Abstract
This study evaluated the prognostic value of glucose/potassium ratio (GPR) in sepsis using MIMIC-IV data (n = 31,717). Patients were stratified by GPR quartiles (Q1: <1.450 to Q4: ≥2.169). Adjusted Cox models demonstrated a dose-dependent increase in mortality in patients with higher GPR levels (P < 0.001), with the highest risk of mortality observed in Q4. Restricted cubic spline analysis revealed a U-shaped relationship between GPR and mortality (30-day optimal cutoff: 1.49). Sensitivity analyses excluding patients with diabetes, acute renal failure, or chronic liver disease confirmed the robustness of results, with consistent findings across subgroups. These findings indicate that GPR independently predicts both short-term (30/90/180-day) and long-term (1-year) mortality in sepsis, providing valuable references for risk stratification.
Similar content being viewed by others
Introduction
Sepsis is a potentially fatal organ dysfunction with high morbidity and mortality due to a dysregulated host response to infection1. According to the Global Burden of Disease Study 2020 published in the Lancet, there were about 48.9 million new cases of sepsis globally in 2017, with 11 million deaths, accounting for 19.7% of all global deaths2. When sepsis progresses to septic shock, its mortality rises further, placing a heavy burden on the healthcare system and society. Sepsis develops rapidly. Early recognition and intervention are key to improving its prognosis. By exploring the predictors of sepsis mortality, an effective early warning system can be constructed to reduce patient mortality.
Glucose metabolism abnormalities often occur concomitantly in critically ill patients, especially sepsis patients3. Hypoglycemia was shown to be positively associated with sepsis severity and mortality, which was more significant in non-diabetic patients4. Hypoglycemia in sepsis was independently linked to a poorer prognosis, especially in diabetic patients5. In addition, abnormal blood potassium is closely associated with prognosis in sepsis patients, with a mortality risk increasing with an increase in serum potassium concentration above 4.4 mmol/L6.. Blood glucose and potassium, as routine clinical indicators, have the advantages of easy access and low costs, and the interaction between the two indicators has led to the introduction of serum glucose/potassium ratio (GPR)7,8. This ratio has demonstrated good value for prediction of risk for a variety of emergent conditions such as traumatic brain injury (TBI)9, acute cerebral hemorrhage10, type A aortic dissection11, acute traumatic spinal cord injury12, and blunt abdominal trauma (BAT) seen in the emergency department (ED)13. However, the direct link of this ratio to prognosis in sepsis patients is still to be identified. This study was aimed at assessing the predictive value of this ratio for prognosis in sepsis patients, and to clarify its association with all-cause mortality, so as to provide a scientific rationale for clinical decision optimization and early intervention.
Methods
Data source and study population
This was a retrospective cohort study using the MIMIC-IV database developed by the MIT Laboratory for Computational Physiology (LCP) in conjunction with Beth Israel Deaconess Medical Center (BIDMC) in Boston, containing detailed clinical data for over 50,000 patients admitted to the BIDMC’s ICU and Emergency Department (ED) in 2008-201914. The database covers a wealth of clinical information such as demographic data, vital signs, laboratory test results, diagnostic information based on ICD-9 and ICD-10, treatment records, and patient outcome data. To ensure that the data were used appropriately, one of the authors of this study (Xueli Yan) passed the necessary accreditation to access the database (accreditation number: 13914865) and extracted variables relevant to the study. Because patient information in the MIMIC-IV database has been anonymized, there is no need to obtain individual patient consent.
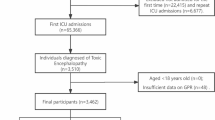
We included 31,717 sepsis patients aged ≥ 18 years initially admitted to the ICU, with a diagnosis based on Sepsis 3.0, i.e., a SOFA score of ≥ 2 with infection1. To ensure complete and reliable data to be analyzed, we set strict exclusion criteria (shown in Fig. 1): (1) patients aged < 18 years, (2) patients with reverse causality or missing data in outcome variables, and (3) patients with missing blood glucose and potassium levels, as these data were critical to the study objective.
Study variables
We collected the data below: (1) demographic data such as gender, race, and age; (2) physiologic indicators: HR, BP, RR, body temperature, and SaO2; (3) hematological and biochemical indicators: blood urea nitrogen, serum calcium, blood creatinine, lactic acid, mean corpuscular volume, platelets, blood potassium, partial thromboplastin time, erythrocyte distribution width, white blood cells (WBCs), and glucose; (4) indicators of clinical severity: APACHE II score, CCI score, OASI, systemic inflammatory response syndrome (SIRS), and Glasgow Coma Scale (GCS) score; (5) existing comorbidities: acute renal failure, acute respiratory distress syndrome, atrial fibrillation, chronic liver disease (CLD), COPD, diabetes, HF, hypertension, myocardial infarction, PVD, renal failure, and septic shock; (6) medications: dopamine, fluconazole, ampicillin sodium/sulbactam sodium, heparin sodium, human albumin, hydrocortisone, insulin, meropenem, norepinephrine, piperacillin sodium/tazobactam sodium, and vancomycin; (7) therapeutic measures: mechanical ventilation, and continuous renal replacement therapy; (8) follow-up time and outcomes: all-cause mortality from patient admission to the ICU to the follow-up endpoint. GPR was calculated by dividing the levels of glucose and potassium in mmol/L. For those frequently admitted to the ICU, only data from their initial ICU stay were included, and measurements and scores were gathered from the initial inspection within 24 h following admission. In addition, indicators with variance inflation factors (VIFs) of more than 5 were removed to circumvent the multicollinearity issue. Variables were excluded from analysis when > 10% missing values occurred; otherwise, multiple imputation (MI) was used to fill in the missing data. The specific missingness rates for each variable are shown in Fig. 2.
Outcomes
The primary outcome for this study was 30-day all-cause mortality after ICU admission, with secondary outcome measures including 90-day, 180-day, and 1-year all-cause mortality after ICU admission.
Statistical analysis
In this study, participants were divided into four groups based on their GPR values. Continuous variables were subjected to the K-S test for normality. They were expressed as Mean ± SD and subjected to intergroup comparison using the t-test when they were normally distributed; otherwise, they were expressed as the median and interquartile range (IQR) and compared among groups using a nonparametric test. The associations between GPR and different study endpoints were quantified with an HR and its 95% CI derived from the Cox proportional hazards (CPH) model. Meanwhile, data were further analyzed to ensure their robustness, and quartiles based on GPR were converted to categorical variables (Q1, Q2, Q3, and Q4). p-value was calculated for a trend test. Potential confounders were adjusted for by Model 1 (unadjusted), Model 2 (adjusted for age, gender, and race), and Model 3 (fully adjusted by incorporating all remaining covariates based on Model 2). The potential nonlinear associations of serum GPR with outcomes were demonstrated by the restricted cubic spline (RCS). Subgroup analysis and an interaction test were performed based on gender, diabetes, acute renal failure, insulin, chronic liver disease, and hydrocortisone. In addition, the robustness of data was further assessed by sensitivity analysis based on exclusion of diabetic patients, patients with acute renal failure, and CLD patients from the cohort, while these associations were explored by logistic regression. R 4.4.2 was used for all analyses, with p < 0.05 (two-tailed) indicating a statistically significant result.
Results
Baseline characteristics
Baseline demographics and clinical attributes are listed by the quartiles of GPR in Table 1. This study included 31,717 sepsis patients in total. They were assigned to four groups according to their GPR values, including Q1 (< 1.450), Q2 (1.450–1.739), Q3 (1.739–2.169), and Q4 (2.169–15.728). Of the study population, males comprised 57.7% (n = 18314), while females accounted for 42.3% (n = 13403); Caucasians comprised 65.5% (n = 20775), and Asians accounted for the smallest percentage at 1.7% (n = 548). The median age was 67 years (IQR: 56–78). As shown in Table 1, HR, RR, body temperature, and BP were significantly higher in the Q4 group vs. the Q1 group, with statistically significant differences (p < 0.001). Meanwhile, in the former group vs. the latter group, most of the disease severity scores, levels of clinical and biochemical indicators, the rate of use of medications, the frequency of intervention, and the incidence of comorbidities were higher (p < 0.001), but no significant difference was observed in serum calcium level between these two groups (p > 0.05). There was also no significant difference with respect to the incidence of COPD and renal failure (p > 0.05).
Association of GPR with mortality in sepsis patients
In Table 2, three models were constructed using multivariate Cox regression analysis to assess the association of GPR with 30DM in sepsis patients. In the fully adjusted model, the risk of 30DM would increase by 12.8% corresponding to each unit increase in GPR (HR = 1.128; 95% CI: 1.097–1.159; p < 0.001). After grouping based on the quartiles of GPR, the Q3 and Q4 groups exhibited a significantly higher mortality risk than the Q1 group (Q3: HR = 1.080; 95% CI: 1.007–1.159; p = 0.031; Q4: HR = 1.262; 95% CI: 1.173–1.358; p < 0.001). At the same time, the 30DM in sepsis patients showed a significant upward trend as GPR increased (p for trend < 0.001). In addition, similar significant associations occurred between GPR and 90DM, 180DM, and 1YM (p < 0.05).
RCS analysis
Notably, there were significant nonlinear U-shaped associations of GPR with 30DM, 90DM, 180DM, and 1YM in patients. Specifically, the lowest risk for 30DM occurred at a GPR of 1.49, and mortality risk tended to increase when GPR was either below or above this value (Fig. 3A). For 90DM (Fig. 3B), 180DM (Fig. 3C), and 1YM (Fig. 3D), the lowest risk occurred at a GPR of 1.44, and similarly, mortality risk tended to increase gradually when GPR was either below or above this value. These results suggest close associations of GPR with these outcome measures in sepsis patients, with a clear optimal threshold range.
(A) Restricted cubic spline regression analysis of GPR index with 30-day mortality; (B) Restricted cubic spline regression analysis of GPR index with 90-day mortality; (C) Restricted cubic spline regression analysis of GPR index with 180-day mortality; (D) Restricted cubic spline regression analysis of GPR index with 1-year mortality.
Survival analysis
In sepsis patients, KM Survival analysis by the quartiles of GPR showed significantly higher 30-day all-cause mortality (ACM) in the fourth quartile group (Q4 group) and Q1 group compared with other groups (p < 0.0001; Fig. 4A). This difference persisted at 90 days (Fig. 4B), 180 days (Fig. 4C), and 1 year (Fig. 4D).
(A) Kaplan-Meier curves for 30-day all-cause mortality stratified by GPR quartiles; (B) Kaplan-Meier curves for 90-day all-cause mortality stratified by GPR quartiles; (C) Kaplan-Meier curves for 180-day all-cause mortality stratified by GPR quartiles; (D) Kaplan-Meier curves for 1-year all-cause mortality stratified by GPR quartiles.
Subgroup analysis (SA)
To clarify the association of GPR with 30DM in specific subgroups, SA was performed for patients based on gender, diabetes, acute renal failure, CLD, insulin use, and hydrocortisone use (Fig. 5) and adjusted for all covariates except the stratification variable in the model. The results showed a generally positive association of GPR with 30DM in subgroups stratified by gender, diabetes, acute renal failure, and insulin use (p < 0.05), although GPR varied by Subgroup. However, GPR has no significant association with 30DM in sepsis patients with comorbid CLD or hydrocortisone use (p > 0.05). The interaction test further showed significant interactions in all the subgroups stratified by diabetes, acute renal failure, CLD, insulin use, and hydrocortisone use (p for interaction < 0.05), except the gender subgroup, which did not show a significant interaction.
Sensitivity analysis
To verify the robustness of the association of GPR with 30DM in sepsis patients, we performed sensitivity analysis (Table 3). First, after the exclusion of diabetic patients, the results showed that a higher GPR was still significantly associated with an increased mortality risk. Subsequently, after further exclusion of patients with acute renal failure or CLD, analysis consistently showed a positive association of GPR with 30DM in sepsis patients. In addition, logistic regression analysis still found a significant association of GPR with the HR for 30DM (p < 0.05), and the risk of 30DM was higher in the Q4 group vs. the Q1 group, with a significant difference in the two groups (p < 0.05). Similarly, GPR tended to be associated with the HRs for 90DM, 180DM, and 1YM (p < 0.05), further confirming the predictive value of GPR for patient prognosis.
Discussion
This study is the first to systematically reveal significant U-shaped associations between GPR and short-term and long-term mortality risk in a large cohort of sepsis patients, and confirms the clinical value of GPR as an independent prognostic marker. Based on multiple analyses, this study not only verified the generalizability of GPR in the prognostic prediction of sepsis but also deeply interpreted its heterogeneity in different clinical subgroups, thereby providing key evidence to support the clinical application of this novel biomarker.
The present RCS analysis is the first attempt to reveal a nonlinear U-shaped association of GPR with mortality risk in sepsis patients. This finding is of great value for clinical and mechanistic research. Notably, the lowest threshold was 1.49 for GPR associated with 30-day mortality risk, and 1.44 for GPR associated with 90-day to 1-year mortality, suggesting a dynamic evolution of requirements for metabolic regulation at different stages of sepsis. The biological basis of this U-shaped association may involve dual pathological mechanisms. Specifically, high GPR may result from a synergistic effect of stress hyperglycemia and hypokalemia, whereas low GPR reflects metabolic imbalance: hypoglycemia with hyperkalemia. High GPR in sepsis is attributed to a significant increase in blood glucose, which is due to increased secretion of catecholamines, cortisol, and glucagon that promote glycogenolysis and hepatic gluconeogenesis while suppressing insulin secretion15,16. It was shown that high blood glucose levels in sepsis patients were positively associated with the levels of inflammatory cytokines such as IL-6, TNF-α, and IL-1β, and negatively associated with the levels of CD4+/CD8+, suggesting that hyperglycemia may exacerbate the inflammatory response and inhibit the immune function, thereby increasing the risk of infections and multiple organ dysfunction17. In addition, the release of large amounts of inflammatory mediators in sepsis exacerbated insulin resistance by interfering with the insulin signaling pathway, resulting in decreased insulin sensitivity of peripheral tissues and decreased glucose utilization18. Hypokalemia was associated with intracellular transfer of potassium ions driven by increased secretion of stress hormones in sepsis19,20. In the presence of a low GPR, hypoglycemia results from impaired gluconeogenesis18 and increased glucose consumption in peripheral tissues21. In the course of sepsis, the occurrence of hyperkalemia involves two pathological processes: on the one hand, over-activated inflammatory response can cause cellular destruction and tissue injury, which contributes to the intracellular efflux of potassium ions, and on the other hand, sepsis-associated acute renal injury (AKI) can lead to impaired renal potassium excretion, further exacerbating the risk of hyperkalemia22,23, and hyperkalemia can induce fatal cardiac arrhythmias24. Restricted cubic spline (RCS) analysis confirmed that glucose/potassium ratio (GPR), as a composite indicator of metabolic disruption in sepsis, was significantly associated with patient prognosis, suggesting that clinicians need to closely monitor the levels of glucose and potassium in blood. The optimal GPR range (1.44–1.49) determined based on statistical association can be converted into a clinical stratification strategy. First, as an initial risk screening tool: calculate baseline GPR for ICU sepsis patients upon admission; if it exceeds the threshold (< 1.44 or > 1.49), clinicians should prioritize metabolic disorder assessment. Second, as a dynamic monitoring tool: establish a 4–6 h blood glucose/potassium monitoring process for patients with baseline GPR outside the threshold, and combine the SOFA score to track disease evolution. Third, as an individualized intervention target: when GPR remains < 1.44, focus on screening for risk of life-threatening hyperkalemia and severe hypoglycemia; when GPR remains > 1.49, strengthen blood glucose control and screen for severe hypokalemia. It should be emphasized that this threshold is not an independent diagnostic criterion but an auxiliary decision-making tool in the sepsis management process. Clinical application requires individualized analysis based on pre-existing diseases, organ function, and response to treatment.
Sepsis patients often have comorbid metabolic disorders or receive treatments that affect glucose-potassium homeostasis. These factors may influence the assessment of the association between GPR and mortality risk. Based on this, we performed subgroup analysis to reveal the heterogeneity of GPR in different clinical situations. The strength of the association of GPR with mortality risk was reduced in the diabetic subgroup compared with non-diabetic patients. This may be related to the pattern of metabolic disruption specific to type 2 diabetes (T2D), with a core pathological mechanism of islet β-cell dysfunction and insulin resistance25, which manifests as metabolic imbalance due to abnormalities of insulin synthesis and release as well as defects in the response of peripheral tissues26. In patients with sepsis complicated by diabetes, an increased GPR mostly reflects homeostasis imbalance in diabetes rather than an alteration that characterizes the acute stress response during sepsis, which provides an important insight into the clinical interpretation of this indicator.
The kidneys are a central regulator of potassium homeostasis in the body. Patients with acute kidney failure (AKF) often present with hyperkalemia, a characteristic electrolyte disturbance27, which leads to a change specific to GPR, thereby significantly affecting the predictive power of GPR in the subgroup with AKF. Despite the interference from AKI, GPR still had statistically significant predictive value for prognosis in this subgroup, highlighting its robustness in assessing metabolic disturbances. However, in the subgroup with CLD, the predictive power of GPR showed significant heterogeneity (p > 0.05), which may be partly due to limited statistical power caused by the insufficient sample size in this subgroup. Furthermore, there may be underlying reasons involved: hypoglycemia caused by decreased hepatic glycogen reserve28 and hypokalemia29 due to disturbance of aldosterone metabolism30. These pathological changes make it difficult for GPR to accurately reflect the metabolic profile of sepsis patients. Similarly, when patients are treated with hydrocortisone, the drug can cause glucose metabolism disturbance by directly impairing pancreatic endocrine function and Suppressing insulin sensitivity of peripheral tissues, leading to steroid-induced diabetes in about 2% of patients31. This metabolic disturbance stems from interference by glucocorticoids and is different from the pattern of metabolic disruption specific to type 2 diabetes. Notably, this drug-specific metabolic alteration may mask sepsis-induced stress hyperglycemia, which in turn affects the effectiveness of clinical interpretation of GPR. The above findings suggest that clinicians should develop treatment options based on GPR in conjunction with patients’ pre-existing diseases rather than GPR alone. Our findings also call for the future development of improved predictive models for specific patient populations to improve the accuracy of prognostic assessment by integrating more metabolic parameters and to provide new ideas for precise individualized treatment and stratified treatment. Meanwhile, to verify the robustness of the association of GPR with 30DM in sepsis patients, we performed sensitivity analysis and found that an increased GPR was positively associated with 30DM, 90DM, 180DM, and 1YM, regardless of whether or not sepsis patients had comorbid diabetes. Similarly, the results remained stable after the exclusion of patients with acute renal failure or CLD, indicating that GPR can be used as an independent risk factor to directly reflect the pathophysiological status of sepsis patients, and that its predictive ability is not affected by comorbidities and can remain stable over different time windows. Therefore, GPR can not only serve as a risk warning signal in the acute phase but also guide the management of long-term follow-up.
Significance and limitations
This study is the first to systematically verify the predictive power of GPR for prognosis in sepsis patients, thus revealing its unique value for clinical application. Compared with complex scoring systems such as the SOFA score, GPR has the significant advantage of eliminating the need for complex calculations by integrating changes in both blood glucose and potassium, thereby enabling clinicians rapidly and dynamically to assess the ICU patient’s condition at the bedside. However, there are still some limitations in this study. First, as it is an observational study, we cannot determine a causal relationship between GPR and all-cause mortality, and it is difficult to control confounders. Although multivariate adjustment and subgroup analysis were used in this study to address potential confounders, the lack of key information such as the site of infection, causative pathogens, treatment regimen, and cause of death in sepsis patients in the database may have led to residual confounding. However, E-value analysis indicates that unmeasured confounding would need to be extremely strong to fully explain away the association between GPR and mortality (point estimate E = 1.39, lower bound E = 1.33), providing additional robust Support for the conclusion. Nonetheless, residual confounding remains a methodological limitation inherent to observational studies. Future multicenter collaborative studies should be conducted to supplement existing evidence. Second, a single measurement of GPR may not identify metabolic fluctuation after therapeutic intervention. It is therefore required to explore the prognostic value of the rate of 24-hour dynamic change in GPR in the future. Third, this study relies on the proportional hazards assumption in the Cox proportional hazards model. Although the robustness of the results was cross-validated through RCS analysis, KM curve separation trends, and sensitivity analysis, no formal schoenfeld residual test was performed. Future studies may further explore time-varying risk patterns. Finally, the generalizability of the MIMIC-IV database may be limited by the following factors: First, differences in demographic characteristics: The 31,717 patients included in this study were primarily from the ICU, a tertiary academic medical center in the United States (Caucasians: 65.5%, Asians: 1.7%; males: 57.7%; a median age of 67 years [IQR: 56–78]), and their distribution by race and age differs significantly from that of ICU populations in Asian/African regions. Second, a difference in the causes of sepsis: Pulmonary sepsis is predominant in the United States, while abdominal sepsis is more common in Asia. This regional difference in etiology may affect the generalizability of GPR for prognostic assessment. Third, differences in clinical practice: The MIMIC-IV database reflects a standardized clinical process in the ICU in the United States, but regions with limited resources often face issues such as restricted antibiotic choices and insufficient life support equipment, which may limit the clinical application of GPR as a marker for intervention guidance. Given these limitations, although this study provides important evidence for the clinical application of GPR, its generalizability still needs to be further verified through large-scale, multicenter studies. The results should be interpreted with caution in clinical practice. Future research will focus on the following: prospective validation of the role of the GPR threshold in improving clinical outcomes; concomitant exploration of the effectiveness of dynamic monitoring data combined with multidimensional indicators in guiding treatment, so as to ultimately optimize risk stratification and precision intervention strategies for sepsis patients.
Conclusion
This study demonstrates for the first time that GPR is an independent predictor of mortality risk in sepsis patients, and its U-shaped association with mortality risk provides a quantitative basis for individualized sepsis management. Due to the easy accessibility of GPR, it can be integrated into a system for prognostic assessment of sepsis in the future to help identify high-risk patients and optimize precise treatment strategies. However, it should be emphasized that the role of GPR in improving prognosis in sepsis patients still needs to be rigorously validated in prospective, multicenter randomized controlled trials.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
27 December 2025
The original online version of this Article was revised: Figure 5 in the original version of this article was duplicated as Figure 3. The original version of the article has been corrected.
References
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315, 801–810. https://doi.org/10.1001/jama.2016.0287 (2016).
Rudd, K. E. et al. Global, regional, and National sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet 395, 200–211. https://doi.org/10.1016/s0140-6736(19)32989-7 (2020).
Tamler, R., LeRoith, D. & Roth, J. Intensive insulin therapy in the medical ICU. N Engl. J. Med. 354, 2069–2071 (2006). author reply 2069–2071.
Kushimoto, S. et al. Impact of blood glucose abnormalities on outcomes and disease severity in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. PLoS One. 15, e0229919. https://doi.org/10.1371/journal.pone.0229919 (2020).
Zohar, Y. et al. The association of diabetes and hyperglycemia with sepsis outcomes: a population-based cohort analysis. Intern. Emerg. Med. 16, 719–728. https://doi.org/10.1007/s11739-020-02507-9 (2021).
Zhao, G., Gu, Y., Chen, Y. & Xia, X. Association of serum potassium levels with mortality in critically ill patients with sepsis during hospitalization. PLoS One. 19, e0314872. https://doi.org/10.1371/journal.pone.0314872 (2024).
Lu, Y., Ma, X., Zhou, X. & Wang, Y. The association between serum glucose to potassium ratio on admission and short-term mortality in ischemic stroke patients. Sci. Rep. 12, 8233. https://doi.org/10.1038/s41598-022-12393-0 (2022).
Zhou, J., Yang, C. S., Shen, L. J., Lv, Q. W. & Xu, Q. C. Usefulness of serum glucose and potassium ratio as a predictor for 30-day death among patients with severe traumatic brain injury. Clin. Chim. Acta. 506, 166–171. https://doi.org/10.1016/j.cca.2020.03.039 (2020).
Marini, J. I. & Sein, M. E. The role of the glucose potassium ratio in the management of traumatic brain injury. Korean J. Neurotrauma. 19, 82–89. https://doi.org/10.13004/kjnt.2023.19.e11 (2023).
Wu, X. Y. et al. Serum glucose and potassium ratio as a predictive factor for prognosis of acute intracerebral hemorrhage. J. Int. Med. Res. 49, 3000605211009689. https://doi.org/10.1177/03000605211009689 (2021).
Chen, Y. et al. The blood glucose-potassium ratio at admission predicts in-hospital mortality in patients with acute type A aortic dissection. Sci. Rep. 13, 15707. https://doi.org/10.1038/s41598-023-42827-2 (2023).
Zhou, W., Liu, Y., Wang, Z., Mao, Z. & Li, M. Serum glucose/potassium ratio as a clinical risk factor for predicting the severity and prognosis of acute traumatic spinal cord injury. BMC Musculoskelet. Disord. 24, 870. https://doi.org/10.1186/s12891-023-07013-5 (2023).
Katipoğlu, B. & Demirtaş, E. Assessment of serum glucose potassium ratio as a predictor for morbidity and mortality of blunt abdominal trauma. Ulus Travma Acil Cerrahi Derg. 28, 134–139. https://doi.org/10.14744/tjtes.2020.88945 (2022).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data. 10, 1. https://doi.org/10.1038/s41597-022-01899-x (2023).
Vanhorebeek, I., Gunst, J. & Van den Berghe, G. Critical care management of Stress-Induced hyperglycemia. Curr. Diab Rep. 18, 17. https://doi.org/10.1007/s11892-018-0988-2 (2018).
Finfer, S. Clinical controversies in the management of critically ill patients with severe sepsis: resuscitation fluids and glucose control. Virulence 5, 200–205. https://doi.org/10.4161/viru.25855 (2014).
Wei, Q. et al. Correlation Analysis of Blood Glucose Level with Inflammatory Response and Immune Indicators in Patients with Sepsis. Dis Markers 8779061, (2022). https://doi.org/10.1155/2022/8779061 (2022).
Marik, P. E. & Bellomo, R. Stress hyperglycemia: an essential survival response! Crit. Care. 17, 305. https://doi.org/10.1186/cc12514 (2013).
Thier, S. O. Potassium physiology. Am. J. Med. 80, 3–7. https://doi.org/10.1016/0002-9343(86)90334-7 (1986).
Back, C. et al. RAAS and stress markers in acute ischemic stroke: preliminary findings. Acta Neurol. Scand. 131, 132–139. https://doi.org/10.1111/ane.12298 (2015).
Weis, S. et al. Metabolic adaptation establishes disease tolerance to sepsis. Cell 169, 1263–1275e1214. https://doi.org/10.1016/j.cell.2017.05.031 (2017).
Su, K., Li, X. T., Hong, F. X., Jin, M. & Xue, F. S. Lidocaine pretreatment attenuates inflammatory response and protects against sepsis-induced acute lung injury via inhibiting potassium efflux-dependent NLRP3 activation. Inflamm. Res. 72, 2221–2235. https://doi.org/10.1007/s00011-023-01810-3 (2023).
Ogawa, Y., Ezaki, S., Shimojo, N. & Kawano, S. Case report: reduced CSF orexin levels in a patient with sepsis. Front. Neurosci. 15, 739323. https://doi.org/10.3389/fnins.2021.739323 (2021).
Montford, J. R. & Linas, S. How dangerous is hyperkalemia?? J. Am. Soc. Nephrol. 28, 3155–3165. https://doi.org/10.1681/asn.2016121344 (2017).
Roden, M. & Shulman, G. I. The integrative biology of type 2 diabetes. Nature 576, 51–60. https://doi.org/10.1038/s41586-019-1797-8 (2019).
Galicia-Garcia, U. et al. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 21 https://doi.org/10.3390/ijms21176275 (2020).
McLean, A., Nath, M. & Sawhney, S. Population epidemiology of hyperkalemia: cardiac and kidney Long-term health outcomes. Am. J. Kidney Dis. 79, 527–538e521. https://doi.org/10.1053/j.ajkd.2021.07.008 (2022).
Krähenbühl, L. et al. Reduced hepatic glycogen stores in patients with liver cirrhosis. Liver Int. 23, 101–109. https://doi.org/10.1034/j.1600-0676.2003.00805.x (2003).
Dominguez, A., Muppidi, V., Leslie, S. W. & Gupta, S. In StatPearls (StatPearls Publishing, 2025).
Quiroz-Aldave, J. E. et al. From liver to hormones: the endocrine consequences of cirrhosis. World J. Gastroenterol. 30, 1073–1095. https://doi.org/10.3748/wjg.v30.i9.1073 (2024).
Beaupere, C., Liboz, A., Fève, B., Blondeau, B. & Guillemain, G. Molecular mechanisms of Glucocorticoid-Induced insulin resistance. Int. J. Mol. Sci. 22 https://doi.org/10.3390/ijms22020623 (2021).
Funding
This study was supported by National Specialist Construction Project of Advantages of Traditional Chinese Medicine of China [grant number: 2024910905]; Provincial Key Disciplines of Chinese Medicine and Ethnic Medicine [grant number: QZYYZDXK(JS)-2023-02]; Science and Technology Program of Guizhou Province [grant number: Guizhou Science and Technology Cooperation Support [2021] General 021]; Project of Department of Science and Technology of Guizhou Province; Basic Research Program of Guizhou Province [grant number: zk[2025] General 132].
Author information
Authors and Affiliations
Contributions
Xueli Yan: Conceptualization, Methodology, Software, Validation, Writing- Original draft preparation; Lan Li: Revise the paper and improve the content, Supervision, Funding acquisition; Xiahui Zhou: Data curation; Ruifeng Huang: Writing- Reviewing and Editing, Funding acquisition. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, X., Li, L., Zhou, X. et al. Associations of serum glucose/potassium ratio with short-term and long-term mortality in sepsis patients: a retrospective cohort study based on the MIMIC-IV database. Sci Rep 15, 34921 (2025). https://doi.org/10.1038/s41598-025-18798-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18798-x
Keywords
This article is cited by
-
Role of Stress Hyperglycemia Ratio and Glucose-to-Potassium Ratio in Identifying Stress-Induced Hyperglycemia in Non-Diabetic Trauma Patients
SN Comprehensive Clinical Medicine (2025)