Abstract
The relationship between allergies and cancer has been a topic of debate for decades. This study conducted a systematic review and meta-analysis of 53 studies to evaluate the association between allergies and cancers. Case–control and cohort studies were analyzed, focusing on cancer incidence. The results showed significant negative correlations between allergies and cancers such as colorectal cancer, lymphoma, pancreatic cancer, leukemia, and brain cancers. For colorectal cancer, the pooled odds ratio (OR) for any allergy was 0.77 (95% CI 0.67–0.87). Asthma was associated with a reduced risk of lymphoma (OR 0.81, 95% CI 0.70–0.94) and gynecological cancers (OR 0.72, 95% CI 0.53–0.97). For Pancreatic cancer, any allergy was associated with an OR of 0.68 (95% CI 0.59–0.77). Hay fever showed a strong inverse association with brain cancer risk (OR 0.66, 95% CI 0.58–0.76). However, atopic allergy was positively linked to an increased risk of lymphoma (OR 2.02, 95%CI 1.10–3.70). The study highlighted significant variations in the effects of different allergy types on cancer risks. These findings suggest that allergies may act as protective factors against certain cancers, while atopic allergy may increase the risk of certain cancers. However, the certainty of evidence, assessed using the GRADE framework, was low to very low, and results should be interpreted with caution. In particular, associations for lung cancer, leukemia, and gastrointestinal cancers were supported by very low-certainty evidence, primarily due to reliance on observational designs, heterogeneity among studies. These findings underscore the complex and heterogeneous relationship between allergies and cancer, and highlight the need for further high-quality research to elucidate the underlying mechanisms and assess clinical implications.
Similar content being viewed by others
Introduction
Allergies and cancer are increasingly prevalent health challenges in both developing and developed countries1. Allergies are caused by the hypersensitivity reaction of the immune system to normally harmless substances in the environment, including hay fever, food allergies, atopic dermatitis, allergic asthma, and anaphylactic shock2. Cancer is one of the leading causes of death worldwide, and according to the latest estimates by the International Agency for Research on Cancer (IARC), there will be 20 million new cases and 9.7 million deaths worldwide in 2022. This disease with high morbidity and mortality has placed a heavy burden on all countries3.
Although allergies and cancers appear to be distinctly different diseases, both are profoundly influenced by the regulatory status of the immune system. As research advances, it has become clear that the immune system plays a pivotal role in the onset and progression of numerous diseases4. Beyond its role as a defense against foreign pathogens, the balance (or imbalance) of the immune system has a profound impact on all aspects of human health5. During an allergic reaction, the immune system mistakenly identifies a harmless allergen as a threat, activating its defense mechanisms. This triggers mast cells and basophils to release large amounts of inflammatory mediators, such as histamines and leukotrienes. These mediators cause a range of allergic symptoms, including skin itching and airway constriction, and in severe cases, can even lead to anaphylactic shock6,7. In summary, allergies stem from immune imbalance, which puts the body into a state of “hyper-defense”, leading to excessive inflammation and self-damage. In contrast, a hallmark of tumor development is the immune system’s inability to recognize and eliminate abnormal cells8. Under normal conditions, immune surveillance can detect and clear early tumor cells, inhibiting their growth and spread9. However, certain tumor cells possess immune evasion mechanisms, allowing them to escape immune detection by expressing immunosuppressive factors (such as PD-L1 and TGF-β) or altering the tumor microenvironment to suppress T-cell activity10,11. These immune evasion strategies are central to current cancer immunotherapy research, which aims to restore immune suppression in tumors by enhancing anti-tumor immune responses12.
Despite growing interest in the immunological interactions between allergic diseases and cancer, existing meta-analyses remain limited in scope, often concentrating on specific allergic phenotypes or single cancer types, and lacking a unified analytical framework. To address these limitations, the present study systematically evaluates the associations between various allergic conditions and multiple cancer outcomes, with the aim of determining whether a history of allergic disease is associated with an increased or decreased risk of specific cancers. This provides a comprehensive and up-to-date assessment to enhance our existing understanding of the relationship between allergies and cancer.
Methods
This Systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline13. The study protocol was registered with PROSPERO (CRD42024602718).
Literature search strategy
The research included searches in databases such as PubMed, Google Scholar, and ScienceDirect. The search covered studies published before November 2024. The search strategy was described in detail in Supplementary Table 1. With a primary focus on the Last 20 years. Older studies were included only if they were deemed particularly relevant. The following restrictions applied:
-
1)
Language: Only studies published in English were considered.
-
2)
Publication Date: The focus was on studies published in the Last 20 years.
-
3)
Study Types: Only peer-reviewed studies and clinical trials were included. Unpublished studies or non-peer-reviewed sources, such as conference abstracts, were not be considered unless particularly significant.
Inclusion and exclusion criteria
This systematic review aimed to evaluate the available research evidence on the relationship between cancer and allergy to reveal the common mechanisms of the two in immune regulation, to evaluate the impact of allergies on cancer comprehensively, and then provide new insight for future treatment directions of cancer. The inclusion and exclusion criteria were outlined as follows.
Inclusion criteria
-
1)
Diagnosis of Cancer: Participants had a confirmed diagnosis of cancer, regardless of cancer type or stage, classified according to standard clinical criteria (e.g., ICD codes).
-
2)
History of Allergic Conditions: Participants had a documented history of at least one allergic condition, which may include allergic rhinitis, asthma, eczema, atopic dermatitis, food allergies, or other allergies.
-
3)
Study Design: The systematic review focused on observational studies, specifically cohort studies and case–control studies, to examine the relationship between allergies and cancer outcomes.
-
4)
Geographic Location: Participants from any geographic location were eligible, provided they met the other inclusion criteria.
Exclusion criteria
-
1)
Acute Allergic Reactions Related to Oncology Treatment: Patients who experienced acute allergic reactions due to cancer treatment were excluded.
-
2)
Severe Comorbidities Affecting Immune Response: Individuals with severe comorbidities, especially those that affect immune response (e.g., autoimmune diseases or immunodeficiency disorders), were excluded, as these may interfere with cancer- or allergy-related outcomes.
-
3)
Non-Allergic Immune Disorders: Studies focused on immune disorders unrelated to allergies, such as autoimmune diseases, were excluded.
-
4)
Case reports, letter to editors, unpublished reports, duplication, in vitro and in vivo studies were not considered as eligible.
-
5)
Studies Without Relevant Comparisons: Studies that did not compare cancer outcomes between patients with and without a history of allergies were excluded.
Data extraction
The data extraction process was carried out in a systematic manner. Initially, two independent reviewers, X. Fan and S. Guo, screened the titles and abstracts of all identified studies based on the pre-specified eligibility criteria. Studies that met the inclusion criteria advanced to full-text review, and any disagreements during the screening process were resolved through discussion or by consulting a third reviewer W. Li. Subsequently, the full-text articles of studies that passed the initial screening were independently reviewed by the same two reviewers to confirm adherence to all inclusion and exclusion criteria. Studies that did not meet these criteria were excluded, and the reasons for exclusion were documented. Data to be extracted included study characteristics such as first author, year, country, study type, study period, cancer identification, exposure assessment, number of cases, number of controls, allergy type, cancer type. Main outcomes focused on data related to cancer incidence, with effect measures presented as odds ratios (OR), hazard ratio (HR), or relative risk (RR), along with 95% confidence intervals (CIs) for each primary outcome. Furthermore, information on confounding factors, adjustments made during analysis, and study Limitations was also collected. All extracted data were recorded in a structured electronic database to ensure completeness and organization. The final dataset encompassed all relevant information necessary for meta-analysis or qualitative synthesis and was stored securely for reference throughout the review process. In the data extraction process, we also paid particular attention to the classification of allergy types. Across the 53 included studies, we identified 26 distinct terms describing allergic conditions (e.g., “any allergy,” “asthma,” “hay fever,” “drug allergy,” “eczema,” “rhinitis,” “food allergy,” etc.). To ensure consistency and analytical power, only allergy subtypes that were reported in more than 10 studies were included in the subgroup meta-analyses. These subtypes were: any allergy (n = 34), asthma (n = 24), hay fever (n = 16), food allergy (n = 15), drug allergy (n = 14), eczema (n = 11), and allergic rhinitis (n = 10). Beyond categorizing allergy subtypes, we also organized the data according to cancer types. The top ten cancer types with the highest number of studies were included in our meta-analysis, and we conducted individual meta-analyses for each cancer type to examine the relationship with allergies. This approach allowed us to systematically assess the association between allergies and cancer risk, progression, or mortality for each specific cancer type. The results of these analyses provide a comprehensive overview of the potential protective or risk-enhancing role allergies may play in various cancers, considering the heterogeneity across cancer types.
Quality assessment
The studies included in this meta-analysis were observation study including cohort study and case–control study. The quality of each included study was assessed using Newcastle–Ottawa scale (NOS) by two independent authors. The scoring criteria for the Newcastle–Ottawa Scale (NOS) are as follows: a total score of 0 to 3 indicates low quality, a score of 4 to 6 indicates moderate quality, and a score of 7 to 9 indicates high quality14.
The GRADE (Grading of Recommendations, Assessment, Development and Evaluations) assessment was used to assess the quality of evidence of the main outcomes. All assessments were performed using the GRADEpro online platform to ensure methodological transparency and reproducibility.
Statistical analysis
For each study reporting the association between allergy type and cancer risk, we calculated the pooled OR, RR, or HR and its 95% CI and combined them to present all results as OR and 95% CI. Data were combined according to the random-effect model in the presence of heterogeneity, otherwise, the fixed-effect model was performed. Statistical heterogeneity was assessed using the chi-squared based Q-test or the I2 method15. To assess the stability of the results, sensitivity analysis was performed using subgroup analysis and leave-one-out analysis (sequentially omitting individual studies). A Baujat plot was further applied to identify studies contributing most to heterogeneity and to evaluate their influence on the pooled estimates. We also performed random-effects meta-regression to examine the influence of potential moderators, including geographic region, allergy assessment method, age, smoking status, alcohol consumption, and gender when substantial heterogeneity (I2 > 70%) was detected. Additionally, To assess the robustness of the findings, a sensitivity analysis excluding studies with a NOS score < 3 was conducted. Funnel plots and Egger’s test were performed to detect potential publication bias16. The trim-and-fill method was employed to assess and, if necessary, correct for potential publication bias. All p-values were two-sided, and values < 0.05 were considered statistically significant. Extracted data were combined into a meta-analysis using STATA 16.0 analysis software (Stata Corporation, College Station, TX).
Results
Literature search results
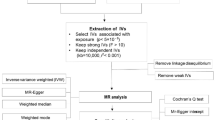
A Literature search was conducted until November 2024 using the PubMed, ScienceDirect, and Google Scholar databases with the search terms:“hypersensitivity”or“allergy”AND“cancer”or“tumor”or“neoplasms”or“malignancy”(Supplementary Table 1). A total of 109 articles from 3 scientific databases were identified: PubMed (66), ScienceDirect (17), and Google Scholar (26). After excluding irrelevant and duplicate studies (duplicate n = 25, irrelevant n = 16, other reasons n = 2), we obtained 66 studies. By evaluating these 66 studies in detail, we found that 13 studies did not meet our inclusion criteria (see Table 1 for specific reasons). Therefore, 53 studies were finally included in the systematic review and meta-analysis (Fig. 1).
Quality assessment
All 53 studies included in this meta-analysis were observational studies, including cohort and case–control designs. Two reviewers independently assessed the methodological quality of each study using the Newcastle–Ottawa Scale (NOS). Among the included studies, 18 were rated as high quality, 29 were rated as moderate quality, and 6 were rated as low quality. (Supplementary Table 2).
Study characteristics
Two authors independently extracted data from full-text articles. Details of the studies were recorded on a standardized form, including the following data elements: first author, year, country, study type, study period, cancer identification, exposure assessment, number of cases, number of controls, allergy type, cancer type.
A total of 53 studies were included in this meta-analysis, including 36 case–control studies and 17 cohort studies. The articles were classified by cancer site and specifically analyzed the top ten most common cancer types included in the studies, including colorectal cancer, breast cancer, lymphoma, pancreatic cancer, lung cancer, prostate cancer, leukemia, brain cancer, gastrointestinal cancers, and gynecological cancers. The exposure types analyzed mainly included: any allergy, atopic allergy, asthma, eczema, food allergy, drug allergy, and hay fever. (Table 2).
Meta-analysis
As outlined in the methods section, after data extraction, we categorized all studies based on cancer types. We included the top ten cancer types with the highest number of studies in our meta-analysis: colorectal cancer (n = 17), breast cancer (n = 13), lymphoma (n = 13), pancreatic cancer (n = 12), lung cancer (n = 10), prostate cancer (n = 9), leukemia (n = 8), brain cancers (n = 8), gastrointestinal cancers (n = 7), and gynecological cancers (n = 7). Individual meta-analyses were then conducted to assess the relationship between each cancer type and allergies.
Colorectal cancer
17 studies investigated the relationship between allergies and colorectal cancer. The combined odds ratio for the 17 studies investigating the association with colorectal cancer was 0.82 (95% CI 0.76–0.89; I2 = 51.5%), indicating a negative correlation between allergies and colorectal cancer. Of the 17 studies, 12 were cohort studies, and 5 were case–control studies (Fig. 2a). In this study, we assessed the potential for publication bias using Egger’s test and a funnel plot. The result of Egger’s test (p-value > 0.05) indicated no significant publication bias. Furthermore, the funnel plot did not show any obvious asymmetry, further supporting the reliability of the results (Supplementary Fig. 1a). Sensitivity analysis using a leave-one-out approach demonstrated that the pooled estimates remained robust, with no single study significantly influencing the overall results (Supplementary Fig. 2a). The Baujat plot was used to illustrate the impact of individual studies on overall heterogeneity and the pooled effect (Supplementary Fig. 3a). The results of subgroup analysis for different allergy types are shown in Fig. 2b and Table 3a. In the subgroup heterogeneity analysis, the study by Choi contributed the most to the observed heterogeneity in the association between allergy and colorectal cancer. After excluding the Choi study, (The Choi study defined allergies based on Korean insurance claims data, which may have led to misclassification of allergic diseases. Additionally, environmental and lifestyle factors specific to the Korean population may have differed from those in other regions.) heterogeneity decreased substantially (I2 = 12.7%), while the overall association remained consistent (Fig. 2c). In addition, we performed a sensitivity analysis excluding studies with a Newcastle–Ottawa Scale (NOS) score lower than 3 to assess the robustness of the pooled estimates. The results were consistent with the original analysis, indicating that our findings were robust and were not significantly affected by the inclusion of low-quality studies (Fig. 2d).
Random-effects meta-analysis of association of colorectal cancer incidence and allergy. (a). Forest plot is stratified by study design. (b). Forest plot is stratified by different allergy type. (c). Forest plot is stratified by different allergy type, results were adjusted based on sensitivity analysis. (d). Forest plot of the association after excluding low‑quality studies (NOS < 3) to assess robustness of the pooled estimate. If HR, RR, or OR are provided, they were used for the calculations. If these measures were not provided, the OR value was calculated based on the raw data from the original articles. OR, odds ratio; RR: relative risk; HR: hazard ratio; CI, confidence interval.
Breast cancer
13 studies investigated the association between allergies and breast cancer. Among them, nine were cohort studies, and four were case–control studies. The pooled odds ratio of the 13 studies was 0.97 (95% CI 0.89–1.05; I2 = 58.2%), indicating little to no association (Fig. 3a). The results from Egger’s test (p > 0.05) and the symmetrical distribution of the funnel plot did not suggest the presence of publication bias (Supplementary Fig. 1b). Sensitivity analysis using a leave-one-out approach demonstrated that the pooled estimates remained robust, with no single study significantly influencing the overall results (Supplementary Fig. 2b). The Baujat plot indicated that Aryaie et al. contributed notably to heterogeneity and the pooled effect, but excluding this study did not reduce the overall heterogeneity (Supplementary Fig. 2b-3b). Subgroup analysis results for different allergy types are presented in Fig. 3b, and no significant associations were observed across the various allergy subtypes (Table 3b). However, sensitivity analysis within the allergy subgroup showed that, after excluding the study by Aryaie et al., the pooled odds ratio for the remaining two studies was 0.90 (95% CI 0.80–1.01; I2 = 52.5%) (Fig. 3c). Based on the Newcastle–Ottawa Scale assessment, all included studies were of moderate to high quality, with no studies rated as low quality. Therefore, no subgroup analysis based on study quality was conducted.
Random-effects meta-analysis of association of breast cancer incidence and allergy. (a). Forest plot is stratified by study design. (b). Forest plot is stratified by different allergy type. (c). Forest plot is stratified by different allergy type, results were adjusted based on sensitivity analysis. If HR, RR, or OR are provided, they were used for the calculations. If these measures were not provided, the OR value was calculated based on the raw data from the original articles. OR, odds ratio; RR: relative risk; HR: hazard ratio; CI, confidence interval.
Lymphoma
13 studies investigated the association between allergies and lymphoma. The pooled odds ratio of the 13 studies was 0.85 (95% CI 0.79–0.92; I2 = 49.6%), indicating a negative association between allergies and lymphoma. Among the studies, seven were cohort studies, and six were case–control studies (Fig. 4a). Egger’s test (p < 0.05) and the asymmetry of the funnel plot suggest the potential for publication bias (Supplementary Fig. 1c). Sensitivity analysis using a leave-one-out approach demonstrated that the pooled estimates remained robust, with no single study significantly influencing the overall results (Supplementary Fig. 2c). The Baujat plot was used to illustrate the impact of individual studies on overall heterogeneity and the pooled effect (Supplementary Fig. 3c). Subgroup analysis results for different allergy types are presented in Fig. 4b and Table 3c. The analysis revealed that heterogeneity primarily arose from differences in study design between cohort and case–control studies (Fig. 4c). (The cohort studies by Hofmann and Mills contributed significantly to the overall heterogeneity. After excluding these studies, the I2 value significantly decreased, indicating that design differences were the key factor driving the increased heterogeneity. After exclusion, the I2 value dropped to 37.7%, and the pooled odds ratio of the remaining four studies was 0.88 (95% CI 0.74–1.05).For the analysis of atopy (four studies) and lymphoma, the pooled odds ratio was 1.36 (95% CI 0.63–2.96; I2 = 62.3%). After excluding the Hofmann study based on study type, the I2 value decreased to 0%, and the pooled odds ratio for the remaining three studies was 2.02 (95% CI 1.10–3.70).) In addition, we performed a sensitivity analysis excluding studies with a Newcastle–Ottawa Scale (NOS) score lower than 3 to assess the robustness of the pooled estimates. The results were consistent with the original analysis, indicating that our findings were robust and were not significantly affected by the inclusion of low-quality studies.(Fig. 4d).
Random-effects meta-analysis of association of lymphoma cancer incidence and allergy. (a). Forest plot is stratified by study design. (b). Forest plot is stratified by different allergy type. (c). Forest plot is stratified by different allergy type, results were adjusted based on sensitivity analysis. (d). Forest plot of the association after excluding low‑quality studies (NOS < 3) to assess robustness of the pooled estimate. If HR, RR, or OR are provided, they were used for the calculations. If these measures were not provided, the OR value was calculated based on the raw data from the original articles. OR, odds ratio; RR: relative risk; HR: hazard ratio; CI, confidence interval.
Pancreatic cancer
12 studies investigated the association between allergies and Pancreatic cancer. The pooled odds ratio of the 12 studies was 0.75 (95% CI 0.69–0.82; I2 = 68.8%), indicating a negative association between allergies and pancreatic cancer. Among the studies, six were cohort studies, and six were case–control studies (Fig. 5a). Egger’s test and the funnel plot suggest the potential for publication bias (p < 0.05) (Supplementary Fig. 1d). Sensitivity analysis using a leave-one-out approach demonstrated that the pooled estimates remained robust, with no single study significantly influencing the overall results (Supplementary Fig. 2d). Based on the Baujat results, studies by Turner, Olson, Engkilde, Cotterchio, and Holly were identified as major contributors to heterogeneity. However, sequential exclusion of these studies did not materially reduce the overall heterogeneity. (Supplementary Fig. 2d-3d). In addition, due to the high level of heterogeneity observed among studies investigating the association between allergy and pancreatic cancer, we conducted both meta-regression and subgroup analyses to identify potential sources of heterogeneity. The meta-regression results suggest that whether adjust alcohol consumption may partially account for the between-study heterogeneity(Supplementary Fig. 4). Subgroup analysis results for different allergy types are presented in Fig. 5b and Table 3d. In the subgroup analysis of atopy and pancreatic cancer (3 studies), heterogeneity was high, with I2 = 77.1%. Sensitivity analysis showed that Huang’s study (This study scored lower (only four stars) on the NOS scale compared to the other two, suggesting that it may have introduced higher heterogeneity due to data quality issues.) was the main source of heterogeneity. After excluding this study, I2 dropped to 0%, and the trend of results remained the same. Similarly, for allergy and pancreatic cancer, Choi’s study contributed the most to heterogeneity. After excluding this study, I2 dropped to 0%, and the trend of results remained the same (Fig. 5c).
Random-effects meta-analysis of association of pancreatic cancer incidence and allergy. (a). Forest plot is stratified by study design. (b). Forest plot is stratified by different allergy type. (c). Forest plot is stratified by different allergy type, results were adjusted based on sensitivity analysis. If HR, RR, or OR are provided, they were used for the calculations. If these measures were not provided, the OR value was calculated based on the raw data from the original articles. OR, odds ratio; RR: relative risk; HR: hazard ratio; CI, confidence interval.
Lung Cancer
Ten studies investigated the relationship between allergy and lung cancer, with a pooled odds ratio of 0.99 (95% CI 0.82–1.19; I2 = 71.5%), suggesting no significant association (Fig. 6a). Egger’s test and funnel plot analysis showed no significant asymmetry, suggesting that publication bias did not affect the results (Supplementary Fig. 1e). Sensitivity analysis using a leave-one-out approach demonstrated that the pooled estimates remained robust, with no single study significantly influencing the overall results (Supplementary Fig. 2e). The Baujat plot indicated that the study by Aryaie et al. contributed substantially to heterogeneity, but its inclusion or exclusion did not alter the direction or significance of the overall pooled effect (Supplementary Fig. 2e-3e). To further explore potential sources of heterogeneity, meta-regression and subgroup analyses were performed for lung cancer, but none of the examined covariates showed a significant effect (Supplementary Fig. 5). Subgroup analysis showed no significant associations between any specific type of allergy and lung cancer (Fig. 6b, Table 3e).Based on the Newcastle–Ottawa Scale assessment, all included studies were of moderate to high quality, with no studies rated as low quality. Therefore, no subgroup analysis based on study quality was conducted.
Random-effects meta-analysis of association of lung cancer incidence and allergy. (a). Forest plot is stratified by study design. (b). Forest plot is stratified by different allergy type. If HR, RR, or OR are provided, they were used for the calculations. If these measures were not provided, the OR value was calculated based on the raw data from the original articles. OR, odds ratio; RR: relative risk; HR: hazard ratio; CI, confidence interval.
Prostate cancer
Nine studies investigated the relationship between allergy and prostate cancer, with a pooled odds ratio of 1.07 (95% CI 0.98–1.17; I2 = 18.2%), suggesting no significant evidence of an association. Among these, seven were cohort studies, and two were case–control studies (Fig. 7a). Egger’s test (p = 0.135) and the symmetrical distribution of the funnel plot indicated no publication bias (Supplementary Fig. 1f.). Sensitivity analysis using a leave-one-out approach demonstrated that the pooled estimates remained robust, with no single study significantly influencing the overall results (Supplementary Fig. 2f.). The Baujat plot was used to illustrate the impact of individual studies on overall heterogeneity and the pooled effect (Supplementary Fig. 3f.). Subgroup analysis indicated a significant positive association between atopy and prostate cancer risk (OR 1.41, 95% CI 1.05–1.88), while no significant associations were found for other allergy types (Fig. 7b, Table 3f). Based on the Newcastle–Ottawa Scale assessment, all included studies were of moderate to high quality, with no studies rated as low quality. Therefore, no subgroup analysis based on study quality was conducted.
Random-effects meta-analysis of association of prostate cancer incidence and allergy. (a). Forest plot is stratified by study design. (b). Forest plot is stratified by different allergy type. If HR, RR, or OR are provided, they were used for the calculations. If these measures were not provided, the OR value was calculated based on the raw data from the original articles. OR, odds ratio; RR: relative risk; HR: hazard ratio; CI, confidence interval.
Leukemia
Eight studies investigated the relationship between allergy and leukemia, with a pooled odds ratio of 0.49 (95% CI 0.35–0.68; I2 = 81.3%), indicating a negative association between allergy and leukemia. Among these, six were cohort studies, and two were case–control studies (Fig. 8a). Egger’s test (p < 0.05) and funnel plot analysis suggested potential publication bias (Supplementary Fig. 1 g). Sensitivity analysis using a leave-one-out approach demonstrated that the pooled estimates remained robust, with no single study significantly influencing the overall results (Supplementary Fig. 2 g). Based on the Baujat plot, the study by Talbot-Smith et al. and Severson et al. were considered the main contributor to heterogeneity. However, excluding either study did not substantially reduce the overall heterogeneity (Supplementary Fig. 2 g-3 g). In addition, given the substantial heterogeneity (I2 = 81.3%) observed among studies examining the association between allergy and leukemia, we performed both meta-regression and subgroup analyses to explore potential sources of heterogeneity. The meta-regression results indicated that geographic region, allergy assessment method, age, gender, alcohol consumption, and smoking status may each partially contribute to the between-study variability (Supplementary Fig. 6). The Q-test for subgroup differences reached statistical significance (Q = 11.48, df = 5, p = 0.0427), suggesting potential variation among allergy types. However, subgroup analysis revealed no significant associations between any specific allergy type and leukemia risk (Fig. 8b, Table 3g). None of the included studies met the criteria for low methodological quality according to the NOS score.
Random-effects meta-analysis of association of leukemia cancer incidence and allergy. (a). Forest plot is stratified by study design. (b). Forest plot is stratified by different allergy type. If HR, RR, or OR are provided, they were used for the calculations. If these measures were not provided, the OR value was calculated based on the raw data from the original articles. OR, odds ratio; RR: relative risk; HR: hazard ratio; CI, confidence interval.
Brain cancer
Eight studies investigated the relationship between allergy and brain cancer, with a pooled odds ratio of 0.77 (95% CI 0.73–0.82; I2 = 39.6%), indicating a negative association between allergy and brain cancer. Among these, five were cohort studies, and three were case–control studies (Fig. 9a). Egger’s test (p < 0.05) and funnel plot analysis suggested evidence of publication bias (Supplementary Fig. 1 h). Sensitivity analysis using a leave-one-out approach demonstrated that the pooled estimates remained robust, with no single study significantly influencing the overall results (Supplementary Fig. 2 h). The Baujat plot was used to illustrate the impact of individual studies on overall heterogeneity and the pooled effect (Supplementary Fig. 3 h). Subgroup analysis showed that most allergy types were significantly associated with a reduced risk of brain cancer (Fig. 9b, Table 3h). Based on the Newcastle–Ottawa Scale assessment, all included studies were of moderate to high quality, with no studies rated as low quality. Therefore, no subgroup analysis based on study quality was conducted.
Random-effects meta-analysis of association of brain cancer incidence and allergy. (a). Forest plot is stratified by study design. (b). Forest plot is stratified by different allergy type. If HR, RR, or OR are provided, they were used for the calculations. If these measures were not provided, the OR value was calculated based on the raw data from the original articles. OR, odds ratio; RR: relative risk; HR: hazard ratio; CI, confidence interval.
Gastrointestinal cancers
Seven studies investigated the relationship between allergy and gastrointestinal cancers, with a pooled odds ratio of 0.84 (95% CI 0.52–1.37; I2 = 96.4%), suggesting little to no evidence of an association. Among these, five were cohort studies, and two were case–control studies (Fig. 10a). Egger’s test and funnel plot analysis did not show significant asymmetry (Supplementary Fig. 1i). The leave-one-out sensitivity analysis indicated that the pooled association between allergy and gastrointestinal cancers risk was notably affected by the exclusion of the study by Zhou et al. (Supplementary Fig. 2i). Consistently, the Baujat plot identified Zhou et al. as a major contributor to both heterogeneity and the overall effect, with I2 decreasing from 96.4% to 33% upon its removal (Supplementary Fig. 2i-3i). In addition, due to the high level of heterogeneity observed among studies investigating the association between allergy and gastrointestinal cancers, we conducted both meta-regression and subgroup analyses to identify potential sources of heterogeneity. The meta-regression results suggest that geographic region may partially account for the between-study heterogeneity (Supplementary Fig. 7). Subgroup analysis revealed no significant associations between specific allergy types and the risk of gastrointestinal cancers (Fig. 10b, Table 3i). However, sensitivity analysis within the allergy subgroup showed that, after excluding the study by Zhou et al., (The quality assessment of this study was only three stars, and the article did not report direct odds ratios, instead calculating them. Additionally, the Zhou study, based on a Chinese population, may have been influenced by regional differences, data collection methods, and varying reporting standards, leading to inconsistent results and high heterogeneity.) the pooled odds ratio was 0.94 (95% CI 0.93–0.95; I2 = 0%), suggesting a potential inverse association between allergy and gastrointestinal cancers (Fig. 10c). In addition, we performed a sensitivity analysis by excluding studies with a Newcastle–Ottawa Scale (NOS) score below 3 to assess the robustness of the pooled estimates. The effect sizes remained largely consistent with the original analysis, while the heterogeneity was substantially reduced (Fig. 10d). This suggests that our findings are robust and that some of the observed heterogeneity may be attributed to lower-quality studies.
Random-effects meta-analysis of association of gastrointestinal cancers incidence and allergy. (a). Forest plot is stratified by study design. (b). Forest plot is stratified by different allergy type. (c). Forest plot is stratified by different allergy type, results were adjusted based on sensitivity analysis. (d). Forest plot of the association after excluding low‑quality studies (NOS < 3) to assess robustness of the pooled estimate. If HR, RR, or OR are provided, they were used for the calculations. If these measures were not provided, the OR value was calculated based on the raw data from the original articles. OR, odds ratio; RR: relative risk; HR: hazard ratio; CI, confidence interval.
Gynecological cancers
Seven studies investigated the relationship between allergy and gynecological cancers (including uterine cancer, cervical cancer, endometrial cancer, and uterine body cancer), with a pooled odds ratio of 0.87 (95% CI 0.69–1.10; I2 = 38.3%), indicating little to no evidence of an association. Among these, six were cohort studies, and one was a case–control study (Fig. 11a). Egger’s test (p = 0.866) and the symmetrical distribution of the funnel plot indicated no publication bias, further confirming the stability of the results (Supplementary Fig. 1j). Sensitivity analysis using a leave-one-out approach demonstrated that the pooled estimates remained robust, with no single study significantly influencing the overall results (Supplementary Fig. 2j). The Baujat plot was used to illustrate the impact of individual studies on overall heterogeneity and the pooled effect (Supplementary Fig. 3j). Subgroup analysis indicated that asthma was significantly associated with a reduced risk of gynecological cancers (OR 0.72, 95% CI 0.53–0.97) (Fig. 11b, Table 3j). In addition, we performed a sensitivity analysis by excluding studies with a Newcastle–Ottawa Scale (NOS) score below 3 to assess the robustness of the pooled estimates. The effect sizes remained largely consistent with the original analysis, while the heterogeneity was substantially reduced (Fig. 11c). This suggests that our findings are robust and that some of the observed heterogeneity may be attributed to lower-quality studies.
Random-effects meta-analysis of association of gynecological cancers incidence and allergy. (a). Forest plot is stratified by study design. (b). Forest plot is stratified by different allergy type. (c). Forest plot of the association after excluding low‑quality studies (NOS < 3) to assess robustness of the pooled estimate. If HR, RR, or OR are provided, they were used for the calculations. If these measures were not provided, the OR value was calculated based on the raw data from the original articles. OR, odds ratio; RR: relative risk; HR: hazard ratio; CI, confidence interval.
GRADE assessment
GRADE assessments were conducted for above meta-analyses. The certainty of evidence was rated as low for colorectal cancer, breast cancer, lymphoma, prostate cancer, brain cancer, and gynecological cancers, and as very low for pancreatic cancer, lung cancer, leukemia, and gastrointestinal cancers. (Table 4).
Discussion
This study utilized a systematic review and meta-analysis approach to comprehensively explore the relationship between allergies and cancer, aiming to elucidate the potential patterns within this complex interaction. Through stringent data selection criteria, we identified the cancer types most frequently studied in association with allergies, with a particular focus on the top ten cancer types: colorectal cancer (n = 17), breast cancer (n = 13), lymphoma (n = 13), pancreatic cancer (n = 12), lung cancer (n = 10), prostate cancer (n = 9), leukemia (n = 8), brain cancers (n = 8), gastrointestinal cancers (n = 7), and gynecological cancers (n = 7). Here, n represents the number of studies included in the analysis for each cancer type. In the preliminary meta-analysis, we observed potential inverse associations between general allergy and several cancer types, including colorectal cancer, lymphoma, pancreatic cancer, leukemia, and brain cancers. Although the pooled analysis suggested a negative association between allergy and leukemia, the substantial heterogeneity (I2 = 81.3%) could not be effectively reduced through sensitivity analyses, subgroup analyses, or meta-regression, indicating that the observed association should be interpreted with caution. Moreover, we did a subgroup analysis based on the subtype of the allergy. And we found for colorectal cancer, any allergy, atopy, and drug allergy, were showed a negative correlation. For brain cancer, most allergy types, particularly any allergy, asthma, hay fever, food allergy, and eczema, were significantly associated with a reduced risk of brain cancer. And for pancreatic cancer, subgroup analysis indicated that any allergy, hay fever, eczema was significantly associated with a reduced risk of pancreatic cancer, while no significant associations were observed for food allergies, drug allergies, or any allergy overall. For lymphoma, subgroup analysis showed that asthma, hay fever, and food allergies were significantly associated with a reduced risk of lymphoma, while no significant associations were observed for eczema, drug allergies, or any allergy overall. These findings suggest no consistent evidence supporting an inverse association between certain allergy subtypes and cancer risk.
Based on the findings of this study, the relationship between cancer and allergies is both multifaceted and complex. In addition, several other studies have explored the association between specific cancers and allergies using meta-analysis. Below we also summarize the main results of these studies. Atopic diseases, asthma, hay fever, and allergies to animals, have been linked to a reduced risk of pancreatic cancer, suggesting a potential protective effect17,18,19,20. However, allergies related to food or drugs do not show any significant association with pancreatic cancer19. In terms of prostate cancer, hay fever and asthma exhibit significant inverse associations with cancer-specific mortality, indicating that these allergic conditions may be associated with improved survival rates in patients with prostate cancer21,22. On the other hand, atopy or any allergy does not appear to be directly linked to a reduced risk of developing prostate cancer itself21. Asthma has been identified as both a risk factor for lung cancer in some studies, while others report a significant inverse association between asthma and lung cancer-specific mortality, indicating a complex relationship between asthma and lung cancer23,24. Regarding glioma, a history of any allergy or allergies of any type appears to offer a protective effect against the development of this type of brain cancer25. Furthermore, asthma has shown significant inverse associations with breast cancer-specific mortality, suggesting that individuals with asthma may have a better prognosis in breast cancer21. Finally, atopic diseases are inversely associated with colorectal cancer (CRC), indicating that allergic conditions may reduce the risk of CRC21. However, a history of allergy does not show a significant association with CRC risk26. These mixed results highlight the need for further research to understand the nuanced relationship between allergic diseases and the risk of different types of malignant cancers.
Traditionally, allergic conditions have been considered a form of chronic inflammation that increases the risk of malignancy. Persistent inflammatory microenvironments may contribute to carcinogenesis through multiple mechanisms, including accelerated cell turnover, increased production of reactive oxygen species (ROS), impaired DNA repair, and remodeling of the extracellular matrix27. For instance, ulcerative colitis has been linked to colorectal cancer, chronic pancreatitis to pancreatic cancer, and gastroesophageal reflux disease to esophageal adenocarcinoma28. However, emerging evidence suggests that allergic inflammation may, in some contexts, be inversely associated with cancer risk. One potential explanation is that allergic responses may enhance immune surveillance by activating specific immune pathways, thereby offering protective effects against tumor development29. Support for this hypothesis has also emerged from preclinical studies. For example, in the observed inverse correlation between asthma and brain cancers, asthma is a type 2 immune-mediated disease characterized by elevated levels of IL-4, IL-5, and IL-13, accompanied by prominent eosinophilic inflammation, IgE production, and activation of mast cells and basophils. These cytokines and effector cells can influence the tumor microenvironment30. A murine model demonstrated that allergic airway inflammation (AAI) delayed glioblastoma progression by reprogramming microglia toward a pro-inflammatory phenotype. This shift was associated with enhanced infiltration of CD4⁺ T cells into the tumor microenvironment and an increase in circulating effector memory T cells31. In line with this, Chatterjee et al. reported that asthma may suppress glioma formation through decorin-mediated inhibition of microglial activity via T cell signaling32. These findings suggest that asthma-associated immune activation may contribute to antitumor immune responses in the central nervous system. In addition, studies have shown that elevated IgE levels in allergic individuals may contribute to antitumor immunity by binding tumor-associated antigens and facilitating antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP)29. Notably, epidemiological evidence suggests that individuals with allergic diseases or elevated serum IgE levels may have a reduced risk of developing a variety of specific site malignancies, including colorectal cancer, pancreatic cancer, and especially gliomas. Glioma has the most consistent negative correlation with these tumors, and some studies have further suggested that higher IgE levels may confer a survival benefit29,33,34. This is also consistent with the findings of this meta-analysis. Conversely, certain allergic mediators may have pro-tumorigenic effects in specific contexts. For instance, histamine has been shown to impair the efficacy of cancer immunotherapy by activating histamine H1 receptors on macrophages, which may dampen antitumor immune responses. A clinical study found that melanoma and lung cancer patients receiving H1 antihistamines during anti-PD-1/PD-L1 therapy experienced significantly improved overall survival compared to those who did not. A similar trend of reduced mortality was observed in breast and colon cancer patients under the same treatment conditions35. These findings, however, appear to contradict our meta-analysis results, which showed a negative association between allergy and such cancer, suggesting that the relationship between allergy and cancer may be context-dependent and influenced by cancer type, immune milieu, and specific allergic pathways.
Collectively, these findings reflect the multifaceted and context-dependent immunological consequences of allergic inflammation. Activated immune cells such as M1 macrophages36, dendritic cells (DCs)37, NK cells38, Th1 cells39, follicular T helper cells (TFH)40, CD8 + T cells, B lymphocytes41, and eosinophils37 typically have an inhibitory effect on tumorigenesis, while tolerogenic cells like M2 macrophages36, tolerogenic DCs, and T and B regulatory lymphocytes39 tend to support carcinogenesis. Moreover, Mast cells exhibit dual roles, balancing immune tolerance with tumor inhibition42. Additionally, immune molecules such as IgE and CCL5 chemokines are associated with anti-cancer effects, whereas IgG4, IL-10, TGF-β, lipocalin-2, and CCL1 chemokines tend to promote cancer progression43. Notably, these immune cells and molecules are also integral to allergic reactions, further complicating the understanding of how allergic responses influence tumorigenesis and progression. For instance, macrophages, DCs, B cells, TFH13 cells, mast cells, and eosinophils are all critical players in allergy pathophysiology, with their roles extending into immune activation, antigen presentation, and cytokine secretion43,44,45. Taken together, the association between allergy and cancer risk, progression, or mortality is not consistent across all cancer types. These seemingly contradictory theories emphasize the relevance of the allergy-cancer relationship and highlight the urgent need for mechanistic studies to elucidate its underlying causal relationships and biological pathways.
In summary, this study synthesizes existing evidence to reveal potential inverse associations between allergies and certain cancer types. While our findings provide preliminary insights into the possible protective effects of allergies against specific cancers, the overall certainty of evidence, evaluated using the GRADE framework, was rated as low to very low. Specifically, the associations observed for lung cancer, leukemia, and gastrointestinal cancers were supported by very low-certainty evidence. This was primarily due to the observational design of all included studies, which limits causal inference. Additionally, substantial heterogeneity across studies, the potential for publication bias, and the presence of residual confounding further reduce the robustness of the findings. Therefore, these associations should be interpreted with caution and warrant confirmation through large-scale prospective cohort studies, randomized controlled trials, and mechanistic in vivo experiments. Despite the above-mentioned limitations, we believe that this study not only provides important insights into the relationship between allergies and cancer, but also lays the foundation for exploring the underlying immunological mechanisms. Future research should focus on addressing these limitations and deepening our understanding of how allergies may influence cancer development, progression, and mortality.
Data availability
The data used in this study are available upon reasonable request by contacting the corresponding author.
References
Ozdemir, C. et al. Lifestyle Changes and Industrialization in the development of allergic diseases. Curr. Allergy Asthma Rep. 24(7), 331–345 (2024).
Gohal, G., Moni, S. S., Bakkari, M. A. & Elmobark, M. E. A review on asthma and allergy: Current understanding on molecular perspectives. J. Clin. Med. 13(19), 5775 (2024).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74(3), 229–263 (2024).
Varadé, J., Magadán, S. & González-Fernández, Á. Human immunology and immunotherapy: Main achievements and challenges. Cell Mol. Immunol. 18(4), 805–828 (2021).
Heymann, F. & Tacke, F. Immunology in the liver — from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13(2), 88–110 (2016).
Ogulur, I. et al. Advances and highlights in biomarkers of allergic diseases. Allergy 76(12), 3659–3686 (2021).
Kay, A. B. Allergy and allergic diseases. N. Engl. J. Med. 344(1), 30–37 (2001).
Gonzalez, H., Hagerling, C. & Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 32(19–20), 1267–1284 (2018).
Ribatti, D. The concept of immune surveillance against tumors: The first theories. Oncotarget 8(4), 7175–7180 (2017).
Cui, J. W. et al. Tumor immunotherapy resistance: Revealing the mechanism of PD-1 / PD-L1-mediated tumor immune escape. Biomed. Pharmacother. 171, 116203 (2024).
Kim SK, Cho SW. The evasion mechanisms of cancer immunity and drug intervention in the tumor microenvironment. Front. Pharmacol. 13 (2022).
Tang, T. et al. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct. Target Ther. 6(1), 72 (2021).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 62(10), e1–e34 (2009).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25(9), 603–605 (2010).
Higgins, J. P. T. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21(11), 1539–1558 (2002).
Macaskill, P., Walter, S. D. & Irwig, L. A comparison of methods to detect publication bias in meta-analysis. Stat. Med. 20(4), 641–654 (2001).
Wang G, Xu Z, Zhu J, et al. Decreased risk in the pancreatic cancer with history of hay fever: A meta-analysis. Front. Public Health. 8 (2020).
Gomez-Rubio, P. et al. Reduced risk of pancreatic cancer associated with asthma and nasal allergies. Gut 66(2), 314–322 (2017).
Gandini, S., Lowenfels, A. B., Jaffee, E. M., Armstrong, T. D. & Maisonneuve, P. Allergies and the risk of pancreatic cancer: A meta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiol. Biomark. Prev. 14(8), 1908–1916 (2005).
Olson, S. H. et al. Allergies and risk of pancreatic cancer: A pooled analysis from the pancreatic cancer case-control consortium. Am. J. Epidemiol. 178(5), 691–700 (2013).
Vojtechova, P. & Martin, R. M. The association of atopic diseases with breast, prostate, and colorectal cancers: A meta-analysis. Cancer Causes Control 20(7), 1091–1105 (2009).
Zhu, J. et al. Association between allergic conditions and risk of prostate cancer: A prisma-compliant systematic review and meta-analysis. Sci. Rep. 6(1), 35682 (2016).
Khan, Q. U. et al. Correlation between allergic rhinitis or hay fever and lung cancer: A systematic review and meta-analysis. Medicine 103(20), e38197 (2024).
Tennis, P., Sherrill, B., Fernandez, C. & Dolan, C. Cancer risk in asthmatic populations. Ann. Allergy Asthma Immunol. 95(4), 354–360 (2005).
Turner, M. C. et al. Allergy and brain tumors in the Interphone study: Pooled results from Australia, Canada, France, Israel, and New Zealand. Cancer Causes Control 24(5), 949–960 (2013).
Ye, J. et al. Allergies and risk of colorectal cancer: A systematic review and meta-analysis of observational studies. Oncotarget 8(9), 14646–14654 (2017).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420(6917), 860–867 (2002).
Muir, A. B. et al. The potential for malignancy from atopic disorders and allergic inflammation: A systematic review and meta-analysis. Clin. Exp. Allergy 50(2), 147–159 (2020).
McCraw, A. J. et al. Insights from IgE immune surveillance in allergy and cancer for Anti-Tumour IgE treatments. Cancers (Basel). 13(17), 4460 (2021).
Fahy, J. V. Type 2 inflammation in asthma–present in most, absent in many. Nat. Rev. Immunol. 15(1), 57–65 (2015).
Poli, A. et al. Allergic airway inflammation delays glioblastoma progression and reinvigorates systemic and local immunity in mice. Allergy 78(3), 682–696 (2023).
Chatterjee, J. et al. Asthma reduces glioma formation by T cell decorin-mediated inhibition of microglia. Nat. Commun. 12(1), 7122 (2021).
Pellizzari, G. et al. IgE re-programs alternatively-activated human macrophages towards pro-inflammatory anti-tumoural states. EBioMedicine 43, 67–81 (2019).
Ferastraoaru, D. et al. AllergoOncology: ultra-low IgE, a potential novel biomarker in cancer-a position paper of the European academy of allergy and clinical immunology (EAACI). Clin. Transl. Allergy. 10(1), 32 (2020).
Li, H. et al. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell 40(1), 36-52.e9 (2022).
Mantovani, A. & Locati, M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization. Arterioscler. Thromb. Vasc. Biol. 33(7), 1478–1483 (2013).
Pan C, Wang Y, Liu Q, et al. Phenotypic profiling and prognostic significance of immune infiltrates in esophageal squamous cell carcinoma. Oncoimmunology. 10(1) (2021).
Ghaemdoust, F., Keshavarz-Fathi, M. & Rezaei, N. Natural killer cells and cancer therapy, what we know and where we are going. Immunotherapy 11(14), 1231–1251 (2019).
Yadav M, Stephan S, Bluestone JA. Peripherally Induced Tregs-Role in Immune Homeostasis and Autoimmunity. Front. Immunol. 4 (2013).
Guo, Z. et al. The role of circulating T follicular helper cells and regulatory cells in non-small cell lung cancer patients. Scand. J. Immunol. 86(2), 107–112 (2017).
Linnebacher, M. & Maletzki, C. Tumor-infiltrating B cells. Oncoimmunology. 1(7), 1186–1188 (2012).
Varricchi, G., de Paulis, A., Marone, G. & Galli, S. J. Future needs in mast cell biology. Int. J. Mol. Sci. 20(18), 4397 (2019).
Di Gioacchino M, Della Valle L, Allegra A, Pioggia G, Gangemi S. AllergoOncology: Role of immune cells and immune proteins. Clin. Transl. Allergy. 12(3) (2022).
Jensen-Jarolim, E. et al. AllergoOncology – the impact of allergy in oncology: <scp>EAACI</scp> position paper. Allergy 72(6), 866–887 (2017).
Jensen-Jarolim, E. et al. AllergoOncology: The role of IgE-mediated allergy in cancer. Allergy 63(10), 1255–1266 (2008).
Fekrazad, S. et al. Allergies and risk of head and neck cancer: A case–control study. Sci. Rep. 14(1), 15006 (2024).
Aryaie, M. et al. The association between allergy and cancer: A case-control study. Asian Pac. J. Cancer Prev. 25(8), 2787–2795 (2024).
Choi, Y. J. et al. Allergic diseases and risk of malignancy of gastrointestinal cancers. Cancers (Basel). 15(12), 3219 (2023).
Kim, J. A. et al. Association of the presence of allergic disease with subsequent risk of liver cancer in a nationwide retrospective cohort among Koreans. Sci. Rep. 12(1), 9856 (2022).
ZHOU X, WANG WH, Shao T, et al. Association of hepatitis B virus infection and allergy with the risk of gastrointestinal cancer. Published online November (2021).
Carneiro, B. G. M. C. et al. Clinical and immunological allergy assessment in cancer patients. Sci. Rep. 11(1), 18110 (2021).
Shirkhoda, M., Nejad, VM., Eslami, B., Zebardast, J., Jalaeefar, A. Association between papillary thyroid cancer and allergy: A Case-Control Study. Int. J. Cancer Manag. In Press(In Press) (2018).
Huang, B. Z. et al. Atopic allergic conditions and pancreatic cancer risk: Results from the multiethnic cohort study. Int. J. Cancer. 142(10), 2019–2027 (2018).
Liao, H. C. et al. Allergy symptoms, serum total immunoglobulin E, and risk of head and neck cancer. Cancer Causes Control 27(9), 1105–1115 (2016).
Kozłowska, R., Bożek, A. & Jarząb, J. Association between cancer and allergies. Allergy Asthma Clin. Immunol. 12(1), 39 (2016).
Helby, J., Bojesen, S. E., Nielsen, S. F. & Nordestgaard, B. G. IgE and risk of cancer in 37 747 individuals from the general population. Ann. Oncol. 26(8), 1784–1790 (2015).
Taghizadeh, N. et al. Objective allergy markers and risk of cancer mortality and hospitalization in a large population-based cohort. Cancer Causes Control 26(1), 99–109 (2015).
Hofmann, J. N. et al. Farm characteristics, allergy symptoms, and risk of non-hodgkin lymphoid neoplasms in the agricultural health study. Cancer Epidemiol. Biomark. Prev. 24(3), 587–594 (2015).
Filippidis, F. T. et al. Association of history of allergies and influenza-like infections with laryngeal cancer in a case-control study. Eur. Arch. Otorhinolaryngol. 272(8), 2063–2069 (2015).
Tambe, N. A. et al. Atopic allergic Conditions and colorectal cancer risk in the multiethnic cohort study. Am. J. Epidemiol. 181(11), 889–897 (2015).
Hollander, P. et al. Autoimmune and atopic disorders and risk of classical hodgkin lymphoma. Am. J. Epidemiol. 182(7), 624–632 (2015).
Shu, X. et al. Atopic conditions and brain tumor risk in children and adolescents—an international case–control study (CEFALO). Ann. Oncol. 25(4), 902–908 (2014).
Cotterchio, M., Lowcock, E., Hudson, T. J., Greenwood, C. & Gallinger, S. Association between allergies and risk of pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 23(3), 469–480 (2014).
Weiss, D. et al. Asthma, allergy and the risk of prostate cancer: Results from the Montreal PROtEuS study. Cancer Epidemiol. 38(6), 695–699 (2014).
Lowcock, E. C., Cotterchio, M. & Ahmad, N. Association between allergies, asthma, and breast cancer risk among women in Ontario. Canada. Cancer Causes Control. 24(5), 1053–1056 (2013).
Dikalioti, S. K. et al. Allergy-associated symptoms in relation to childhood non-Hodgkin’s as contrasted to Hodgkin’s lymphomas: A case–control study in Greece and meta-analysis. Eur. J. Cancer. 48(12), 1860–1866 (2012).
Chae, Y. K. et al. Association between common allergic symptoms and cancer in the NHANES III female cohort. PLoS ONE 7(9), e42896 (2012).
Grimmer, J. F. et al. Hemangioma is associated with atopic disease. Otolaryngol.-Head Neck Surg. 146(2), 206–209 (2012).
Stott-Miller, M. et al. A history of allergies is associated with reduced risk of oral squamous cell carcinoma. Cancer Causes Control 23(12), 1911–1919 (2012).
Engkilde, K., Thyssen, J. P., Menné, T. & Johansen, J. D. Association between cancer and contact allergy: A linkage study. BMJ Open 1(1), e000084 (2011).
Maisonneuve, P. et al. Past medical history and pancreatic cancer risk: Results from a multicenter case-control study. Ann. Epidemiol. 20(2), 92–98 (2010).
ElMasri, W. M., Tran, T. H. & Mulla, Z. D. A case-control study of asthma and ovarian cancer. Arch. Environ. Occup. Health. 65(2), 101–105 (2010).
Becker, N. et al. Birth order, allergies and lymphoma risk: Results of the European collaborative research project Epilymph. Leuk Res. 31(10), 1365–1372 (2007).
Olson, S. H. et al. Allergies, variants in IL-4 and IL-4Rα genes, and risk of pancreatic cancer. Cancer Detect Prev. 31(5), 345–351 (2007).
Cozen, W. et al. The effect of atopy, childhood crowding, and other immune-related factors on non-Hodgkin lymphoma risk. Cancer Causes Control 18(8), 821–831 (2007).
Wigertz, A. et al. Allergic conditions and brain tumor risk. Am. J. Epidemiol. 166(8), 941–950 (2007).
Prizment, A. E. et al. History of allergy and reduced incidence of colorectal cancer, iowa women’s health study. Cancer Epidemiol. Biomark. Prev. 16(11), 2357–2362 (2007).
Wang, H. et al. Atopic diseases, immunoglobulin E and risk of cancer of the prostate, breast, lung and colorectum. Int. J. Cancer. 119(3), 695–701 (2006).
Becker, N., Deeg, E., Rüdiger, T. & Nieters, A. Medical history and risk for lymphoma: Results of a population-based case-control study in Germany. Eur. J. Cancer. 41(1), 133–142 (2005).
Eriksson, N. E., Mikoczy, Z. & Hagmar, L. Cancer incidence in 13811 patients skin tested for allergy. J. Invest. Allergol. Clin Immunol. 15(3), 161–166 (2005).
Turner, M. C. et al. Cancer mortality among US men and women with asthma and hay fever. Am. J. Epidemiol. 162(3), 212–221 (2005).
Lindelöf, B., Granath, F., Tengvall-Linder, M. & Ekbom, A. Allergy and cancer. Allergy 60(9), 1116–1120 (2005).
Bosetti, C. et al. Allergy and the risk of selected digestive and laryngeal neoplasms. Eur. J. Cancer Prev. 13(3), 173–176 (2004).
Talbot-Smith, A. Allergy, atopy, and cancer: A prospective study of the 1981 Busselton Cohort. Am. J. Epidemiol. 157(7), 606–612 (2003).
Holly, E. A. Prior history of allergies and pancreatic cancer in the San Francisco Bay Area. Am. J. Epidemiol. 158(5), 432–441 (2003).
Hedderson, M. M., Malone, K. E., Daling, J. R. & White, E. Allergy and risk of breast cancer among young women (United States). Cancer Causes Control 14(7), 619–626 (2003).
Briggs, N. C., Levine, R. S. & Brann, E. A. Allergies and risk of non-Hodgkin’s lymphoma by subtype. Cancer Epidemiol. Biomark. Prev. 11(4), 401–407 (2002).
Negri, E. et al. Allergy and other selected diseases and risk of colorectal cancer. Eur. J. Cancer. 35(13), 1838–1841 (1999).
Petroianu, A., Chaves, D. N. B. & De, O. O. Comparative incidence of allergy in the presence or absence of cancer. J. Int. Med. Res. 23(5), 358–363 (1995).
Eriksson, N. E., Holmén, A., Högstedt, B., Mikoczy, Z. & Hagmar, L. A prospective study of cancer incidence in a cohort examined for allergy. Allergy 50(9), 718–722 (1995).
Dai, Q. et al. Prior immunity-related medical conditions and pancreatic-cancer risk in Shanghai. Int. J. Cancer. 63(3), 337–340 (1995).
Mills, P. K., Beeson, W. L., Fraser, G. E. & Phillips, R. L. Allergy and Cancer: Organ site-specific results from the adventist health study. Am. J. Epidemiol. 136(3), 287–295 (1992).
La, V. C., D’Avanzo, B., Negri, E. & Franceschi, S. History of selected diseases and the risk of colorectal cancer. Eur J Cancer Clin Oncol. 27(5), 582–586 (1991).
McDuffie, H. H. Atopy and primary lung cancer. Chest 99(2), 404–407 (1991).
La, V. C. et al. Medical history, diet and pancreatic cancer. Oncology 47(6), 463–466 (1990).
Severson, R. K. et al. Acute myelocytic leukemia and prior allergies. J. Clin. Epidemiol. 42(10), 995–1001 (1989).
McWhorter, W. P. Allergy and risk of cancer. A prospective study using nhanesi followup data. Cancer 62(2), 451–455 (1988).
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82074104) and the National Natural Science Foundation of China (8247143489) and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences(2022-I2M-2–002).
Author information
Authors and Affiliations
Contributions
S.G and X.F performed the literature search, study inclusion, and data extraction for the included studies. S.G and X.F, W.L. participated in the concept, design, statistical analysis, and manuscript writing. J.B. and E.M. supervised the study process and reviewed the data. H.J. provided critical guidance on the methodology and interpretation of the results. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, S., Fan, X., Li, W. et al. Correlation between allergy and cancer: a systematic review and meta-analysis. Sci Rep 15, 34901 (2025). https://doi.org/10.1038/s41598-025-18646-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18646-y