Abstract
Little is known about phase angle (PhA), a bioelectrical impedance analysis (BIA) indicator of cellular health, and muscle asymmetry in the limbs of children. The purpose of this study is to clarify PhA and muscle mass asymmetry in the limbs of children. We examined the muscle mass and PhA in the extremities of 1199 healthy 8-year-olds (604 males and 595 females) from large cohort study targeting children conducted in Japan. Muscle mass and PhA were measured in each limb by using a BIA device and the limb asymmetry of these body compositions was assessed. The muscle mass and PhA were significantly higher in the dominant upper limbs. In the lower limbs, muscle mass was greater on the right side in both sexes, whereas the PhA showed no significant asymmetry in males and was higher on the left side in females. The degree of asymmetry in the PhA was greater than that in muscle mass, especially in the upper limbs. These results provide a standard reference for asymmetry in muscle mass and PhA in the extremities for this age group. Further studies are needed to clarify the relationship between limb dominance and PhA.
Similar content being viewed by others
Introduction
Limb muscle mass asymmetry is a common phenomenon among healthy individuals. It is influenced by limb dominance, age, and sex1,2. Upper extremity asymmetry is usually more pronounced, depending on the dominant upper limb. In contrast, the lower extremities show functional asymmetry related to habitual patterns of use3,4. However, most of these reports have been based on adults, and very few have investigated muscle mass asymmetry in children’s limbs; therefore, the extent to which asymmetry in limb muscle mass is typically present in children remains unclear.
Additionally, the phase angle (PhA) derived from bioelectrical impedance analysis (BIA) represents the geometric relationship between resistance and reactance, serving as the raw data for BIA5. PhA has recently been recognized as an indicator of cell health, integrity, and muscle quality6,7,8. Although whole-body PhA has been extensively studied, local measurements, especially in the extremities, have recently received increased attention due to their relevance in assessing partial muscle quality and functional performance8,9. First, there are very few reports on limb asymmetry in the PhA, and very little is known about it, particularly in children. The assessment of PhA in pediatric populations provides valuable insights into growth, development, and body composition, making it a potentially powerful tool for monitoring the health status and physical development of children.
Given that muscle mass and strength asymmetry can reflect developmental trends, lifestyle factors, and health status, evaluating PhA asymmetry in the extremities of children can provide new insights into normal developmental patterns and early indicators of imbalance.
We have conducted a cohort study of children at our institution. In this cohort study, we evaluated the body compositions of 8-year-old participants, including their PhAs. This study aimed to investigate the muscle mass and PhA asymmetry in the limbs of healthy children using a cohort of cases. We hypothesized that muscle mass in the limbs of children, as well as in adults, and even PhA, would be significantly greater on the right side or dominant side. By examining these partial measurements, we aimed to provide a standard reference for asymmetry in muscle mass and PhA in the extremities for this age group and to explore the potential of muscle mass and PhA asymmetry as developmental indicators.
Materials and methods
Study design
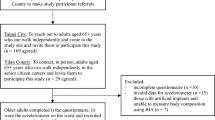
The Japan Environment and Children’s Study (JECS) is a national project funded directly by the Ministry of Environment, Japan. This birth cohort study was conducted to elucidate the influence of environmental factors during the fetal period and early childhood on children’s health, with a follow-up period of approximately 13 years. Participants in this cohort study are recruited at the time of their mother’s pregnancy, but participation is voluntary. The details of the JECS protocol and baseline data are available elsewhere10. Specifically, document surveys were conducted every 6 months after birth, and face-to-face surveys were conducted periodically, typically every few years. During the planning stage, a face-to-face survey was conducted when the participants were 8 years old. The JECS conducts height and weight measurements, urine tests, and computer-based developmental tests in 8-year-old children. In addition to the face-to-face survey with uniform content conducted nationwide at age 8 years, we independently conducted an Adjunct Study. This Adjunct Study included postural stability tests, footprint surveys, questionnaire surveys on the results of sports tests conducted at each school, ophthalmological or oral investigations, and body composition tests (including bioelectrical impedance analysis, or BIA) using a body composition analyzer. Participants and their respective data were used in this study. This study was approved by the Ethics Committee of university of Yamanashi (approval number: 2020). Written informed consent was obtained from the guardians of all the participants in accordance with the Declaration of Helsinki. All surveys were conducted at venues set up within our university.
Participants
2055 children (8 years old) were participated in the Adjunct Study conducted at venues set up in our university between July 2019 and November 2022 and no participants had a history of trauma or neuromuscular disorders that could affect limb muscle mass or PhA. Of these 2055 participants 1279 who were examined correctly for body composition were included in the study. Based on past reports11,12, the maximum value of PhA is considered to be around 10. Therefore, values greater than this were considered outliers, and eighty cases with PhA greater than 10 were excluded. Finally, 1199 8-year-old children (604 males and 595 females) were surveyed. All the study population consisted of ethnically homogeneous Japanese individuals, all born and raised in Japan.
Body composition assessment
Body composition assessments were conducted in a room set up inside the venue by a group of well-trained staff in both morning and afternoon sessions. Out of concern for dehydration, no restrictions were imposed on drinking or eating prior to the examination. The heights of the participants were measured and recorded in centimeters to the nearest millimeter. Body weight, body fat percentage, and predicted muscle mass were assessed using an MC-780U body composition analyzer (TANITA, Tokyo, Japan), which generated these analyses based on bioelectrical impedance analysis. At the same time, the participant wore shorts and a T-shirt. The soles of the participants’ feet and the measuring equipment were thoroughly wiped with sanitizing wipes each time.
Muscle mass and PhA were measured separately for each limb using a body composition analyzer. The dominant upper limb was determined based on parental interviews, defining the dominant hand as the one primarily used for writing or throwing a ball.
Statistical analysis
All statistical analyses were performed using JMP Pro Software version 16, released in 2021 by SAS Institute Japan Co., Ltd. (Tokyo, Japan).
As not all muscle mass and PhA data were normally distributed, extremity muscle mass and PhA were compared between the dominant and non-dominant sides of upper limb using the Wilcoxon signed-rank test. In the lower extremities, the muscle mass and PhA were compared between the right and left sides using the Wilcoxon signed-rank test. Differences in the degree of asymmetry between muscle mass and PhA were assessed using Cohen d and the mean differences. Statistical significance was set at a P value of less than 0.05.
Results
The body sizes and dominant upper limb of all participants are presented in Table 1. None of the participants had large body sizes.
The muscle mass was significantly greater, and the differences between the dominant and non-dominant upper limbs and the left and right lower limbs are presented in Table 2. In the upper extremities, the dominant and right sides had significantly greater muscle mass than the non-dominant and left sides in both sexes. In the lower extremities, the muscle mass was also significantly greater on the right side in both sexes.
The phase angles of the extremities and the differences between the groups are presented in Table 3. In the upper extremities, PhA was significantly greater on the dominant and right sides than on the non-dominant and left sides in both sexes. In the considerably greater lower extremities, there was no difference between the left and right sides in males, whereas the left side had a significantly greater PhA in females.
Table 4 lists the results, which indicate more asymmetrical differences in the PhA and muscle mass. The effect sizes and mean differences were greater for the right or dominant upper limb in the upper limbs. For the lower limbs, the effect size and mean differences were minimal for both PhA and muscle mass.
Discussion
In this study, we measured the body composition of 1199 healthy 8-year-old children and found that the upper-limb PhA and muscle mass were significantly greater in the right or dominant upper limb. In the lower limbs, significant asymmetry was observed in both the PhA and muscle mass, albeit to a lesser extent. Asymmetry in the extremities was greater for PhA than for muscle mass. No previous study has examined the asymmetry between the PhA and muscle mass in the extremities in such a large number of children.
Although humans are characterized by a symmetrical body, divided by a plane that runs along the longitudinal axis and separates the body into left and right parts, unlike other primates, the functional preference for one of the upper limbs establishes directional asymmetry13,14. This indicates that bilateral discrepancies are consistently present across populations. The right upper limb is functionally dominant in most humans, and the frequency of left-handedness is estimated to be 10–13%15. In humans, a functional preference for the right upper limb results in pronounced domination of the left upper limb in terms of its morphological features. Pande et al. studied 10 fetuses and showed that the average weight of each muscle and bone in the upper limbs was greater on the dominant side16. Singh also revealed that distinct asymmetries exist in the limbs of rabbits and frogs and reported that stronger muscles in the dominant limb are congenitally inherited17. Moreover, regular sports practice may lead to asymmetrical development of the musculature in the dominant limb18,19.
These reports suggest that muscle asymmetry in limbs is influenced by both congenital factors and factors acquired through lifestyle and exercise habits. In our study, the right or dominant side had significantly more muscle mass in the upper limbs. Considering that the participants were not engaged in high-intensity sports at the time of the study, the results were attributed mainly to congenital factors.
However, several studies have linked muscle asymmetry with disease pathogenesis. Bishop et al. stated that inter-limb strength differences may be detrimental to performance in jumping, kicking, and cycling2. Ohba et al. suggested that in adolescent idiopathic scoliosis with a constructive thoracic curve, right shoulder imbalance is an independent risk factor for upper extremity skeletal muscle asymmetry20. However, these reports do not account for the inherent asymmetry in muscle mass, even under healthy conditions. Our results suggest that muscle mass asymmetry is present in healthy children and may serve as a standard for evaluating muscle mass asymmetry in various pathological conditions in the future.
Although PhA has recently been recognized as an indicator of cell health, integrity, and muscle quality, reports of PhA asymmetry in children’s limbs are scarce and poorly understood. In this study, the right or dominant upper limb showed a greater PhA on the upper extremity than on the contralateral side. D’Hondt et al. reported significantly more pronounced BIA-based side-to-side differences in elite youth tennis players than in a non-athletic reference population at the upper limb level. In contrast, no significant between-group differences were found in the lower limbs21. They stated that an explorative study should trigger future research to further scrutinize the role of PhA as a promising field method for monitoring bodily asymmetries in sports performance and athletic health. Stagi et al. reported that PhA appears to play a role in maintaining body composition symmetry, thus indicating a possible positive effect of sports that was not previously detected22. However, these reports were insufficient to evaluate asymmetry in the standard PhA because of the small number of participants. In this report, the PhA of the right or dominant upper limb was already larger than that of the contralateral hand at an age when the children had not yet been trained in sports, suggesting that congenital factors may contribute to PhA asymmetries. In addition, the effect size between the dominant and non- dominant upper limb was larger for the PhA than for muscle mass. This result suggests that the dominance of the dominant upper limb over the non-dominant upper limb is more related to PhA than to muscle mass. Whether daily use of the dominant upper limb increases PhA or whether the limb with a large PhA is the dominant side cannot be clarified by this study alone, and further research is needed.
This study has a few limitations. First, uniformity was lacking under the conditions under which body composition was measured. PhA is an evaluation index based on information obtained when a 50-kHz electric current is applied to the inside and outside of cells, and it varies with the amount of water in the body and the time that has elapsed after a meal. Standard BIA test protocols recommend fasting for 4 h prior to the test, avoiding exercise for 12 h prior, avoiding alcohol consumption for 24 h prior, consuming 1 L of water up to 1 h before the test, and avoiding caffeine consumption on the day of the test to obtain the most accurate results. If these standardized conditions are not followed, fluctuations in participants’ hydration status during measurement may significantly affect PhA values23. Participants in this study were not required to adhere to the restrictions regarding fasting duration or water intake before the examination, and the potential impact of this factor on the results cannot be overlooked. Patients with extremely high PhA values were excluded from this study. Such outliers may be related to the lack of uniformity in body composition measurement methods. And the inherent uncertainties in BIA device data are indeed significant issues. Second, unfortunately, the detailed algorithms and specific formulas used by the TANITA MC-780U body composition analyzer for calculating muscle mass are proprietary and not publicly disclosed by the manufacturer. Third, Dual-energy X-ray absorptiometry (DXA) is often considered a good reference method for body composition assessment. However, for a large cohort study like current study, BIA offers significant practical advantages, including no radiation exposure, ease of operation without requiring specialized qualifications, and overall convenience and we adopted BIA. While BIA is practical, it is generally known that BIA, particularly for muscle mass assessment, can exhibit higher variability compared to DXA, and the interpretation of data derived from BIA should be done with caution. Fourth, this study was only able to perform univariate analysis of the asymmetry of body composition in the limbs. Ideally, multivariate analysis should also have been performed to examine factors related to limb asymmetry, but this was impossible due to the nature of the measurement items in this adjunct study.
Conclusion
We performed BIA on the limbs of 8-year-old healthy children and found that both muscle mass and PhA were significantly greater in the right or dominant upper limb. The degree of asymmetry was greater in the PhA than in the muscle mass. These results provide a standard reference for asymmetry in muscle mass and PhA in the extremities for this age group. Further studies are needed to clarify the relationship between limb dominance and PhA.
The conclusions of this article are solely the responsibility of the authors and do not represent the official views of the government.
Data availability
All data generated or analyzed during the study are included in the published paper.
References
Koger, R. et al. Asymmetry in body composition parameters of the upper and lower extremity among healthy Austrian women. Anthropol. Anz. 80, 285–294 (2023).
Bishop, C., Turner, A. & Read, P. Effects of inter-limb asymmetries on physical and sports performance: A systematic review. J. Sports Sci. 36, 1135–1144 (2018).
Montefiori, E. et al. MRI-based anatomical characterisation of lower-limb muscles in older women. PLoS ONE 15, e0242973 (2020).
Kulas, A. S. et al. Bilateral quadriceps and hamstrings muscle volume asymmetries in healthy individuals. J. Orthop. Res. 36, 963–970 (2018).
Di Vincenzo, O., Marra, M. & Scalfi, L. Bioelectrical impedance phase angle in sport: A systematic review. J. Int. Soc. Sports Nutr. 16, 49 (2019).
Akamatsu, Y. et al. The phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J. Cachexia Sarcopenia Muscle. 13, 180–189 (2022).
Yamada, Y. et al. Electrical properties assessed by bioelectrical impedance spectroscopy as biomarkers of age-related loss of skeletal muscle quantity and quality. J. Gerontol. A. Biol. Sci. Med. Sci. 72, 1180–1186 (2017).
Hirano, Y., Yamada, Y., Matsui, Y., Ota, S. & Arai, H. Lower limb muscle quality and phase angle contribute to the reduced walking speed among older adults. Geriatr. Gerontol. Int. 22, 603–609 (2022).
Hatanaka, S. Relationship between phase angle and lower-extremity function in older adults: Itabashi Longitudinal Study on Aging. Nutrition 119, 112289 (2024).
Kawamoto, T. et al. Working Group of the Epidemiological Research for Children’s Environmental Health, Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 14, 25 (2014).
Bosy-Westphal, A. et al. Phase angle from bioelectrical impedance analysis: Population reference values by age, sex, and body mass index. JPEN J. Parenter. Enteral. Nutr. 30(4), 309–316 (2006).
Barbosa-Silva, M. C. G., Barros, A. J. D., Wang, J., Heymsfield, S. B. & Pierson, R. N. Jr. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 82(1), 49–52 (2005).
Dongen, S. V. Fluctuating asymmetry and developmental instability in evolutionary biology: Past, present and future. J. Evol. Biol. 19, 1727–1743 (2006).
Corballis, M. C. The evolution and genetics of cerebral asymmetry. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364, 867–879 (2009).
Raymond, M. & Pontier, D. Is there geographical variation in human handedness?. Laterality 9, 35–51 (2004).
Pande, B. S. & Singh, I. One-sided dominance in the upper limbs of human fetuses as evidenced by asymmetry in muscle and bone weight. J. Anat. 109, 457–459 (1971).
Singh, I. One-sided dominance in the limbs of rabbits and frogs, as evidenced by asymmetry in bone weight. J. Anat. 109(Pt 2), 271–275 (1971).
Theodorou, E., Grivas, T. B. & Hadjicharalambous, M. The influence of the dominant leg in body asymmetries in children and adolescent male soccer players. Pediatr. Rep. 16, 684–695 (2024).
Dimitrova, A. & Yankova-Pandourska, I. Asymmetry of lean body mass accumulation in 12-year-old tennis players. (Preliminary results). Acta Morphol. Anthropol. 24, 63–67 (2017).
Ohba, T. et al. Upper extremity skeletal muscle mass asymmetry exacerbated by shoulder imbalance in Lenke1A adolescent idiopathic scoliosis. J. Clin. Med. 11, 1–8 (2022).
D’Hondt, J. et al. Bioelectrical impedance analysis as a means of quantifying upper and lower limb asymmetry in youth elite tennis players: An explorative study. Eur. J. Sport Sci. 22, 1343–1354 (2022).
Stagi, S., Moroni, A., Micheletti Cremasco, M. & Marini, E. Body composition symmetry in long-term active middle-aged and older individuals. Int. J. Environ. Res. Public Health. 18, 5956 (2021).
Aburto-Corona, J. A., Calleja-Núñez, J. J., Moncada-Jiménez, J. & de Paz, J. A. The effect of passive dehydration on phase angle and body composition: A bioelectrical impedance analysis. Nutrients 16, 2202 (2024).
Acknowledgements
We are grateful to all the participants of the JECS-Y and all the individuals involved in the data collection. We would also like to thank Editage (www.editage.com) for their English language editing services.
Funding
The Japan Environment and Children’s Study was funded by the Ministry of the Environment, Japan.
Author information
Authors and Affiliations
Consortia
Contributions
M.W. performed conceptualization, formal analysis , investigation , writing original draft preparation. T.F. and J.I. performed review & editing. R.S., S. O., A. K., M. K.and H. Y. performed project administration. Z. Y. and H. H. were superviser.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wako, M., Fujimaki, T., Ichikawa, J. et al. Limb muscle mass and phase angle asymmetry in 8-year-old children. Sci Rep 15, 33577 (2025). https://doi.org/10.1038/s41598-025-18695-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18695-3