Abstract
This study aims to investigate the association between multidimensional frailty phenotypes and recurrent pregnancy loss (RPL) in reproductive-aged women. This cross-sectional study analyzed data from 6,438 reproductive-aged women participating in the National Health and Nutrition Examination Survey from 1999 to 2018. RPL was defined as experiencing two or more pregnancy losses, including elective abortions and stillbirths. Frailty was assessed using a validated 49-item Frailty Index (FI), which encompasses seven domains: cognitive health, physical health, mental health, comorbidities, healthcare utilization, laboratory parameters, and performance metrics. Participants were categorized as robust, pre-frail, or frail. We employed weighted multivariable logistic regression models, hierarchically adjusted for covariates, to evaluate the associations between frailty and RPL. Additionally, sensitivity analyses and subgroup analyses were conducted to confirm the robustness and specificity of our findings. A total of 6,438 reproductive-aged women were included in the analysis, frailty severity demonstrated a dose-dependent association with RPL risk. The overall RPL prevalence was 21.73%, with increasing rates observed across frailty strata: 18.75% (robust, FI < 0.10), 23.08% (pre-frail, 0.10 ≤ FI < 0.25), and 29.03% (frail, FI ≥ 0.25). Multivariable models revealed persistently elevated RPL risks for pre-frail ( OR = 1.23, 95% CI:1.01–1.52) and frail women (OR = 1.51, 95% CI:1.11–2.05) versus robust counterparts after full adjustment for sociodemographic and clinical confounders (p trend < 0.001). Subgroup analyses confirmed robustness across demographic and health strata, except for educational attainment (interaction p = 0.046). Threshold regression identified a nonlinear relationship: below FI = 0.183, each SD increase in frailty index amplified RPL odds exponentially (OR = 67.11, p < 0.001), whereas no significant association emerged beyond this inflection point. This study underscores frailty severity as an independent risk factor for RPL in reproductive-aged women, highlighting the need for standardized frailty screening and early interventions during the pre-frailty stage to mitigate RPL risk.
Similar content being viewed by others
Introduction
Recurrent pregnancy loss (RPL), clinically defined as two or more pregnancy losses prior to 20 weeks of gestation according to the latest guidelines, persists as a significant clinical challenge, affecting 1–5% of women within reproductive age and imposing substantial physical and psychosocial morbidity1,2,3,4. While advancements have elucidated established etiological contributors—including chromosomal anomalies, autoimmune dysregulation, and structural uterine abnormalities—up to 50% of RPL cases remain idiopathic, underscoring persistent deficiencies in characterizing multifactorial pathophysiological mechanisms5,6. Contemporary theoretical frameworks posit that systemic physiological instability, rather than discrete pathological entities, may lead to a higher risk of pregnancy loss7,8,9. This hypothesis is inherently crucial for understanding reproductive physiology. The population of reproductive-aged women, characterized by a wide range of health trajectories—from stable homeostasis to subtle dysregulation—provides a valuable context for examining how systemic physiological instability may affect reproductive outcomes10,11. However, the role of this physiological diversity in the pathogenesis of RPL has not been sufficiently explored, which restricts opportunities for mechanistic discoveries and targeted interventions.
Frailty is a multidimensional construct that has historically been defined in geriatric populations to indicate depleted physiological reserves and reduced adaptability to stress12. Recently, it has gained attention as an important indicator of health vulnerability in younger populations13. Within obstetrical contexts, frailty may encapsulate latent dysfunctions spanning metabolic perturbations, chronic inflammatory activation, endocrine axis disruption, and compromised allostatic load—collectively undermining maternal adaptive capacity during gestation14. Although preliminary observational studies have associated preconception frailty phenotypes with adverse outcomes such as preterm delivery and hypertensive disorders of pregnancy, its potential role in RPL etiology remains largely unexplored15. This knowledge gap is particularly consequential given that recurrent pregnancy failure may emerge from bidirectional interactions between cumulative physiological frailty and maladaptive gestational responses, especially in individuals subjected to repeated pregnancy-related stressors without sufficient recovery intervals16.
This study aims to investigate the association between frailty and RPL in reproductive-aged women, a demographic spanning peak fertility periods and diverse preconception health profiles. Using a validated multidimensional frailty instrument, we analyze the independent relationship between frailty severity and RPL history, adjusting for established confounders. By combining frailty metrics with clinical data, we aim to establish frailty as a biomarker of preconception vulnerability, refining risk stratification and informing resilience-focused interventions.
Methods
Study population
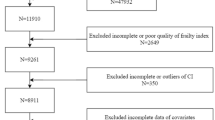
This cross-sectional study utilized data from the National Health and Nutrition Examination Survey (NHANES) 1999–2018, a nationally representative program assessing health and nutritional status in the United States through interviews, physical examinations, and laboratory tests17. The study protocol was approved by the National Center for Health Statistics Research Ethics Review Board, and all participants provided written informed consent. From an initial pool of 101,316 participants across ten NHANES survey cycles (1999–2018), we applied a multi-stage selection protocol to derive a cross-sectional sample of reproductive-aged women (18-45years). The selection process first excluded 49,893 males and 36,376 individuals outside the target age range, resulting in 15,047 eligible women. Subsequent phases excluded 7,252 participants with incomplete fertility-related data (pregnancy loss history, oral contraceptive use, or female hormone information) and 1,357 individuals lacking complete sociodemographic/health covariates (BMI, marital status, poverty-income ratio, smoking, alcohol use, education, hypertension, or diabetes). The final analytical sample comprised 6,438 women with complete data across all study variables, ensuring methodological rigor while preserving the nationally representative profile of reproductive-aged women in the United States. A detailed flowchart of the study population selection process is presented in Fig. 1.
The definition of recurrent pregnancy loss
RPL was operationally defined as ≥ 2 discrete pregnancy loss events, regardless of whether they occurred consecutively or intermittently, aligning with current clinical consensus criteria1,18. This classification was derived from computer-assisted interview data collected at the Mobile Examination Center, where participants self-reported their complete reproductive histories. The calculation method involved subtracting the number of live births (responses to “How many of your deliveries resulted in a live birth?”) from the overall count of reported pregnancies (responses to “How many times have you been pregnant?”). In this study, “initiated pregnancies” refers to all reported pregnancies, including both planned and unplanned pregnancies, without distinguishing between confirmed and unconfirmed pregnancies. Cases with a difference ≥ 2 were categorized as RPL, encompassing all pregnancy termination types including miscarriages, stillbirths, ectopic pregnancies, and elective abortions19. This threshold-based definition excluded currently pregnant individuals to avoid misclassification, ensuring temporal separation between pregnancy outcomes.
The definition of frailty index (FI)
Frailty was quantified using a validated FI based on the cumulative deficit model, operationalized as the ratio of observed health deficits to the total number of potential deficits, yielding a continuous scale from 0 (optimal health) to 1 (complete frailty)20. The FI incorporated approximately 49 items spanning seven health domains to provide a comprehensive health profile: cognitive function (memory impairment), physical dependency (difficulties in activities of daily living), depressive symptoms (assessed by PHQ-9), comorbidities (including arthritis, diabetes, cardiovascular conditions, and renal failure), healthcare utilization patterns (hospitalizations, medication use), physical performance metrics (grip strength and body mass index), and laboratory parameters (HbA1c, hemoglobin, lymphocyte counts)21,22. Participants with ≥ 80% completeness in item responses were included to ensure data reliability. Consistent with established clinical thresholds, FI scores were categorized as robust (FI < 0.10), pre-frail (0.10 ≤ FI < 0.25), or frail (FI ≥ 0.25) to reflect progressive vulnerability to adverse outcomes23,24.
Other variables
To account for potential confounding factors, this study incorporated a comprehensive set of covariates spanning sociodemographic, clinical, and behavioral domains, consistent with established methodologies in population health research. Demographic covariates included age measurement, race/ethnicity categorized as Mexican American, Non-Hispanic White, Non-Hispanic Black, or Other, educational attainment stratified into less than high school, high school/equivalent, or more than high school, marital status recoded as having a partner(married/living with partner), no partner (widowed/separated/divorced), or unmarried, and poverty-income ratio (PIR) expressed as a continuous variable25. Clinical characteristics encompassed body mass index (BMI) calculated from objectively measured height and weight, diabetes status defined by physician diagnosis, hypoglycemic medication use, HbA1c ≥ 6.5%, or fasting glucose ≥ 7.0 mmol/L, hypertension status confirmed through physician diagnosis, antihypertensive medication use, systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg. Education level was classified as less than high school, high school or equivalent, and college or above. Smoking history were categorized as “No” and “Yes”, alcohol consumption was classified by whether ≥ 4 drinks/day26,27. Female-specific covariates incorporated reproductive history variables including ever-use of birth control pills and exogenous female hormones18.
Statistical analysis
To ensure the representativeness of our findings for the target U.S. population, all analyses incorporated appropriate sampling weights accounting for NHANES’s complex multi-stage stratified sampling design. Participants were classified using established clinical thresholds for frailty: Robust (FI ≤ 0.10), Pre-frail (0.10 < FI < 0.25), and Frail (FI ≥ 0.25), with the Robust group serving as the reference category in regression analyses. Continuous variables are expressed as weighted means ± standard error (SE), while categorical variables are presented as weighted proportions. Group comparisons employed weighted t-tests or ANOVA for continuous variables and weighted chi-square tests for categorical variables. Four progressively adjusted weighted multivariate logistic regression models examined associations between frailty categories and outcomes: Model 1 (Crude) contained only frailty terms; Model 2 added adjustments for age, BMI, race, smoking status, alcohol consumption, poverty-to-income ratio (PIR), education level, and marital status; Model 3 further incorporated hypertension and diabetes; Model 4 included all preceding covariates plus birth control pill and female hormone use. Results are presented as Odds Ratios (OR) with 95% Confidence Intervals (CI). Statistical significance of trends across frailty categories was formally evaluated by assigning ordinal scores (Robust = 1, Pre-frail = 2, Frail = 3) and testing the significance of this continuous trend variable within each model.
Continuous frailty index scores were examined for non-linear relationships using restricted cubic splines within Generalized Additive Models, with knot placement optimized via the Akaike Information Criterion. Threshold effects were investigated using piecewise linear regression modeling. Likelihood-ratio tests were then applied to compare the goodness-of-fit of the single-slope model with that of the segmented model. Subgroup analyses were performed with weighted multivariable logistic regression models that adjusted for all confounders except the stratifying variable. In the sensitivity analysis, RPL was redefined as experiencing three or more losses. We performed weighted multivariable logistic regression, trend tests, subgroup analyses, and interaction tests to ascertain whether the association between the frailty index and RPL is affected by varying definitions of RPL.
All analyses were conducted using R software (version 4.4.0), utilizing specialized packages designed for complex survey designs. The “survey” package managed all weighted analyses, including descriptive statistics, hypothesis testing, and regression models. Non-linear splines and generalized additive models were implemented using the “rms” and “mgcv” packages. Additionally, piecewise threshold effects were assessed with the “segmented” package, with statistical significance determined by a two-tailed p-value of less than 0.0528,29,30.
Results
Baseline characteristics of the study population
Table 1 presents the baseline characteristics of 6,438 reproductive-aged women (18–45 years) stratified by frailty status: 2,408 in the robust group (FI < 0.10), 3,468 in the pre-frail group (0.10 ≤ FI < 0.25), and 562 in the frail group (FI ≥ 0.25). The overall prevalence of RPL was 21.73%. A significant gradient increase in RPL risk was observed with worsening frailty status: rates were 18.75% in the robust group, 23.08% in the pre-frail group, and 29.03% in the frail group. Additionally, participants stratified by frailty severity showed statistically significant differences (p < 0.05) in clinical characteristics including age, BMI, race, marital status, PIR, smoking status, education level, hypertension, diabetes, and female hormone use.
Univariate logistic regression analysis of RPL
Univariate logistic regression analysis of RPL (Table 2) identified several significant risk and protective factors. Significant risk factors for RPL (OR > 1, p < 0.05) included non-Hispanic Black race (versus Mexican American), other racial groups, unmarried status, smoking, drinking, hypertension, pre-frail status (vs. robust), and frail status (vs. robust).
Association between frailty index and RPL
We constructed four sequentially adjusted multivariate logistic regression models to evaluate the independent association between frailty and RPL. Table 3 details the ORs and 95% CIs derived from these analyses. In the unadjusted model (Model 1), pre-frail women exhibited an 30% higher risk of RPL compared to robust women (OR = 1.30; 95% CI: 1.07–1.58; p = 0.01), while frail women demonstrated a 77% increased risk (OR = 1.77; 95% CI: 1.36–2.30; p < 0.001). After comprehensive adjustment for sociodemographic and clinical confounders across Models 2–4, the risk estimates attenuated progressively but remained statistically significant (p < 0.05). In the fully adjusted model (Model 4), pre-frail women maintained a 23% elevated RPL risk (OR = 1.23; 95% CI: 1.01–1.52; p = 0.047), whereas frail women sustained a 51% higher risk (OR = 1.51; 95% CI: 1.11–2.05; p = 0.009). The persistent dose-dependent relationship across all models (p for trend < 0.001) confirms that frailty severity constitutes an independent risk factor for RPL.
The non-linear relationship between frailty index and RPL
Threshold regression analysis identified a statistically significant inflection point in the frailty-RPL association at FI = 0.183 (Likelihood Ratio Test vs. linear model: P = 0.007) (Fig. 2). Below this threshold, each standard deviation increment in frailty index corresponded to substantially increased odds of RPL (OR = 67.11, 95% CI: 12.62–356.81, P < 0.001). Conversely, no significant association was detected beyond this critical value (OR = 2.02, 95% CI: 0.35–11.74, P = 0.434). This segmented pattern—characterized by exponentially elevated risk at lower frailty levels (FI < 0.183) and attenuated effects in severe frailty-demonstrated superior fit (P = 0.007) over the significant yet constrained linear association (OR = 10.91, 95% CI: 4.95–24.06, P < 0.001) (Table 4).
Subgroup analysis and interaction effects
Subgroup analyses indicated a positive association between the frailty index and RPL risk (Fig. 3). Across subgroups including age, BMI, PIR, marital status, smoking history, alcohol consumption, hypertension, diabetes, and contraceptive use, effect estimates consistently exceeded 1, confirming the robustness of this association. Formal interaction testing demonstrated significant effect modification solely across educational attainment strata (p for interaction = 0.046), while none of the remaining 11 subgroups exhibited statistically significant interactions (p for interaction > 0.05), thereby strengthening the robustness of the frailty-RPL relationship.
Sensitivity analysis
We redefined RPL as experiencing three or more losses and conducted statistical analyses to examine whether the association between the frailty index and RPL is influenced by different definitions. The results, presented in Table S1, indicate that in the fully adjusted model (Model 4), the risk of RPL among women with pre-frailty remains elevated by 58% (OR = 1.58; 95% CI: 1.19–2.08; p = 0.002), while the risk among frail women increases by 78% (OR = 1.78; 95% CI: 1.09–2.92; p = 0.024). Trend tests demonstrate that the risk rises with increasing severity of frailty (P for trend = 0.003). Subgroup analyses reveal that the positive correlation between the frailty index and the risk of RPL loss remains stable across all subgroups, and interaction tests did not yield statistically significant results in any subgroup (p for interaction > 0.05), thus confirming the robustness of this association (Figure S1).
Discussion
This study investigated the association between frailty and RPL in reproductive-aged women. Our analysis demonstrates that increasing frailty severity exhibits a graded relationship with elevated RPL risk, with pre-frail and frail states conferring substantially higher vulnerability compared to robust health. Notably, this association persisted independent of sociodemographic, clinical, and lifestyle factors, underscoring frailty as an autonomous pathophysiological determinant. Crucially, we identified a nonlinear risk threshold (FI ≈ 0.18), below which even marginal increases in frailty correspond to disproportionate rises in RPL susceptibility. These findings collectively establish frailty as a clinically significant risk dimension for RPL, challenging conventional paradigms that overlook subclinical physiological decline in reproductive health assessment.
Frailty is a clinically recognizable state of vulnerability arising from the age-associated decline in physiological reserves and function across multiple organ systems31. This progressive deterioration compromises the body’s capacity to maintain homeostasis, predisposing individuals to adverse health outcomes following minor stressors32. A robust positive association exists between frailty and advancing age33. Consequently, substantial research has focused on elucidating connections between frailty and susceptibility to multiple geriatric syndromes. Epidemiological evidence consistently demonstrates that frailty significantly elevates vulnerability to diverse diseases and exacerbates their clinical trajectories34. Shu et al. reported that physically frail older adults exhibit substantially higher risks of cognitive decline (OR: 5.76; 95% CI: 1.20–27.6) and memory impairment (OR: 5.53; 95% CI: 1.64–18.7) compared to non-frail counterparts35. Chen et al. identified a dose-response relationship between frailty severity and cardiovascular risk, with frail older cardiovascular disease outpatients exhibiting twice the mortality risk of robust individuals36. Additionally, frailty independently correlates with increased risks of diabetes mellitus and asthma exacerbations in aging populations37,38. To our knowledge, our study is the first to demonstrate a significant association between frailty and elevated risk of RPL among reproductive-aged women. Frail women exhibited a 51% higher RPL risk compared to robust counterparts (OR = 1.51; 95% CI: 1.11–2.05; P < 0.001). Gotaro et al. demonstrated a significant positive association between higher gravidity and elevated frailty severity39. Multiparity may constitute a potential physiological stressor, potentially accelerating multisystem functional decline through cumulative metabolic dysregulation, chronic inflammatory states, and depletion of critical nutrient reserves – thereby propelling frailty progression40. In this context, RPL represents a distinct high-risk gestational condition where repeated embryonic loss may be closely linked to preexisting subclinical health deficits in maternal systemic resilience.
Widespread dysregulation of multisystem physiological homeostasis may significantly increase susceptibility to RPL in individuals with frailty. The core mechanism involves persistent activation of chronic low-grade inflammation, wherein elevated pro-inflammatory cytokines directly disrupt the embryonic implantation microenvironment and compromise placental development41. Concurrent dysregulation of metabolic-endocrine networks – manifested as sarcopenia, impaired glucose tolerance, and hypothalamic-pituitary-ovarian/adrenal axis dysfunction – further impairs endometrial receptivity and luteal phase integrity41,42,43. Furthermore, progressively accumulated oxidative stress diminishes reactive oxygen species scavenging capacity, inducing gamete DNA fragmentation, embryonic developmental arrest, and trophoblast dysfunction44. These pathophysiological alterations collectively attenuate immune tolerance at the maternal-fetal interface while synergizing with microcirculatory disturbances to impede proper placentation.
Frailty fundamentally signifies diminished resilience in meeting gestational physiological demands. The FI—a comprehensive assessment tool grounded in the cumulative deficit model—constructs a continuous frailty spectrum by quantifying 49 variables across seven health domains: cognitive function, physical dependence, depressive symptomology, comorbidity burden, healthcare utilization, physical performance, and laboratory parameters45,46. Its clinical stratification robustly categorizes individuals as robust, pre-frail, or frail, accurately reflecting progressive depletion of physiological reserves24. The robust association between FI and RPL reveals that synergistic accumulation of multisystem health deficits may compromise gestational sustainability, a mechanism transcending singular pathological attribution. Clinically, this evidence transforms FI into a critical peri-conceptual risk stratification metric: pre-frail women require early intervention targeting chronic disease management, psychological support, and physical optimization, while frail individuals necessitate intensified perinatal monitoring including serial placental function assessments and fetal growth surveillance. Ultimately, FI-informed hierarchical care reorganizes clinical paradigms from reactive treatment to proactive health preservation, generating innovative preventive pathways for RPL.
The control and interpretation of biases are crucial in clinical research. As a cross-sectional study, the biases in our research primarily stem from several key areas. Firstly, the definition of RPL varies among guidelines. The Royal College of Obstetricians and Gynaecologists defines RPL as three or more miscarriages, a standard maintained in their 2023 guidelines47. In contrast, the European Society of Human Reproduction and Embryology and the 2024 Australian Clinical Management Guidelines define RPL as two or more losses4,48. Recent articles in The Lancet challenge the distinctions between sporadic and recurrent miscarriages, advocating for improved support following even a single loss instead of waiting for three instances5,49,50. Given these developments, we adopted a definition of RPL as two or more losses to ensure that women experiencing two miscarriages receive adequate attention and assessment of their frailty status. Another source of bias arises from our inclusion of individuals with elective terminations in the calculation of RPL outcomes. These two factors jointly contributed to the higher incidence of RPL observed in our study. Due to inherent limitations within the database, we could not exclude these cases during the inclusion and exclusion process. To enhance the robustness of our findings, we expanded our definition of RPL to include individuals who have experienced three or more losses and conducted sensitivity and stratified analyses. Our results indicated a robust association between frailty and increased RPL risk, thereby reinforcing the validity of our findings. Additionally, our study incorporated an unmarried population and identified unmarried status as a risk factor for RPL. While the inclusion of elective terminations may contribute to this association, several biological and social factors provide independent explanatory pathways. These include: endometrial injury and cervical compromise resulting from surgical procedures; a higher prevalence of health-risk behaviors (e.g., smoking, nutritional deficiencies) and delayed prenatal care; increased susceptibility to reproductive tract infections leading to inflammatory cascades; and chronic stress due to limited social support affecting hypothalamic-pituitary-adrenal axis regulation and uteroplacental function1,5,41,51,52,53. In the subgroup analysis, frailty was positively correlated with elevated RPL risk across all marital statuses, and the interaction test was not significant, indicating that marital status did not significantly alter the association between frailty and RPL.
The cross-sectional design of this study inherently limits our ability to ascertain the temporal sequence between frailty and RPL at the individual level. While acknowledging that RPL itself could theoretically influence frailty status, population-level age analysis offers suggestive insights: the mean and median ages at RPL occurrence (35.7 and 31 years, respectively) exceeded those at the onset of pre-frailty (34.5 and 29 years) and frailty (35.37 and 30 years). This pattern implies that, on average, frailty manifestations may precede RPL in this reproductive-aged cohort, aligning with our objective to identify antecedent risk factors. Crucially, as a cross-sectional study, our primary finding demonstrates a significant association between frailty and increased RPL risk, not causation. Such studies serve a vital role in efficiently screening potential risk factors and generating hypotheses for longitudinal validation.
This study provides the first evidence from a large reproductive-aged population showing an independent link between FI and RPL, addressing an important research gap between reproductive health and physiological resilience. The analysis reveals a clear dose-response pattern where increasing frailty levels correspond to progressively higher RPL risk, with most subgroups showing consistent results through stratified analysis. A significant finding is the identification of a key threshold effect: modest worsening in mild frailty states leads to disproportionately large risk increases, while beyond certain FI levels, this relationship stabilizes. This nonlinear pattern provides new insights beyond previous linear assumptions, offering a scientific basis for preconception frailty screening.
However, limitations in the study findings require caution in translating these results to clinical guidance. The cross-sectional design prevents establishing causality and may not fully account for potential confounding factors such as polycystic ovary syndrome and endometriosis. Reliance on self-reported pregnancy history and the inclusion of the number of elective terminations in the calculation of RPL outcomes could introduce bias, and current FI assessments lack pregnancy-specific biomarkers (like clotting function or endometrial markers) that might strengthen clinical relevance. Database constraints also limit broader application due to small numbers in severe frailty subgroups and absence of genetic data. Additionally, the frailty index was analyzed as a composite measure; sensitivity analyses exploring domain-specific contributions (e.g., physical, cognitive, psychological) to RPL risk were not performed, limiting mechanistic insights. Notably, the nonlinear pattern suggests that interventions might show reduced effectiveness at advanced frailty stages. Future research should use longitudinal designs with repeated measurements to better understand how frailty pathways affect pregnancy outcomes over time.
Conclusion
Frailty severity shows an independent association with RPL risk in reproductive-aged women, with RPL in this study defined as experiencing two or more pregnancy losses, including elective abortions and stillbirths. This relationship demonstrates dual patterns: a dose-response gradient and a nonlinear relationship pattern—where initial increments in frailty correspond to disproportionately elevated RPL risk. Therefore, integrating standardized frailty screening into preconception and prenatal care protocols, along with early interventions during the clinically relevant pre-frailty stage, may contribute to mitigating the risk of RPL.
Data availability
The NHANES data are publicly available at https://wwwn.cdc.gov/nchs/nhanes/default.aspx. Further inquiries can be directed to the corresponding author.
References
Dimitriadis, E., Menkhorst, E., Saito, S., Kutteh, W. H. & Brosens, J. J. Recurrent pregnancy loss. Nat. Rev. Dis. Primers. 6, 98. https://doi.org/10.1038/s41572-020-00228-z (2020).
Devall, A. J. & Coomarasamy, A. Sporadic pregnancy loss and recurrent miscarriage. Best Pract. Res. Clin. Obstet. Gynecol. 69, 30–39. https://doi.org/10.1016/j.bpobgyn.2020.09.002 (2020).
Genovese, H. G. & McQueen, D. B. The prevalence of sporadic and recurrent pregnancy loss. Fertil. Steril. 120, 934–936. https://doi.org/10.1016/j.fertnstert.2023.08.954 (2023).
Suker, A., Li, Y., Marren, A. & Robson, D. Plain Language summary of the ACCEPT guideline for the management of recurrent pregnancy loss. Aust N Z. J. Obstet. Gynaecol. https://doi.org/10.1111/ajo.70000 (2025).
Quenby, S. et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 397, 1658–1667. https://doi.org/10.1016/s0140-6736(21)00682-6 (2021).
Yu, N., Kwak-Kim, J. & Bao, S. Unexplained recurrent pregnancy loss: novel causes and advanced treatment. J. Reprod. Immunol. 155, 103785. https://doi.org/10.1016/j.jri.2022.103785 (2023).
Garel, K. A., White, K. O. & Treder, K. M. Optimizing pregnancy loss care. Obstet. Gynecol. Clin. N. Am. 52, 289–302. https://doi.org/10.1016/j.ogc.2024.12.005 (2025).
Monticciolo, I., Guarano, A., Inversetti, A., Barbaro, G. & Di Simone, N. Unexplained recurrent pregnancy loss: clinical application of immunophenotyping. Am. J. Reproductive Immunol. (New York N Y : 1989). 92 (e13939). https://doi.org/10.1111/aji.13939 (2024).
Walter, K. Early pregnancy loss. Jama 329, 1426. https://doi.org/10.1001/jama.2023.4973 (2023).
Tint, A. et al. Health characteristics of reproductive-aged autistic women in ontario: A population-based, cross-sectional study. Autism: Int. J. Res. Pract. 25, 1114–1124. https://doi.org/10.1177/1362361320982819 (2021).
Schon, S. B., Cabre, H. E. & Redman, L. M. The impact of obesity on reproductive health and metabolism in reproductive-age females. Fertil. Steril. 122, 194–203. https://doi.org/10.1016/j.fertnstert.2024.04.036 (2024).
Dent, E. et al. Management of frailty: opportunities, challenges, and future directions. Lancet (London England). 394, 1376–1386. https://doi.org/10.1016/s0140-6736(19)31785-4 (2019).
Loecker, C., Schmaderer, M. & Zimmerman, L. Frailty in young and Middle-Aged adults: an integrative review. J. Frailty Aging. 10, 327–333. https://doi.org/10.14283/jfa.2021.14 (2021).
Hao, W. et al. Reproductive factors and their association with physical and comprehensive frailty in middle-aged and older women: a large-scale population-based study. Human reproduction open hoae038, (2024). https://doi.org/10.1093/hropen/hoae038 (2024).
Sun, N., Yang, K., Wang, H. & Zhou, W. Investigating genetic links between biological aging and adverse pregnancy outcomes. Biogerontology 26, 56. https://doi.org/10.1007/s10522-025-10198-y (2025).
Giouleka, S. et al. Investigation and management of recurrent pregnancy loss: A comprehensive review of guidelines. Obstet. Gynecol. Surv. 78, 287–301. https://doi.org/10.1097/ogx.0000000000001133 (2023).
Lau, D. T. et al. Data related to social determinants of health captured in the National health and nutrition examination survey. Am. J. Public. Health. 113, 1290–1295. https://doi.org/10.2105/ajph.2023.307490 (2023).
Gonzalez-Martin, R. et al. Association of blood cadmium and lead levels with self-reported reproductive lifespan and pregnancy loss: the National health and nutrition examination survey 1999–2018. Environ. Res. 233, 116514. https://doi.org/10.1016/j.envres.2023.116514 (2023).
Fang, H. et al. Association of coexposure to perfluoroalkyl and polyfluoroalkyl compounds and heavy metals with pregnancy loss and reproductive lifespan: the mediating role of cholesterol. Ecotoxicol. Environ. Saf. 286, 117160. https://doi.org/10.1016/j.ecoenv.2024.117160 (2024).
Jayanama, K. et al. Relationship of body mass index with frailty and all-cause mortality among middle-aged and older adults. BMC Med. 20 https://doi.org/10.1186/s12916-022-02596-7 (2022).
Jiang, Z., Wang, J., Cai, X., Wang, P. & Liu, S. L-shaped association of serum α-Klotho and frailty among the middle-aged and older adults: results from NHANES 2007–2016. BMC Geriatr. 23, 716. https://doi.org/10.1186/s12877-023-04324-z (2023).
Liu, X. et al. Association between frailty index and mortality in depressed patients: results from NHANES 2005–2018. Sci. Rep. 15, 3305. https://doi.org/10.1038/s41598-025-87691-4 (2025).
Zhou, J., Li, Y., Zhu, L. & Yue, R. Association between frailty index and cognitive dysfunction in older adults: insights from the 2011–2014 NHANES data. Front. Aging Neurosci. 16, 1458542. https://doi.org/10.3389/fnagi.2024.1458542 (2024).
Yue, G. et al. Association between frailty with kidney stones disease among adults aged 20 years and older population: a cross-sectional study of NHANES 2007–2020. Eur. J. Med. Res. 30, 412. https://doi.org/10.1186/s40001-025-02672-7 (2025).
Wang, S. et al. Association between pregnancy loss and depressive symptoms in women: a population-based study. BMC Psychiatry. 24, 526. https://doi.org/10.1186/s12888-024-05948-0 (2024).
Feng, X., Wu, S., Ke, B. & Liang, Y. Elevated TyG index associated with increased prevalence of gallstones in a united States cross-sectional study. Front. Public. Health. 12, 1351884. https://doi.org/10.3389/fpubh.2024.1351884 (2024).
Xiao, Q. et al. L-shaped association of serum 25-hydroxyvitamin D concentrations with cardiovascular and all-cause mortality in individuals with osteoarthritis: results from the NHANES database prospective cohort study. BMC Med. 20, 308. https://doi.org/10.1186/s12916-022-02510-1 (2022).
Duan, Y. & Zhang, R. Risk factors and prediction model of delirium in elderly patients after hip arthroplasty. Pak J. Med. Sci. 40, 1077–1082. https://doi.org/10.12669/pjms.40.6.9306 (2024).
Cheng, N., Chen, Y., Jin, L. & Chen, L. Nonlinear association between visceral fat metabolism score and heart failure: insights from LightGBM modeling and SHAP-Driven feature interpretation in NHANES. BMC Med. Inf. Decis. Mak. 25, 223. https://doi.org/10.1186/s12911-025-03076-7 (2025).
Liu, F. et al. Nonlinear relationship between oxidative balance score and hyperuricemia: analyses of NHANES 2007–2018. Nutr. J. 23, 48. https://doi.org/10.1186/s12937-024-00953-1 (2024).
Howlett, S. E., Rutenberg, A. D. & Rockwood, K. The degree of frailty as a translational measure of health in aging. Nat. Aging. 1, 651–665. https://doi.org/10.1038/s43587-021-00099-3 (2021).
Dent, E. et al. Recent developments in frailty identification, management, risk factors and prevention: A narrative review of leading journals in geriatrics and gerontology. Ageing Res. Rev. 91, 102082. https://doi.org/10.1016/j.arr.2023.102082 (2023).
Kim, D. H. & Rockwood, K. Frailty in older adults. N. Engl. J. Med. 391, 538–548. https://doi.org/10.1056/NEJMra2301292 (2024).
Clark, D., Kotronia, E. & Ramsay, S. E. Frailty, aging, and periodontal disease: basic biologic considerations. Periodontology 2000. 87, 143–156. https://doi.org/10.1111/prd.12380 (2021).
Nishiguchi, S. et al. Differential association of frailty with cognitive decline and sarcopenia in community-dwelling older adults. J. Am. Med. Dir. Assoc. 16, 120–124. https://doi.org/10.1016/j.jamda.2014.07.010 (2015).
Chen, L. et al. Physical frailty, adherence to ideal cardiovascular health and risk of cardiovascular disease: a prospective cohort study. Age Ageing. 52 https://doi.org/10.1093/ageing/afac311 (2023).
Zeng, X. Z. et al. Epidemiological status and associated factors of frailty and pre-frailty in older adults with asthma in china: A National cross-sectional study. Front. Public. Health. 11, 1136135. https://doi.org/10.3389/fpubh.2023.1136135 (2023).
Assar, M. E. & Laosa, O. Rodríguez mañas, L. Diabetes and frailty. Curr. Opin. Clin. Nutr. Metab. Care. 22, 52–57. https://doi.org/10.1097/mco.0000000000000535 (2019).
Kojima, G., Ogawa, K., Iliffe, S., Taniguchi, Y. & Walters, K. Number of pregnancies and trajectory of frailty index: english longitudinal study of ageing. J. Am. Med. Dir. Assoc. 21, 1249–1253e1241. https://doi.org/10.1016/j.jamda.2020.04.010 (2020).
Aviram, A., Barrett, J. F. R., Melamed, N. & Mei-Dan, E. Mode of delivery in multiple pregnancies. Am. J. Obstet. Gynecol. MFM. 4, 100470. https://doi.org/10.1016/j.ajogmf.2021.100470 (2022).
Alecsandru, D., Klimczak, A. M., Garcia Velasco, J. A., Pirtea, P. & Franasiak, J. M. Immunologic causes and thrombophilia in recurrent pregnancy loss. Fertil. Steril. 115, 561–566. https://doi.org/10.1016/j.fertnstert.2021.01.017 (2021).
Uța, C. et al. Alloimmune causes of recurrent pregnancy loss: cellular mechanisms and overview of therapeutic approaches. Med. (Kaunas Lithuania). 60 https://doi.org/10.3390/medicina60111896 (2024).
Amrane, S. & McConnell, R. Endocrine causes of recurrent pregnancy loss. Semin. Perinatol. 43, 80–83. https://doi.org/10.1053/j.semperi.2018.12.004 (2019).
Zhang, X., Gao, J., Yang, L., Feng, X. & Yuan, X. Oxidative stress and its role in recurrent pregnancy loss: mechanisms and implications. J. Mol. Histol. 56, 55. https://doi.org/10.1007/s10735-024-10332-z (2024).
Kojima, G., Iliffe, S. & Walters, K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 47, 193–200. https://doi.org/10.1093/ageing/afx162 (2018).
Zhang, D. et al. Frailty and risk of mortality in older cancer survivors and adults without a cancer history: evidence from the National health and nutrition examination survey, 1999–2014. Cancer 128, 2978–2987. https://doi.org/10.1002/cncr.34258 (2022).
Regan, L., Rai, R., Saravelos, S. & Li, T. C. Recurrent MiscarriageGreen-top guideline 17. Bjog 130, e9–e39. https://doi.org/10.1111/1471-0528.17515 (2023).
Bender Atik, R. et al. ESHRE guideline: recurrent pregnancy loss: an update in 2022. Hum Reprod Open hoad002, (2023). https://doi.org/10.1093/hropen/hoad002 (2023).
Coomarasamy, A. et al. Recurrent miscarriage: evidence to accelerate action. Lancet 397, 1675–1682. https://doi.org/10.1016/s0140-6736(21)00681-4 (2021).
The, L. Miscarriage: worldwide reform of care is needed. Lancet 397, 1597. https://doi.org/10.1016/s0140-6736(21)00954-5 (2021).
Alfsen, G. C. & Hernæs, L. Dead infants and unmarried women–from forensic pathology reports 1910-12. Tidsskr Nor. Laegeforen. 133, 2493–2497. https://doi.org/10.4045/tidsskr.13.0897 (2013).
Flink-Bochacki, R. et al. Examining intendedness among pregnancies ending in spontaneous abortion. Contraception 96, 111–117. https://doi.org/10.1016/j.contraception.2017.05.010 (2017).
Giakoumelou, S. et al. The role of infection in miscarriage. Hum. Reprod. Update. 22, 116–133. https://doi.org/10.1093/humupd/dmv041 (2016).
Acknowledgements
We express our deep gratitude to all participants of the National Health and Nutrition Examination Survey (NHANES) for their invaluable contributions, without which this research would not have been possible. Their willingness to share personal information and time is instrumental in advancing our understanding of population health trends.
Funding
Northern Jiangsu Clinical Medicine Research Institute’s 2024 Projects (HAKY202400225); Huai’an Basic Research Plan (Joint Special Project) Health Research Project (HABL2023064).
Author information
Authors and Affiliations
Contributions
A.Z. conceptualized the study; M.G. and Y.Z. conducted the statistical analysis; Y.Y. and F.W. wrote the main manuscript . All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The National Health and Nutrition Examination Survey (NHANES), jointly conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS), underwent comprehensive ethics review and was approved by the NCHS Research Ethics Review Board (NCHS ERB). Written informed consent was obtained from each participant. Because the present work constitutes secondary analyses of de-identified data, no additional institutional review board oversight was required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, A., Gong, M., Zhu, Y. et al. The association between frailty and recurrent pregnancy loss in reproductive-aged women: a cross-sectional study. Sci Rep 15, 33582 (2025). https://doi.org/10.1038/s41598-025-18713-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18713-4