. In this paper, the term “MPs” will be used to refer collectively to both large microplastics (lMPs, 5 –1 mm) and microplastics (MPs, 1 mm–1 μm), unless otherwise specified. MPs are classified as primary or secondary based on their origin: primary MPs result directly from industrial processes, such as plastic pellets9, while secondary MPs arise from the fragmentation of plastic debris or from textiles during washing and wear10. Notably, physicochemical variables can speed up the deterioration of plastics through mechanical stimulation, biological, thermal, and photo-oxidative degradation11.
Freshwater ecosystems are less studied in the context of plastic pollution compared to other environments, and significant knowledge gaps remain regarding the sources, pathways, fate, and distribution of plastics within them12. Aquatic biota plays a crucial role in the transport, temporary storage, and transformation of plastics in these ecosystems13. A broad variety of aquatic organisms, including birds14, fish15, bivalves16, crustaceans17 and other invertebrates ingest MPs leading to various toxic effects18. These include behavioural modification, metabolic disorders, and compromised immune responses, each of which poses significant threats to the composition and stability of aquatic ecosystems18. Identifying sentinel species for monitoring plastics’ occurrence and their effects on ecosystems is essential for understanding their environmental impact and developing management strategies, as shown by previous studies12,16,19.
Invasive species are a major driver of biodiversity loss globally, with invasive crayfish being among the most widely introduced species in freshwater ecosystems20,21. Europe currently hosts 12 invasive crayfish species, four of which — Faxonius limosus (Rafinesque, 1817), Pacifastacus leniusculus (Dana, 1852), Procambarus clarkii (Girard, 1852), and Procambarus virginalis Lyko, 2017 — are listed as species of Union Concern22,23. Originating from North America, they were introduced in Europe and respectively in Italy for bait, fish forage, and food purposes24. Three species - F. limosus, P. clarkii, and P. leniusculus - already coexist in Lake Maggiore24,25. Faxonius limosus (the spiny-cheek crayfish) was firstly found in Italy in Lake Iseo26,27 and later in Lake Maggiore25,28. Procambarus clarkii (the red swamp crayfish) was introduced in Italy in 198929. It thrives due to its rapid reproduction, high fecundity rates, opportunistic diet, and aggressive nature30. It also transmits the deadly crayfish plague (caused by Aphanomyces astaci Schikora, 1906), which poses a significant threat to native crayfish31. Pacifastacus leniusculus (the signal crayfish) was first recorded in Italy in the Adige River in 199732 and in Lake Maggiore (Swiss side) in 201733. Notwithstanding all of them are among the most invasive crayfish worldwide20,34, since they have a remarkable adaptability, allowing them to thrive in a wide range of environmental conditions and an exceptional degree of tolerance to environmental stresses, they are frequently used as bioindicators in aquatic ecosystems19,35.
Lake Maggiore, characterized by growing urbanization, industrial and touristic activities is an interesting survey area and becoming a hotspot of invasive crayfish. In response to the increasing concerns about the harmful impacts of MPs36 and invasive crayfish on freshwater ecosystems and human health28, the aim of our pilot study was to assess quantitatively and qualitatively the MPs content in the intestine of three invasive crayfish species whose coexistence is evident in the Swiss sector of Lake Maggiore23,24.
Specifically, our hypotheses are: H1) we predict that the three coexisting invasive crayfish species will show different MP contamination rates, reflecting potential differences due to interspecific interactions; H2) we expect a positive correlation between crayfish size and the size of MPs ingested, with larger species ingesting a broader range of MPs19,37; H3) we hypothesize that the three species will ingest different MP types, consistent with species-specific feeding habits and previously observed associations38.
Materials and methods
Study area and samplings
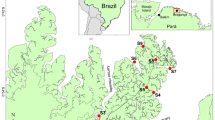
Sampling of invasive crayfish was carried out in Lake Maggiore (46° 5.88’ N − 08° 42.88’ E), one of the large and deep lakes of the Po Valley, in the north-western area (Piedmont, Italy). Only the Swiss part of the lake was considered, and only the Locarno surroundings (Fig. 1). Lake Maggiore, being included within the LTER Subalpine lakes macrosite (https://deims.org/8ffe6c61-5473-4e56-9a6e-827baad941e5), is regularly monitored for water chemistry and biology (mainly plankton and fish) within the International Commission for the Protection of Italian-Swiss Waters (CIPAIS) (www.cipais.org) since the 1980s39.
Map of the sampling area located in the Swiss sector of Lake Maggiore. Red polygon: area where invasive crayfish were taken. The map was manually drawn using Adobe Fresco (version 4.8.1) on an iPad 10th generation. Adobe Fresco is available at: https://www.adobe.com/products/fresco.html.
The study area extends from the town of Locarno (lakeside promenade) towards the Bolle di Magadino Nature Reserve, a protected area at the confluence of River Ticino into Lake Maggiore, up to Magadino municipality. From the shore of the lake to the inner part of the territory, the area extends to watercourses that connect the mainland to the River Ticino. From spring into early autumn, the Locarno Bay and lakeside promenade, surrounded by houses, to Tenero is bustling with tourists. Continuing towards Magadino, the Minusio and Magadino harbours and a large camping area characterised the lake littoral. On site trails allow the entrance to the Bolle di Magadino Nature Reserve (protected since the 1970s), known to be one of the last natural deltas in Switzerland, and representing a locally very popular Reserve.
For summer samplings, a total of 52 cylindrical flexible/collapsible nylon traps (30 × 60 cm) with double entrances and bait (ca. 20 catfood g/trap) were used, set al.ong the coast in the 0.5–3 m depth zone for 24 h. No native crayfish species inhabits the lake shores24.
The crustacean species studied in this work are not protected under European or national legislation and do not fall within the categories of homeothermic wild fauna (birds and mammals) or other protected species listed in Annex IV of Directive 92/43/EEC or Annex I of Directive 2009/147/EC. Consequently, ethical approval from an institutional committee was not required. All methods were carried out in accordance with relevant institutional and national guidelines and regulations. This study is reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org).
After capture, traps were removed to avoid injuries and cannibalism among individuals, and to allow the release of by-catches of non-target species. Native species other than crayfish were released on site to safeguard local biodiversity. In the laboratory, crayfish were gradually anaesthetised and euthanized by exposure freezing temperatures (approximately − 20 °C), following a method commonly used in crustacean studies to minimize stress and avoid unnecessary suffering19.
Biometric analysis
Invasive crayfish were later divided by sex, measured — total body length in mm (TL) and weight in g, cephalothorax length in mm (CL), both chelae length in mm and weight in g — and their species belonging was confirmed through complementary morphological analyses, using both naked eye examination and stereomicroscopy, alongside molecular analyses. A digital scale (Mettler Toledo PG 603-S: precision 0.001 g) and a digital calliper (Mitutoyo Digimatic: precision 0.1 mm) were used for biometric analyses.
A pool of 30 individuals per species was created after the captures to ensure a sufficient sample size to perform biological, chemical and statistical analyses. To create the pool, individuals of each species were chosen based on size: in detail, for the F. limosus pool, individuals larger than 4 cm (TL) were chosen, whereas for P. leniusculus and P. clarkii, individuals larger than 7 cm. Both male and female were included in each pool.
Molecular analysis
To isolate and purify genomic DNA from tissue specimens, six individuals (3 males and 3 females) of each species of different size from the total pool of 30 individuals per species, were selected. The PureLinkTM Genomic DNA kit (Invitrogen, ThermoFisher Scientific) was used to extract DNA from samples, following the manufacturer’s instruction. For taxonomic identification, we targeted a fragment of the mitochondrial gene encoding for the cytochrome c oxidase subunit I (COI), using the invertebrate universal primers LCO1490 and HCO219840. PCR assays were carried out in a reaction volume of 50 µl, applying protocols and conditions already described41. Amplification products were checked by agarose gel electrophoresis and sequenced by Sanger sequencing at Macrogen Europe (Milan, Italy). Electropherograms were checked in FinchTV 1.4.0 (https://digitalworldbiology.com/FinchTV). Forward and reverse sequences were trimmed and merged using Sequencher 5.0 (http://www.genecodes.com). Merged sequences were, then, blasted in GenBank to confirm the taxonomic identity of each sample (https://blast.ncbi.nlm.nih.gov/) and, once verified, deposited (Table S1).
Microplastic analysis
To perform the digestion and MP content analyses, only the intestines of the crayfish previously frozen were preserved for examination. After defrosting, the posterior part of the digestive system was manually extracted by carefully removing the telson with the attached intestine, which was then weighed. The intestines of 10 individuals for each species (3 replicates, tot 30 individuals; n = 3 pools of 10 specimens) were pooled and homogenized, using a Potter-Elvehjem Tissue Homogenizer.
Homogenates were filtered using a vacuum pump on 8 μm cellulose nitrate membrane filters (Sartorius™ 50 mm). The filters were then treated with 15% hydrogen peroxide (H2O2) to complete the digestion of the organic residues left.
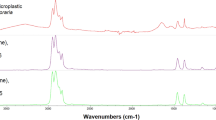
Suspected MPs were transferred from the filters to clean filters using tweezers and a stereomicroscope. The quantification and classification of each plastic particle in terms of shape, size, color, and polymer were carried out individually using the spectrometer µFTIR (Spotlight 200i equipped with Spectrum Two, PerkinElmer). The analysis was carried out using attenuated total reflectance (ATR) with 32 scans within the wavelength range of 600 to 4000 cm− 1 wavelength range. Spectra were compared with standard libraries (PerkinElmer) using the Spectrum 10 software. Following a visual examination of spectrum peaks by the operator, a matching score ≥ 0.70 was considered. MPs were categorised, based on their shape, in fibers, fragments, films, or pellets, and on their size42. To classify the MPs size was followed the ISO/TR 21,960 classification8. Although the µFTIR system allows the identification of particles larger than 10 μm, the visual sorting under a stereomicroscope limits the recovery of very small debris. In our samples, the smallest MP successfully identified had a maximum length of 0.16 mm (160 μm).
The quantity of synthetic particles was expressed both as number of items/individuals (items/ind.) and as items/g as mean value with standard deviation.
Quality assurance/quality control (QA/QC)
All procedures were conducted under a laminar flow hood to minimize airborne plastic contamination. Glass equipment and Petri dishes were used exclusively, and filtered solutions (Milli Q water and H2O2) were employed throughout. Dissections were performed using sterilized steel scissors and tweezers, with researchers wearing cotton lab coats and nitrile gloves to ensure contamination-free handling.
To monitor the eventual atmospheric contamination by plastics (especially fibers), nitrate cellulose membrane filters (blanks), identical to those used for the samples, with a pore size of 8 μm, were processed in parallel to samples during homogenization and filtration. No plastics were detected in the blanks of F. limosus and P. leniusculus. In contrast, two polyester fibers (one black and one transparent) were found in the blank filter of P. clarkii. As two black polyester fibers were also detected in the P. clarkii samples, these were subtracted from the final count42.
Statistical analysis
For each dataset the normality was assessed by Shapiro-Wilk test and the homoscedasticity was evaluated by Levene’s test. When the test indicated a deviation from normality, a logarithmic transformation was applied.
Then, to verify for significant differences within cephalothorax length (CL), total body length (TL) and weight (TW) across the three crayfish species, a one-way Analysis of Variance (ANOVA) was performed followed by Dunnett’s post-hoc test. The same analysis was also applied to evaluate potential differences in MPs concentration and size across the three species.
Regression analysis was performed for both sexes pooled to determine the relationship between total body length (TL in cm) and weight (TW in g) for each specimen of each species and commonly considered in crayfish instead of age determination43. Given that total body weight correlates with intestinal weight and total body length correlates with cephalothorax length, Pearson’s correlation test was performed only on intestinal weight and cephalothorax length to examine the relationship between the biometric features of crayfish and the MP characteristics found in their intestines (MP concentration: as items/ind. and as items/g, and MP size).
A Permutational Multivariate Analysis of Variance (PERMANOVA) using distance matrices (Adonis function in R) was applied to test for differences in shape, color and polymer composition across crayfish species.
The statistical significance level was set at p < 0.05. Statistical analyses were performed using GraphPad Prism software (version 8.0.1) and R version 4.3.1 (2023-06-16 ucrt).
Results
Species identification/confirmation
Molecular analyses confirmed the classification of the specimens. Specifically, three males and three females per species from the pool of 30 individuals were used for molecular investigations. All the blasted sequences exhibited a query coverage of 100.0%, an e-value of 0.0, and an identity percentage of 100.0%, ensuring the reliability of the molecular data.
Biometric features of crayfishes
The total number of the three invasive crayfish caught during the sampling period was 90, divided into 30 individuals for each of the three invasive crayfish species. A summary of body size ranges is provided in Fig. 2 (A-C) and features in Table S2, where a larger size variability is observed for F. limosus compared to the other two species. The regression analysis of weight vs. total length yielded regression coefficients indicating positive allometric growth in all three invasive species (Fig. 2D-F).
Procambarus clarkii and P. leniusculus reached greater sizes and weights than F. limosus. Most specimens (67%) of F. limosus showed a size range between 5 and 8 cm, and a total average weight of 13.7 g, while most individuals of the other two species (77% of P. clarkii and 87% of P. leniusculus) show a size range between 7 and 10 cm and a total average weight of about 23 g. Maximum body size is 10.4 cm for F. limosus, and about 11.2 cm for P. clarkii and P. leniusculus. CL showed a size range between 2 and 4 cm for F. limosus (average length = 3.57 cm) and a similar dimensional range (3–5 cm) for P. clarkii (average length = 4.54 cm) and P. leniusculus (average length = 4.37 cm). Maximum body weight is 44.6 g (F. limosus), 46.1 g (P. clarkii) and 61.14 g (P. leniusculus), respectively. Subsequently, an ANOVA analysis was conducted to verify for significant differences within CL, TL and TW across the three crayfish species. Statistical significance was found for each morphological feature (CL: f = 16.83, df = 2, p < 0.001; LT: f = 15.14, df = 2, p < 0.001; TW: f = 7.87, df = 2, p < 0.001). Specifically, Dunnett’s post-hoc test detected significant differences between F. limosus and the other two species (CL: F. limosus vs. P. clarkii, p = 2.91 × 10− 5, and F. limosus vs. P. leniusculus, p < 0.001; LT: F. limosus vs. P. clarkii, p < 0.001, and F. limosus vs. P. leniusculus, p < 0.001; TW: F. limosus vs. P. clarkii, p < 0.001, and F. limosus vs. P. leniusculus, p < 0.001).
Regression analysis of total body weight vs. length revealed positive allometric growth across all three species. Notably, the weight-length relationship showed a more pronounced allometric growth pattern in P. clarkii and P. leniusculus, suggesting that weight increased at a significantly faster rate than body length in these species (Fig. 2E-F).
Microplastics contamination in invasive crayfish
In this pilot study, the three invasive species found in the same area of Lake Maggiore exhibited mean MP concentrations in their intestine of 0.37 ± 0.31 items/ind. corresponding to 6.13 ± 4.01 items/g in F. limosus, 0.17 ± 0.10 items/ind. (2.17 ± 1.22 items/g) in P. leniusculus, and 0.07 ± 0.06 items/ind. (0.91 ± 0.79 items/g) in P. clarkii (Fig. 3A). Comparing MP concentrations across the three species, no significant statistical differences were found (ANOVA: f = 2.1, df = 2, p = 0.204). MP occurrence (Table S3) was 3/3 pools (100%) for F. limosus, 3/3 pools (100%) for P. leniusculus, and 2/3 pools (67%) for P. clarkii.
Regarding MP sizes, they were in the range 0.18–2.45 mm (average 0.54 mm) in F. limosus, 0.16–1.10 mm (average 0.55 mm) in P. leniusculus, and 0.22–0.61 mm (average 0.41 mm) in P. clarkii (Fig. 3B). No significant differences were observed within MPs sizes across the three species (ANOVA: f = 0.112, df = 2, p = 0.897). More in detail, according to ISO/TR 21,960 classification, two MP size ranges were identified: 1 mm–1 μm and 5 –1 mm. The relative proportions were 82% and 18% in F. limosus, 67% and 33% in P. leniusculus, while 100% P. clarkii showed exclusively the smaller size range. All the characteristics of MPs were shown in Table S3, while in Figure S1 and S2 are respectively reported the main obtained MP spectra and the raw µFTIR images of particles.
Pearson’s correlation test showed no significant correlations between the biometric features of invasive crayfish vs. MP features (size and number of items) (Fig. 4).
Overall, fibers were the most prevalent shape, accounting for 79% of the total, followed by fragments (16%) and films (5%). Specifically, F. limosus showed 91% MPs as fibers and 9% as films (Fig. 5A). Pacifastcus leniusculus displayed both fibers and fragments as equally represented, whereas in P. clarkii 100% MPs were fibers. MP shape did not show significant differences across the three species (Adonis-PERMANOVA: R2 = 0.273, p = 0.064).
Analyzing color distribution, overall, black emerged as the most common color accounting for 28% of the total, followed by blue (22%) (Fig. 5B). Both transparent and orange colors tied at 17% each, while pink, light blue, and green each comprised 6% of the total. In F. limosus, black (36%) was the main color, followed by orange (27%), transparent (18%), blue and green (9% each). In P. leniusculus, blue was the most common color (40%), and the others were light blue (20%), transparent (20%), and black (20%). In P. clarkii, MPs were only blue and pink, with each accounting for 50%. Color did not show any significant difference across the three species (Adonis: R2 = 3.011, p = 0.060).
Concerning polymers, overall, polyester (PEST) was the most frequent accounting for 44%, followed by polyacrylate (PAK) with 39%, polyurethane (PU), polyethylene (PE), polyvinyl chloride (PVC) each 6%. Specifically in F. limosus, PAK represented the 55% of MPs, PEST the 36% and PU the 9% (Fig. 6). In P. leniusculus, PEST was the 60%, followed by PE and PVC, which each contributed for 20%. In P. clarkii, PAK and PEST each account for 50%. Polymer composition did not differ significantly across the three species (Adonis: R2 = 1.188, p = 0.338).
Discussion
Lake Maggiore, one of the largest and deepest subalpine Italian lakes, is known for its floating plastic contamination ranged from a minimum of 0.02 plastics/m³ in September to a maximum of 0.29 plastics/m³ in December36. Our results suggest the lake as an essential model area for understanding the impact of multiple environmental pressures, especially for MP contamination, due to its capacity to collect pollutants from a vast surrounding basin. MPs in freshwaters come from various sources, including plastic bag fragments, ropes, and synthetic textile fibers. The increasing interest in MPs’ pollution highlights the crucial role plays by lakes as sink for plastics12,44. In this study, we found that all three invasive crayfish species (F. limosus, P. leniusculus, and P. clarkii) ingested MPs, with F. limosus showing the highest average concentrations, while no significant differences were observed in type, color, or shape of MPs among species.
Invasive crayfish have recently attracted attention for their potential role in monitoring MP pollution, particularly due to their benthic habits and wide distribution19,45. Evidence from Lake Maggiore further underscores the complex interactions between invasive crayfish and MP contamination in freshwater systems28,36. However, this study did not aim to evaluate their ecological indicator value or assess MP levels in surrounding abiotic compartments. Instead, our objective was to perform a comparative analysis of MP ingestion among three coexisting invasive species, providing new insights into species-specific exposure and polymer composition within their intestinal content.
Biometric characteristics and competitive interaction
All three invasive crayfish species were found in close proximity within the same stretch of coast. To the best of our knowledge, this coexistence appears to be quite uncommon. Studies on the coexistence of invasive crayfish in some aquatic ecosystems have already been reported46,47,48. However, the coexistence of three invasive crayfish species has rarely been reported49, probably because the presence of P. clarkii seemed to affect the feeding habits and trophic niches of other two invasive crayfish, P. virginalis and F. limosus. Procambarus clarkii has been found closer to the watercourse entering Lake Maggiore (Magadino channel), while the other species predominantly inhabit the lake shore area. The niche separation arises from P. clarkii attitude to compete for space and resources against the other two species48,49 thanks to more rapid growth and sexual maturation, greater fecundity and breeding frequency, and tolerance of warmer or more degraded habitats50.
It is widely recognized that P. clarkii and P. leniusculus reached larger sizes compared to F. limosus24,25. Adults of the former two species typically range from 5.5 to 15 cm in size, whereas F. limosus rarely exceeds 10 cm in nature, in contrast with the research by51 where the species can grow beyond 10 cm in length due to its strong adaptation to the prevalent environmental conditions in Estonia. Our biometric data are consistent with those already published in previous studies24,48,52 confirming the intrinsic differences in size between the three species. Nevertheless, the smaller size attained by F. limosus could also be due to increased intraspecific competition with the other two crayfish species, as shown by several authors53,54. Pacifastacus leniusculus and P. clarkii have a strong ability to outcompete F. limosus in mixed populations55. At the same time, invasive crayfish constitutes a food source for various native and invasive fish and birds56,57. This predator-prey interaction may significantly influence their growth, demographic patterns, and the overall community composition and behavior25,26.
State of the Art and quantification of MPs in invasive crayfish
This study marks the first presentation of comparative results on MP uptake across three coexisting invasive crayfish. Most notably, it also represents the first report of MP uptake in F. limosus. To date, research on MP ingestion in invasive crayfish is limited, with most studies focusing predominantly on P. clarkii, while no data were reported before on F. limosus (Table 1). For other invasive crayfish, there is only a single study on Faxonius cristavarius Taylor, 200058. This knowledge gap underscores the importance of broadening research efforts to better understand interspecific differences in MP accumulation across species and different environments.
The present work shows that invasive crayfish exhibit low concentrations of MPs, thus aligning with a recent study by36 reporting relatively low levels of plastic contamination in the surface waters of Lake Maggiore, ranging from a minimum of 0.02 plastics/m3 to a maximum of 0.29 plastics/m3. These levels, originating exclusively from secondary sources, are low compared to those observed in other lakes worldwide36.
Our first aim was to evaluate potential species-specific differences in MP uptake. Regarding species-specific content, few information exists on dietary preferences in relation to the coexistence of these three species and how this may influence MP ingestion. Contrary to our expectation (H1), our study did not reveal significant interspecific differences in MP uptake. Among crayfish species, F. limosus recorded the highest MP concentration, although this finding was not statistically significant. This higher content may be attributed to its detritivorous feeding habits, especially in competition with more aggressive species. Faxonius limosus primarily feeds on detritus from sediments49 which are known to accumulate high MP level12, potentially explaining the highest concentration recorded in this species49. reported the coexistence of F. limosus with P. clarkii and another invasive crayfish (P. virginalis) and observed that the P. clarkii occupied a distinct trophic niche. Procambarus clarkii showed a more carnivorous behaviour, preying on other crayfish and fish, than F. limosus, feeding predominantly on detritus, whereas P. virginalis exhibited an intermediate feeding behavior49. Similarly, P. leniusculus is an omnivore with a generalist diet60, whereas P. clarkii, although primarily consuming fresh macrophytes and detritus, showed a more pronounced carnivorous habit61.
Comparing our MPs concentrations in crayfish with previous studies (Table 1), we found lower concentrations in both P. clarkii and P. leniusculus, while for F. limosus, no comparative data are available. For P. clarkii, our analysis revealed lower values compared to those reported in three studies conducted in different ecosystems: two in aquaculture in China37,59 and one in Lake Candia in Italy1937. reported higher MPs concentrations in the digestive tract of crayfish in Shanghai, while59 found MPs accumulation in all body parts except the flesh, with significantly higher concentrations in the stomach and gut compared to the gills. The low MP concentrations found in these studies may be due to varying pollution levels influenced by hydrodynamics and plastic usage, with rice-fish culture paddies being isolated by ridges that block microplastic entry37,59. Compared to19, our values for MPs per individual were also lower in the digestive tract and MP content was influenced by crayfish weight, with smaller individuals showing higher MP concentrations, suggesting that feeding habits and metabolic rates could influence MP retention. However, our concentration per gram of tissue is higher than that recorded in the same study as well as we did not find any relationships between crayfish size/MP size.
The recorded MP values in P. leniusculus are much lower than those reported by38 in crayfish gut and tails from watercourses within the Yorkshire Dales National Park. MP concentrations were higher in crayfish guts than in tails, and a positive correlation with MP concentration and urbanization degree was observed38. However, our results are more in line with the study by60, which found 1–2 items/ind. in crayfish from the Wieprza River in Poland, though our values remain slightly lower. Despite F. limosus showing the highest MP concentrations among the species in our study, these values remain lower than those reported for P. leniusculus and P. clarkii37,38,59.
Our findings from Lake Maggiore suggest that crayfish reached similar concentrations of Perca fluviatilis (perch; 1.73 ± 1.83 items/ind62. , , but much lower than MP concentrations of some sessile taxa, such as bivalves which can reach up to 6 items/ind. in Lake Maggiore63, reinforcing the evidence that filter-feeding organisms retain more MPs16.
Synthesizing our insights, it seemed that variability in MP contamination depended on the environmental context and the surrounding land use. Differences in MP concentrations could be attributed to factors such as local plastic use and pollution levels64. However, to gain a clearer understanding of species-specific uptake differences, further studies should evaluate multiple species coexisting in the same environment to assess potential influences of feeding habits and competition.
Qualitative characterization of MPs across three invasive coexisting crayfish
Contrary to our hypothesis (H2), we did not find significant differences in MP size distribution across species since most of the MPs were < 1 mm. This finding aligns with previous studies reporting that crayfish predominantly ingest MPs < 1 mm, regardless of species19,37,58. These MPs mainly derived from the breakdown of larger plastic debris and are easily ingested by aquatic organisms16,65. Additionally, the excretion of MPs by aquatic animals contributed to their redistribution within aquatic ecosystems, potentially increasing their input and further fragmenting them into smaller particles during digestion process66.
The lack of correlation between biometric parameters and MP characteristics (size and number of items) may be attributed to the limited sample size that can hinder our results. Other studies have reported no significant relationship between crayfish size and MP size ingested60. Interestingly, although not statistically significant, among the studied species, F. limosus which shows the smallest size, concentrates more MPs as reported by19] and [38 for P. clarkii. This last species shows an age-related shift in diet: juveniles feed primarily on macroinvertebrates, which ingest large MP amounts18, while adults consume more detritus and plants67. This may explain why juveniles ingest more MPs, differing from the usual pattern of higher MP levels at higher trophic levels, both in freshwater and marine environments19,68.
Once again contrary to our expectation (H3), the other qualitative characteristics of MPs did not significantly differ across invasive crayfish. Fibers were the most prevalent form, consistent with previous studies both on freshwater biota16,69 and on abiotic matrices12,70. This prevalence suggests a mainly secondary origin, as fibers, fragments and films, indicative of plastic degradation, were the main shapes detected, while primary MPs, such as pellets, were absent36. Regarding color, dark-colored MPs, such as blue and black, were mainly detected, consistent with findings in other invasive crayfish19,60; whereas other studies observed mainly white or transparent MPs37,58,59. Interestingly59, observed that red and transparent MPs were more prevalent in the digestive systems of crayfish (P. clarkii) than in the environment. This pattern may be influenced not only by the crayfish’s feeding habits but also by the traits of their prey, as crayfish are more likely to accidentally ingest MPs that resemble their prey in color59.
PEST and PAK were the most abundant polymers, alongside other common types such as PE and PVC. In this context, it is important to note that no nylon (polyamide) particles from crayfish traps were observed in analysed specimens (Table S3). Previous studies on invasive crayfish have reported PEST, PE, PP, and PET as the most prevalent polymers19,37. Similarly to our findings, other taxa from Lake Maggiore, such as bivalves, have been found to ingest primarily PEST and PAK fibers63. MP uptake in aquatic organisms can be also influenced by bioavailability of pollutants, with studies indicating that the shape, color, and polymeric composition of MPs are often similar across various species and abiotic matrices19,37.
Lake Maggiore is influenced by multiple environmental pressures from urbanization, industrial activities, and tourism, which contribute to the presence of MPs71. Urban expansion has led to increased population and infrastructure demand, resulting in higher waste production72. MP diversity in the lake stems from synthetic fibers from laundry73, ineffective wastewater treatment74, recreational activities, seasonal plastic waste from tourism36, and atmospheric deposition10.
The exposure and uptake of MPs in crayfish in the range 1 mg/L − 100 mg/L pose toxic effects mainly investigated in laboratory conditions using generally P. clarkii and highlight immunological, neurological, oxidative stress, reproductive and developmental effects, as well as microbiome dysbiosis75,76. In general, smaller and irregular MPs tend to have more harmful effects on aquatic crustaceans and their impact is further amplified when they coexist with other contaminants68. Given the growing evidence of microfiber uptake in aquatic organisms, further research is needed to assess their ecotoxicological impact, which remains understudied compared to other MP shapes, such as the uncommon pellets and beads77.
Although our study focuses on invasive crayfish that, by law, should not be commercialized22,78, from a human perspective MP presence in crayfish raises significant concerns, as they may act as vectors of heavy metals and pathogens making these species unsafe for consumption60. noted that only the edible portions of crayfish, such as the abdomen and the chelae, are typically consumed, while the stomach and the intestines, where MPs are commonly detected, are discarded. However, the potential transfer of MPs and associated contaminants into crayfish muscle tissue remains uncertain, underscoring the need for further research to evaluate possible risks to human health.
Conclusions
The hydrographic basin of Lake Maggiore plays a key role in the transportation of MPs, collecting runoff from various sources and conveying pollutants into the lake. Given these dynamics, MPs become ubiquitous, affecting different areas of the lake and its aquatic organisms. This makes Lake Maggiore an ideal site for monitoring the combined effects of human activities on freshwater ecosystems and understanding the pathways through which MPs enter and circulate within aquatic environments and organisms.
Our findings indicate no significant interspecific differences in MP concentration or qualitative characteristics across F. limosus, P. leniusculus, and P. clarkii. To better understand whether MP content differences arise from their coexistence and trophic interactions, future studies should assess how species occurrence affects diet under controlled conditions.
Although MP concentrations in crayfish were relatively low, their presence remains concerning due to the potential co-transport of other contaminants through the food web. Most MPs found were PEST and PAK fibers (< 1 mm), suggesting a likely domestic source of plastic pollution, and highlighting the role of household waste and fabric degradation in MPs presence in the lake. Overall, the MP polymeric composition may be due to several anthropogenic sources (urban, industrial, and touristic activities).
Future research should focus on evaluating the broader ecological impacts of MP pollution in the lake, particularly its interactions with other stressors such as additional pollutants, biodiversity loss, and climate change. Developing effective strategies to protect the lake’s ecosystem should remain a priority.
Invasive crayfish hold significant potential as sentinels of MP pollution in dynamic aquatic environments owing to their widespread distribution across various ecosystems. Furthermore, the removal of invasive crayfish for management purposes could remove ingested MPs from the ecosystem, although this should not be interpreted as ecological remediation, as the primary sources of pollution persist and required removal. Future studies on MP accumulation in other organs will help clarify the potential contribution of such management actions.
Data availability
Data are available from the corresponding author on reasonable request.
References
Haase, P. et al. The recovery of European freshwater biodiversity has come to a halt. Nature 620, 582–588. https://doi.org/10.1038/s41586-023-06400-1 (2023).
Reid, A. J. et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 94, 849–873. https://doi.org/10.1111/brv.12480 (2019).
Windsor, F. M. et al. A catchment-scale perspective of plastic pollution. Glob. Change Biol. 25 (4), 1207–1221. https://doi.org/10.1111/gcb.14572 (2019).
Quadroni, S., Cesarini, G., De Santis, V. & Galafassi, S. Interconnected impacts of water resource management and climate change on microplastic pollution and riverine biocoenosis: A review by freshwater ecologists. J. Environ. Manag. 372, 123363. https://doi.org/10.1016/j.jenvman.2024.123363 (2024).
Havel, J. E., Kovalenko, K. E., Thomaz, S. M., Amalfitano, S. & Kats, L. B. Aquatic invasive species: challenges for the future. Hydrobiologia 750, 147–170. https://doi.org/10.1007/s10750-014-2166-0 (2015).
Barrowclough, D. & Deere Birkbeck, C. Transforming the global plastics economy: the political economy and governance of plastics production and pollution. Soc. Sci. 11, 26. https://doi.org/10.3390/socsci11010026 (2020).
PlasticsEurope Plastics—The Facts 2024. (2024). https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2024/
ISO/TR 21960. Plastics; Environmental Aspects. State of Knowledge and Methodologies (ISO Plastics: Mount Vernon, NY, USA, 2020).
Issac, M. N. & Kandasubramanian, B. Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut Res. 28, 19544–19562. https://doi.org/10.1007/s11356-021-13184-2 (2021).
De Falco, F., Cocca, M., Avella, M. & Thompson, R. C. Microfiber release to water, via laundering, and to air, via everyday use: a comparison between polyester clothing with differing textile parameters. Environ. Sci. Technol. 54, 3288–3296. https://doi.org/10.1021/acs.est.9b06892 (2020).
Singh, B. & Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 93, 561–584. https://doi.org/10.1016/j.polymdegradstab.2007.11.008 (2008).
Cera, A., Cesarini, G. & Scalici, M. Microplastics in freshwater: what is the news from the world? Diversity 12, 276. https://doi.org/10.3390/d12070276 (2020).
D’Avignon, G., Gregory-Eaves, I. & Ricciardi, A. Microplastics in lakes and rivers: an issue of emerging significance to limnology. Environ. Rev. 30, 228–244. https://doi.org/10.1139/er-2021-0048 (2022).
Holland, E. R., Mallory, M. L. & Shutler, D. Plastics and other anthropogenic debris in freshwater birds from Canada. Sci. Total Environ. 571, 251–258. https://doi.org/10.1016/j.scitotenv.2016.07.158 (2016).
Azevedo-Santos, V. M. et al. Plastic ingestion by fish: A global assessment. Environ. Pollut. 255, 112994. https://doi.org/10.1016/j.envpol.2019.112994 (2019).
Cesarini, G., Corami, F., Rosso, B. & Scalici, M. Microplastics, additives, and plasticizers in freshwater bivalves: preliminary research of biomonitoring. Water 15, 2647. https://doi.org/10.3390/w15142647 (2023a).
Iannilli, V. et al. Plastic abundance and seasonal variation on the shorelines of three volcanic lakes in central italy: can amphipods help detect contamination? Environ. Sci. Pollut Res. 27, 14711–14722. https://doi.org/10.1007/s11356-020-07954-7 (2020).
De Sá, L. C., Oliveira, M., Ribeiro, F., Rocha, T. L. & Futter, M. N. Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future? Sci. Total Environ. 645, 1029–1039. https://doi.org/10.1016/j.scitotenv.2018.07.207 (2018).
Pastorino, P. et al. The invasive red swamp crayfish (Procambarus clarkii) as a bioindicator of microplastic pollution: insights from lake Candia (northwestern Italy). Ecol. Indic. 150, 110200. https://doi.org/10.1016/j.ecolind.2023.110200 (2023).
Lodge, D. M. et al. Global introductions of crayfishes: evaluating the impact of species invasions on ecosystem services. Annu. Rev. Ecol. Evol. Syst. 43, 449–472. https://doi.org/10.1146/annurev-ecolsys-111511-103919 (2012).
Manfrin, C., Souty-Grosset, C., Anastácio, P. M., Reynolds, J. & Giulianini, P. G. Detection and control of invasive freshwater crayfish: from traditional to innovative methods. Diversity 11, 5. https://doi.org/10.3390/d11010005 (2019).
EU Regulation, 1143/2014. European Parliament and of the Council on the prevention and management of the introduction and spread of invasive alien species, https://eur-lex.europa.eu/eli/reg/2014/1143/2019-12-14
Commission Implementing Regulation EU 2022/1203. 12 July 2022 amending Implementing Regulation (EU) 2016/1141 to update the list of invasive alien species of Union concern,.https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022R1203
Boggero, A. et al. New records of the spiny-cheek crayfish faxonius limosus (Rafinesque, 1817): expansion in subalpine lakes in North-western Italy. BioInvasions Rec. 12, 445–456. https://doi.org/10.3391/bir.2023.12.2.09 (2023).
Garzoli, L., Mammola, S., Ciampittiello, M. & Boggero, A. Alien crayfish species in the deep subalpine lake maggiore (NW-Italy), with a focus on the biometry and habitat preferences of the spiny-cheek crayfish. Water 12, 1391. https://doi.org/10.3390/w12051391 (2020).
Aquiloni, L., Tricarico, E. & Gherardi, F. Crayfish in italy: distribution, threats and management. Int. Aquat. Res. 2, 1–14 (2010). http://www.intelaquares.com/doc/1b.pdf
Morpurgo, M. et al. Distribuzione dei gamberi d’acqua Dolce in Italia. Studi Trentini Sci. Naturali. 87, 125–132 (2010).
Boggero, A. et al. An integrated evaluation of the invasiveness risk posed by non-native crayfish in lake maggiore (Northwest Italy). Manag Biol. Invasions. 16, 135–152. https://doi.org/10.3391/mbi.2025.16.1.09 (2025).
Lo Parrino, E., Ficetola, G. F., Manenti, R. & Falaschi, M. Thirty years of invasion: the distribution of the invasive crayfish Procambarus Clarkii in Italy. Biogeographia–The J. Integr. Biogeogr. 35. https://doi.org/10.21426/B635047157 (2020).
Souty-Grosset, C. et al. The red swamp crayfish Procambarus Clarkii in europe: impacts on aquatic ecosystems and human well-being. Limnologica 58, 78–93. https://doi.org/10.1016/j.limno.2016.03.003 (2016).
Caprioli, R. et al. Aphanomyces Astaci genotypes involved in recent crayfish plague outbreaks in central Italy. Dis. Aquat. Org. 130, 209–219. https://doi.org/10.3354/dao03275 (2018).
Machino, Y. Présence de l’écrevisse de Californie (Pacifastacus leniusculus) En Italie. L’Astaciculteur De France. 52, 2–5 (1997).
Boggero, A., Dugaro, M., Migliori, L. & Garzoli, L. Prima segnalazione Del Gambero Invasivo Pacifastacus Leniusculus (Dana 1852) nel Lago maggiore (Cantone ticino, Svizzera). Boll Soc. Tic Sci. Nat. 106, 103–106 (2018).
O’Hea Miller, S. B., Davis, A. R. & Wong, M. Y. The impacts of invasive crayfish and other non-native species on native freshwater crayfish: a review. Biology 13, 610. https://doi.org/10.3390/biology13080610 (2024).
Suárez-Serrano, A., Alcaraz, C., Ibanez, C., Trobajo, R. & Barata, C. Procambarus Clarkii as a bioindicator of heavy metal pollution sources in the lower Ebro river and delta. Ecotoxicol. Environ. Saf. 73, 280–286. https://doi.org/10.1016/j.ecoenv.2009.11.001 (2010).
Binelli, A. et al. Monthly variability of floating plastic contamination in lake maggiore (Northern Italy). Sci. Total Environ. 919, 170740. https://doi.org/10.1016/j.scitotenv.2024.170740 (2024).
Lv, W. et al. Microplastic pollution in rice-fish co-culture system: A report of three farmland stations in Shanghai. China Sci. Total Environ. 652, 1209–1218. https://doi.org/10.1016/j.scitotenv.2018.10.321 (2019).
Dent, A. R. et al. Microplastic burden in invasive signal crayfish (Pacifastacus leniusculus) increases along a stream urbanization gradient. Ecol. Evol. 13, e10041. https://doi.org/10.1002/ece3.10041 (2023).
Rogora, M. et al. Temporal changes in nutrients in a deep oligomictic lake: the role of external loads versus climate change. J. Limnol. 80, 2051. (2021). https://doi.org/10.4081/jlimnol.2021.2051
Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299 (1994).
Kamburska, L. et al. A new misleading colour morph: is Marmorkrebs the only marbled. crayfish? BioInvasions Rec. 13, 949–961. https://doi.org/10.3391/bir.2024.13.4.09 (2024).
Magni, S. et al. The fate of microplastics in an Italian wastewater treatment plant. Sci. Total Environ. 652, 602–610. https://doi.org/10.1016/j.scitotenv.2018.10.269 (2019).
Sokal, R. R. & Rohlf, F. J. Biometrica. Blume Ediciones, Madrid, Spain: 832 pp. (1981).
Pan, T. et al. Review of microplastics in lakes: sources, distribution characteristics, and environmental effects. Carbon Res. 2, 25. https://doi.org/10.1007/s44246-023-00057-1 (2023).
Pastorino, P., Esposito, G., Prearo, M. & Sonne, C. The role of invasive alien species as bioindicators for environmental pollution. Curr. Opin. Environ. Sci. Health. 100620 https://doi.org/10.1016/j.coesh.2025.100620 (2025).
Anastácio, P. M. et al. Indicators of movement and space use for two co-occurring invasive crayfish species. Ecol. Indic. 53, 171–181. https://doi.org/10.1016/j.ecolind.2015.01.019 (2015).
Larson, E. R., Twardochleb, L. A. & Olden, J. D. Comparison of trophic function between the globally invasive crayfishes Pacifastacus Leniusculus and Procambarus Clarkii. Limnol 18, 275–286. https://doi.org/10.1007/s10201-016-0505-8 (2017).
Bruno, M. C., Burgio, S., Conti, A. & Marcellucci, C. There can be only one: the two-year spread of Procambarus clarkii in a Faxonius limosus-infested small perialpine lake in Trentino (Northeast Italy). In: International Association of Astacology Symposium 24, Zagreb, Croatia, September 16–20, 2024, p. 96. https://hdl.handle.net/10449/87076 (2024).
Veselý, L. et al. Trophic niches of three sympatric invasive crayfish of EU concern. Hydrobiologia 848, 727–737. https://doi.org/10.1007/s10750-020-04479-5 (2021).
Mueller, K. W. Reproductive habits of non-native red swamp crayfish (Procambarus clarkii) at pine lake, Sammamish. Wash. Northwest. Sci. 81, 246–250. https://doi.org/10.3955/0029-344X-81.3.246 (2007).
Kaldre, K., Paaver, T., Hurt, M. & Gross, R. Continuing expansion of non-indigenous crayfish species in Northern europe: first established spiny-cheek crayfish faxonius limosus (Refinesque, 1817) population in Estonia. BioInvasions Rec. 9, 127–132. https://doi.org/10.3391/bir.2020.9.1.17 (2020).
Scalici, M., Cappelletti, C., Maule, A., Casellato, S. & Ciutti, F. Life history traits of the American spiny-cheek crayfish in two subalpine lakes. Vie Milieu. 69, 193–200 (2019). http://hdl.handle.net/10449/65856
Fořt, M., Hossain, M. S., Kouba, A., Buřič, M. & Kozák, P. Agonistic interactions and dominance establishment in three crayfish species non-native to Europe. Limnol 74, 73–79. https://doi.org/10.1016/j.limno.2018.11.003 (2019).
Kouba, A. et al. Survival, growth, and reproduction: comparison of marbled crayfish with four prominent crayfish invaders. Biol 10, 422. https://doi.org/10.3390/biology10050422 (2021).
Lang, I., Paz-Vinas, I., Cucherousset, J. & Loot, G. Patterns and determinants of phenotypic variability within two invasive crayfish species. Freshw. Biol. 66, 1782–1798. https://doi.org/10.1111/fwb.13792 (2021).
De Santis, V. & Volta, P. Spoiled for choice during cold season? Habitat use and potential impacts of the invasive Silurus Glanis L. in a deep, large, and oligotrophic lake (Lake maggiore, North Italy). Water 13, 2549. https://doi.org/10.3390/w13182549 (2021).
Giordano, J. & Battisti, C. The non-native red swamp crayfish Procambarus Clarkii as prey for waterbirds: a note from Torre Flavia wetlands (Central Italy). Alula 194 https://doi.org/10.60990/alula.2023.23 (2023).
Gray, A., Mayer, K., Gore, B., Gaesser, M. & Ferguson, N. Microplastic burden in native (Cambarus appalachiensis) and non-native (Faxonius cristavarius) crayfish along semi-rural and urban streams in Southwest Virginia. USA Environ. Res. 258, 119494. https://doi.org/10.1016/j.envres.2024.119494 (2024).
Zhang, D. et al. Microplastic pollution in water, sediment, and specific tissues of crayfish (Procambarus clarkii) within two different breeding modes in jianli, Hubei Province. China Environ. Pollut. 272, 115939. https://doi.org/10.1016/j.envpol.2020.115939 (2021).
Dobrzycka-Krahel, A., Skóra, M. E. & Pladzyk, A. Plastic debris in the stomach of the invasive signal crayfish Pacifastacus Leniusculus from a Baltic coastal river. Water 16, 903. https://doi.org/10.3390/w16060903 (2024).
Gherardi, F. & Barbaresi, S. Feeding preferences of the invasive crayfish, Procambarus Clarkii. Bull. Fr. Pêche Piscic. 387, 7–20. https://doi.org/10.1051/kmae:2007014 (2007).
Galafassi, S., Sighicelli, M., Pusceddu, A., Bettinetti, R., Cau, A., Temperini, M.E., … Volta, P. (2021). Microplastic pollution in perch (Perca fluviatilis, Linnaeus 1758) from Italian south-alpine lakes. Environmental Pollution, 288, 117782.
Della Torre, C. et al. First comparative assessment of contamination by plastics and non-synthetic particles in three bivalve species from an Italian sub-alpine lake. Environ. Pollut. 330, 121752. https://doi.org/10.1016/j.envpol.2023.121752 (2023).
He, B. et al. Factors influencing MPs presence in urban waterways, in: (eds He, B., Liu, A., Ayoko, G., Egodawatta, P., Wijesiri, B. & Goonetilleke, A.) Environmental Risks Posed by Microplastics in Urban Waterways. SpringerBriefs in Water Science and Technology, Singapore, 13–24 (2023).
Roch, S., Friedrich, C. & Brinker, A. Uptake routes of microplastics in fishes: practical and theoretical approaches to test existing theories. Sci. Rep. 10, 3896. https://doi.org/10.1038/s41598-020-60630-1 (2020).
Cau, A. et al. Benthic crustacean digestion can modulate the environmental fate of microplastics in the deep sea. Environ. Sci. Technol. 54, 4886–4892. https://doi.org/10.1021/acs.est.9b07705 (2020).
Scalici, M. & Gherardi, F. Structure and dynamics of an invasive population of the red swamp crayfish (Procambarus clarkii) in a mediterranean wetland. Hydrobiologia 583, 309–319. https://doi.org/10.1007/s10750-007-0615-8 (2007).
D’Costa, A. H. Microplastics in decapod crustaceans: accumulation, toxicity and impacts, a review. Sci. Total Environ. 832, 154963. https://doi.org/10.1016/j.scitotenv.2022.154963 (2022).
Gallitelli, L. et al. Transport and deposition of microplastics and mesoplastics along the river course: A case study of a small river in central Italy. Hydrol 7, 90. https://doi.org/10.3390/hydrology7040090 (2020).
González-Pleiter, M. et al. Fibers spreading worldwide: microplastics and other anthropogenic litter in an Arctic freshwater lake. Sci. Total Environ. 722, 137904. https://doi.org/10.1016/j.scitotenv.2020.137904 (2020).
Sighicelli, M. et al. Microplastic pollution in the surface waters of Italian subalpine lakes. Environ. Pollut. 236, 645–651. https://doi.org/10.1016/j.envpol.2018.02.008 (2018).
Lella, L. in Monitoring the SDGs in Piedmont Region, Italy. 92–93 (eds Stamos, I.) (Publications Office of the European Union, 2023).
Sbarberi, R. et al. Comparison of plastic pollution between waters and sediments in four Po river tributaries (Northern Italy). Sci. Total Environ. 912, 165–177. https://doi.org/10.1016/j.scitotenv.2023.168884 (2024).
Galafassi, S., Nizzetto, L. & Volta, P. Plastic sources: A survey across scientific and grey literature for their inventory and relative contribution to microplastics pollution in natural environments, with an emphasis on surface water. Sci. Total Environ. 693, 133499. https://doi.org/10.1016/j.scitotenv.2019.07.305 (2019).
Zhang, X. et al. Accumulation of polyethylene microplastics induces oxidative stress, Microbiome dysbiosis and immunoregulation in crayfish. Fish. Shellfish Immunol. 125, 276–284. https://doi.org/10.1016/j.fsi.2022.05.005 (2022).
Hamed, M., Said, R. E., Shaalan, W. M., Elbaghdady, H. A. M. & Sayed, A. E. D. H. Immunological, neurological, and intestinal changes in red swamp crayfish (Procambarus clarkii) exposed to the combined toxicity of pyrogallol and microplastics. Mar. Pollut Bull. 213, 117641. https://doi.org/10.1016/j.marpolbul.2025.117641 (2025).
Cesarini, G. et al. Teratogenic effects of environmental concentration of plastic particles on freshwater organisms. Sci. Total Environ. 898, 165564. https://doi.org/10.1016/j.scitotenv.2023.165564 (2023b).
IPBES & Summary for Policymakers of the Thematic Assessment Report on Invasive Alien Species and their Control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Roy, H. E., Pauchard, A., Stoett, P., Renard Truong, T., Bacher, S., Galil, B. S., Hulme, P. E., Ikeda, T., Sankaran, K. V., McGeoch, M. A., Meyerson, L. A., Nuñez, M. A., Ordonez, A., Rahlao, S. J., Schwindt, E., Seebens, H., Sheppard, A. W., Vandvik, V. (eds.). IPBES secretariat, Bonn, Germany, 52 pp. (2023). https://doi.org/10.5281/zenodo.7430692
Acknowledgements
We sincerely thank the anonymous reviewers for their constructive comments and suggestions, which helped us to improve the clarity and quality of this manuscript.
Funding
The activities here described were partly funded by the International Commission for the Protection of Italian-Swiss Waters (CIPAIS) (Cooperation agreement 769/2019, 812/2021). R.S. and L.K acknowledge the support of the Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for tender No. 3138 of 16 December 2021, rectified by Decree n. 3175 of 18 December 2021 of the Italian Ministry of University and Research funded by the European Union – NextGenerationEU. Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUPB83C22002930006, Project title “National Biodiversity Future Center - NBFC”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Giulia Cesarini: Conceptualization; Methodology; Validation; Formal analysis; Data curation; Visualization; Writing – original draft; Writing – review & editing. Marco Orlandi: Formal analysis; Data curation; Writing – original draft; Writing – review & editing. Riccardo Sbarberi: Methodology; Formal analysis; Data curation; Writing – review & editing. Raffaella Sabatino: Formal analysis; Investigation; Writing – original draft; Writing – review & editing. Stefano Magni: Validation; Methodology; Writing – review & editing. Andrea Binelli: Methodology; Resources; Writing – review & editing. Nicole Santi: Investigation; Writing – review & editing. Denise Schiavetta: Investigation; Data curation; Writing – review & editing. Lyudmila Kamburska: Investigation; Writing – review & editing. Mirko Zanini: Investigation; Writing – review & editing. Silvia Zaupa: Investigation; Writing – review & editing. Angela Boggero: Conceptualization; Validation; Resources; Visualization; Writing – original draft; Writing – review & editing; Supervision; Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics and permits
The present work did not require any ethics. Catch permits were requested and obtained from: Switzerland, Canton Tessin – Ufficio Federale dell’Ambiente (permits 30 October 2017, 28 May 2018 and updates to 2023). Then, transport of live crayfish permits was asked to the Swiss Federal Office for the Environment (FOEN) - Water Division (BAFU 417.511.11–832: 28 May 2018, 02 March 2023). No formal AVMA guidelines exist for the euthanasia of decapod crustaceans. The method employed was selected based on published procedures aimed at ensuring appropriate handling and minimizing potential suffering in invertebrate research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cesarini, G., Orlandi, M., Sbarberi, R. et al. Microplastics and invasive crayfish: emerging interactions and ecological implications from three coexisting species in a subalpine lake. Sci Rep 15, 33395 (2025). https://doi.org/10.1038/s41598-025-18595-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18595-6