Abstract
Despite the inherent high salinity tolerance of mature carob (Ceratonia siliqua L., Fabaceae) trees, young seedlings are particularly vulnerable to salt stress, which can hinder their growth and development. Although hydrogen sulfide (H₂S) has been widely studied as a regulator of salinity tolerance in herbaceous plants, its role in woody legumes remains largely unexplored. This study aimed to explore the potential of hydrogen sulfide (H2S) pretreatment to enhance the salinity tolerance of young carob seedlings. A pot experiment was conducted using a factorial design to evaluate the effects of sodium hydrosulfide (NaHS, an H2S donor) at 0, 75, and 200 µM under saline conditions (0, 50, and 100 mM NaCl). The results indicated that H2S pretreatment enhanced the biomass of both shoots and roots under both saline and non-saline conditions, with the potential to compensate growth loss due to salt stress. Additionally, stress markers such as hydrogen peroxide (H2O2) and malondialdehyde (MDA) increased by about 23% and 28%, respectively, in seedlings treated with 75 µM NaHS after exposure to 100 mM NaCl stress. The enhanced salinity stress tolerance was presumably associated with increased antioxidant enzyme activity, including superoxide dismutase (SOD, ≈ 24%), ascorbate peroxidase (APX, ≈ 118%), and peroxidase (POD, ≈ 50%) after 75 µM NaHS pretreatment in plants exposed to 100 mM NaCl. Moreover, NaHS pretreatment reduced sodium ion (Na⁺) accumulation in both leaves and roots. These findings indicate that H2S enhances the salinity tolerance of carob seedlings, helping them better withstand suboptimal growing conditions.
Similar content being viewed by others
Introduction
Soil salinization and salinity stress are becoming inevitable threats to agricultural production, affecting approximately 800 million hectares worldwide1,2. Salinization of agricultural land is evidently accelerating in the arid and semi-arid regions of the world not only in association with the climate change factors, but also because of inappropriate land use and management.
Salinity is a major threat to plant growth mostly by causing osmotic and oxidative stress along with ion toxicity3. Soils are considered saline when the electrical conductivity of its saturated extract reaches or exceeds 4 dS/m. Salt stress typically leads to cell membrane damage, increased production of reactive oxygen species (ROS), reduced photosynthesis, and interference with nutrient absorption4,5,6. In response to salinity, plants activate defence mechanisms that involve both enzymatic and non-enzymatic antioxidant systems to mitigate these adverse effects7,8.
Carob (Ceratonia siliqua L.) is an evergreen tree belonging to the Fabaceae plant family, native to the Mediterranean region and Southwest Asia9. Primarily cultivated for their seeds and pods (pulp), carob trees produce a gum that can be extracted from the endosperm of their seeds known as carob bean gum (CBG). Aside from the gum which is widely utilized as a thickener and stabilizer in canned foods, carob biomass also finds application as a livestock feed supplement, and its pods can be milled and used as cocoa powder substitute. Carob syrup, also obtained from carob pods, serve as a versatile sweetener and flavouring agent in various foods and beverages10,11.
Carob, known for its resilience to harsh conditions such as salinity and drought12. It holds significant ecological importance, especially in afforestation efforts targeting regions characterized by saline and poor-quality soils, where soil erosion and desertification are prevalent. Carob cultivation is an essential component of ecosystem restoration in these areas13. Despite the general resilience of carob trees to saline environment, the carob seedlings’ establishment in saline soil is insufficient14,15. For successful growth and establishment in such conditions, carob seedlings require support to survive the critical early stages. In search for priming agents to improve the seedling performance of carob plants, hydrogen sulfide (H₂S) seemed to be of potential aid.
Hydrogen sulfide acts as a signalling molecule that regulates plant growth and responses to abiotic stresses, including heavy metal toxicity, cold, drought, and salinity16,17,18. It alleviates the negative impacts of salinity through mechanisms such as enhancing the potassium-to-sodium ratio, protecting the photosynthetic machinery, boosting the antioxidant system, and stimulating osmotic regulators and polyamines19. While much of the research on H₂S has focused on herbaceous plants, its effects on woody plants like carob deserve further exploration. Hence, the current research aimed to explore the impacts of H₂S pretreatment and salinity stress on the growth and physiological responses of carob seedlings with a focus on antioxidant systems.
Materials and methods
Plant materials and treatments
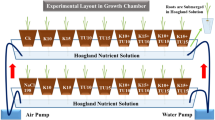
The current study was carried out in a greenhouse at Shiraz University’s College of Agriculture (52°32′ E, 29°36′ N; 1810 m above sea level). Seeds were collected from carobs’ natural habitats in Fars Province in Iran. The systematic identification was carried out and a herbarium specimen is available at School of Agriculture, Shiraz University. Seeds were chemically scarified using 96% sulfuric acid for 20 min to break dormancy, before being thoroughly rinsed with distilled water. The seeds were planted in plastic bags (15 × 30 cm) filled with a peat-perlite mixture (1:1 v/v ratio). The seedlings were grown under greenhouse conditions with a day/night temperature of 25 ± 3 °C/18 ± 3 °C and a relative humidity of 50–60%.
Each plastic bag contained a single seed and irrigated with tap water for the first two weeks following plantation. Subsequently, a sequence of diluted nutrient solutions was applied: 1/7 strength for two weeks, 1/6 for one week, 1/4 for one week, 1/3 for one week, 1/2 for one week, and finally, a complete nutrient solution for the remainder of the experiment. The composition and concentrations of elements in the nutrient solution adhered to the specifications provided in Supplementary Table S1. The pH of the nutrient solution was kept at 6.5 ± 0.2 and each pot received 100 mL of fertigation solution every two days. To prevent salt accumulation and subsequent increases in substrate electrical conductivity (EC), tap water was applied after every third nutrient solution application, with excess water (20% of the irrigation volume) allowed to drain. The experiment was designed as a factorial study within a completely randomized setup, with two factors: salinity stress induced by sodium chloride (NaCl) at three concentrations (0, 50, and 100 mM) and sodium hydrosulfide (NaHS) as an H₂S donor at three levels (0, 75, and 200 µM). Treatments were repeated four times, with four plants per replicate. NaHS treatments were introduced (nutrient solutions supplemented with different concentrations of NaHs) into the culture medium after seed sowing and applied every five days until just before the onset of salinity stress. Salinity stress was introduced four months after seed sowing by gradually increasing NaCl levels in the pots to reach the desired concentrations. To prevent osmotic shock, salinity was incrementally raised: on the first day of treatment, plants received a 10 mM NaCl solution, followed by 25 mM on the second day, and progressively increased to 40 mM, 50 mM, 75 mM, and finally 100 mM. The salinity treatments were continued for 45 days when various parameters were assessed to evaluate the effects of the different treatments. The experimental design and the applied treatment times are illustrated in Supplementary Fig. S1.
Growth rate
Growth rate was assessed by determining the length of seedling at both the initiation and end of the 45-days salinity treatment, and the results were reported in centimetres per month (cm month− 1).
Shoot and root dry weight
At the end of the growth experiment, four plants from each treatment (one plant from each replicate) were randomly selected and removed from the bags for analysis. Following a thorough wash with distilled water, the plants were divided into two parts: root and shoot. Subsequently, the samples were oven-dried for 48 h at 80 °C to obtain their constant dry weights (DWs, g) using a digital scale.
The activity of enzymatic antioxidants
A week before the end of the experiment, four plants from each treatment group were randomly chosen (one plant from each replicate) and in total four fully expanded leaves were collected to measure enzymatic antioxidant activity. The fresh leaf samples were immediately frozen in liquid nitrogen and stored at -80 °C. To extract enzymes, 0.5 g of leaf material was ground into a fine powder using liquid nitrogen with a mortar and pestle. This powder was then mixed with 2 ml of extraction buffer, which consisted of 50 mM potassium phosphate buffer at pH 8.0, 10% (w/v) polyvinylpyrrolidone (PVP), 0.1 mM EDTA, and 1 mM dithiothreitol (DTT). The homogenate was centrifuged at 12,000 × g for 30 min at 4 °C, and the supernatant was then carefully collected for subsequent analysis.
Superoxide dismutase (SOD) activity was measured using the method described by Dhindsa et al.20. To begin, 0.1 mL of the enzyme extract was added to a test tube containing a reaction mixture composed of 13 mM L-methionine, 25 mM nitroblue tetrazolium chloride (NBT), 0.1 mM EDTA, 50 mM sodium carbonate, and 2 mM riboflavin in 50 mM phosphate buffer at pH 7.8. The mixture was then illuminated with two 15 W fluorescent lamps for 15 min. A control sample, which included the entire reaction mixture without the enzyme and produced maximum colour, was also prepared. The reaction was halted by turning off the lights and placing the tubes in darkness, with a complete but non-irradiated reaction mixture used as a blank. Absorbance was recorded at 560 nm, with one unit of SOD activity defined as the amount of enzyme needed to reduce the absorbance by 50% relative to the control without the enzyme. The results were expressed as units of enzyme activity per mg of protein per minute (U mg− 1 protein). Proteins were quantified using Bradford method21.
Guaiacol peroxidase (POD) activity was measured by mixing 50 µL of the crude enzyme extract with 2 mL of a reaction solution containing 50 mM potassium phosphate buffer (pH 7.0), 13 mM guaiacol, and 5 mM hydrogen peroxide (H2O2). The oxidation of guaiacol to tetraguaiacol, which results in an increase in absorbance, was tracked at 470 nm over the course of one minute. The extinction coefficient for guaiacol oxidation was 26.6/(mM×cm). Peroxidase activity was expressed as the amount of guaiacol oxidized, in millimoles per minute per milligram of protein (U mg− 1 protein), according to the method of Chance and Maehly22.
Ascorbate peroxidase (APX) activity was determined spectrophotometrically following the method of Nakano and Asada23. The method involved tracking the decrease in absorbance at 290 nm, which corresponds to the oxidation of ascorbate. The rate of ascorbate oxidation was measured from 1 to 60 s after initiating the reaction by adding H₂O₂. The reaction mixture contained 1 milliliter of solution with 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.15 mM H₂O₂, 0.1 mM EDTA, and 50 µL of enzyme extract. APX activity was reported as micromoles (µmol) of ascorbate oxidized per minute per milligram of protein (µmol ASC min− 1 mg− 1 protein).
Hydrogen peroxide (H2O2) and malondialdehyde (MDA)
Quantification of hydrogen peroxide (H₂O₂) was carried out using the method described by Junglee et al.24. This involved measuring the concentration of H2O2 by monitoring the oxidation of potassium iodide at 350 nm. The H2O2 levels were expressed in micromoles per gram of fresh weight (µmol g− 1 FW).
To assess membrane lipid peroxidation, the concentration of malondialdehyde (MDA), a marker of unsaturated fatty acid peroxidation, was measured following the procedure outlined by Ali et al.25. First, 0.2 g of freeze-dried leaf tissue was homogenized in 5 ml of 0.1% trichloroacetic acid (TCA) and then centrifuged at 10,000 × g for 5 min. One milliliter of the supernatant was mixed with 2.5 ml of 0.5% thiobarbituric acid (TBA) in 20% TCA and incubated at 95 °C for 30 min. The reaction was then rapidly stopped by cooling the mixture on ice, followed by centrifugation at 10,000 × g for 30 min. Absorbance readings were taken at 532 nm and 600 nm, with the MDA concentration calculated by subtracting the non-specific absorbance at 600 nm from the absorbance at 532 nm, using an extinction coefficient of 155/(mM×cm) and the results are expressed as µmol g− 1 FW.
Leaf and root Na and K concentration
One gram of dried samples was homogenized and then ashed at 550 °C in a porcelain crucible for 6 h. The obtained white ash was treated with 2 M hot hydrochloric acid (HCl), filtered, and carefully diluted to a final volume of 50 mL with distilled water. This extract was then used to determine the sodium and potassium contents using a flame photometer, following the method described by Kalra26.
Data analysis
All statistical analyses were performed using R version 4.3.3 (R Core Team, 2023). The normality of each measured parameter was assessed using the Shapiro-Wilk test (p > 0.05). The measured parameters which were found to be non-normally distributed were log-transformed to achieve normality prior to analysis. A two-way analysis of variance (ANOVA) was conducted to evaluate the effects of NaCl (0, 50, 100 mM), NaHS (0, 75, 200 µM), and their interaction on all measured parameters. Given the significant interaction effects (p < 0.05) for all traits, slicing was performed using Tukey’s Honestly Significant Difference (HSD) test. Comparisons were made as follows: (1) NaHS levels within each NaCl level, with significant differences denoted by lowercase letters (a, b, c), where ‘a’ represents the highest mean; (2) NaCl levels within each NaHS level, with significant differences denoted by uppercase letters (A, B, C), where ‘A’ represents the highest mean. Additionally, main effects of NaCl and NaHS were compared using Tukey’s HSD test, with lowercase letters (a, b, c), assigning ‘a’ to the highest means. Statistical analyses were conducted using the R packages `readxl`27, `emmeans`28, `multcomp`29 and, `agricolae`30. Results were saved in text files for each trait and summarized in a CSV file for ANOVA tables. Principal component analysis (PCA) was applied to all measured physiological and biochemical traits. All variables were standardized by z-transformation prior to PCA, and sampling adequacy was verified by the Kaiser–Meyer–Olkin (KMO) measure and Bartlett’s test of sphericity. Components were retained according to the Kaiser criterion (eigenvalues > 1), and variables were checked for low communality (< 0.5) to be excluded. The biplot was generated in R useing ggplot231.
Results
Growth parameters
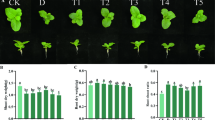
The interaction between NaHS and salinity significantly influenced the height of carob seedlings (Supplementary Table S2). Under the 100 mM salinity treatment, the plant height decreased to the lowest value compared with other treatments (2 cm/month growth rate), and this treatment resulted in significantly lower values than the control and 50 mM treatments (Fig. 1). Notably, the NaHS pretreatment partially alleviated this decline in seedling height. The NaHS treatments of both concentrations (75 µM and 200 µM) significantly increased plant height at all salinity levels. Specifically, at 100 mM salinity level, in seedlings treated with 75 and 200 µM NaHS, the plant height was higher compared to untreated seedlings by ≈ 30% and ≈ 27%, respectively. The homogeneity group with highest growth (height) was observed at 0 and 50 mM salinity levels, along with NaHS pretreatments at concentrations of 75 and 200 µM (Fig. 1).
Interaction effect of NaHS and salt stress on plant height of carob seedlings treated with different levels of NaCl (0, 50, 100 mM) and NaHS (0, 75, 200 µM). Bars represent means ± standard deviations (n = 4). Lowercase letters (a–c) indicate significant differences among NaHS levels within each NaCl level, and uppercase letters (A–C) indicate significant differences among NaCl levels within each NaHS level, based on Tukey’s HSD test (p < 0.05).
Shoot and root dry weight
The interaction effects of NaHS and salinity had a significant effect on the shoot and root dry weight of carob seedlings (P ≤ 0.05) (Supplementary Table S2). There was no significant difference between root and shoot DWs in the control and the 50 mM salt treatment, except for the 75 µM NaHS. Under 100 mM salinity stress treatment, the shoot DW (≈ 0.72 g) and root DW (≈ 0.53 g) reached their significantly lowest levels among the treatments, resulting in a decrease of about 35% and 22%, respectively, compared to the control (Fig. 2, A and B). Nevertheless, both NaHS pretreatments at this salinity level, significantly improved the biomass. Specifically, the application of 75 µM NaHS enhanced the shoot and root DW by about 48% and 20%, respectively, compared to the 100 mM salt-treated plants without NaHS. The highest shoot DW (≈ 1.6 g) and root DW (≈ 0.9 g) were achieved in response to the 75 µM NaHS treatment (without salt), and these values were significantly different compared to control at both 0 and 50 mM NaCl (Fig. 2, A and B).
Interaction effect of NaHS and salt stress on (A) shoot and (B) root dry weight of carob seedlings treated with different levels of NaCl (0, 50, 100 mM) and NaHS (0, 75, 200 µM). Bars represent means ± standard deviations (n = 4). Lowercase letters (a–c) indicate significant differences among NaHS levels within each NaCl level, and uppercase letters (A–C) indicate significant differences among NaCl levels within each NaHS level, based on Tukey’s HSD test (p < 0.05).
Malondialdehyde and hydrogen peroxide
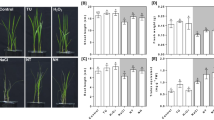
NaHS, salinity, and their interaction significantly influenced MDA and H2O2 contents in carob leaves (Supplementary Table S3). Under 100 mM salinity, H2O2 (≈ 70%) and MDA (≈ 90%) contents increased compared to the control (Fig. 3, A and B). Seedling pretreatment with NaHS (75 µM) before the 100 mM salt treatment reduced H2O2 (≈ 23%) and MDA (≈ 25%) compared to plants exposed to 100 mM salt stress alone. The 50 mM salt stress did not significantly affect the H2O2 and MDA levels in comparison to control plants (Fig. 3, A and B).
Interaction effect of NaHS and salt stress on (A) malondialdehyde (MDA) and (B) hydrogen peroxide (H2O2) content of carob seedling’s leaves treated with different levels of NaCl (0, 50, 100 mM) and NaHS (0, 75, 200 µM). Bars represent means ± standard deviations (n = 4). Lowercase letters (a–c) indicate significant differences among NaHS levels within each NaCl level, and uppercase letters (A–C) indicate significant differences among NaCl levels within each NaHS level, based on Tukey’s HSD test (p < 0.05).
Antioxidant enzymes
The interaction between salinity and NaHS pretreatment significantly influenced the activity of leaf enzymatic antioxidants (Supplementary Table S4). Under 100 mM NaCl stress, there was an increase in APX (2.63-fold), POD (≈ 217%), and SOD (≈ 265%) activities in the leaves of non-treated seedlings compared to the control (Fig. 4, A-C). However, under 100 mM NaCl stress seedlings pre-treated with both 75 and 200 µM NaHS exhibited significantly higher activity of enzymatic antioxidants than non-treated plants. Remarkably, APX, POD, and SOD demonstrated the highest activity levels in response to the 75 µM NaHS plus 100 mM NaCl treatment.
Interaction effect of NaHS and salt stress on the activity of (A) ascorbate peroxidase (APX), (B) peroxidase (POD) and (C) superoxide dismutase (SOD) in the leaves of carob seedlings treated with different levels of NaCl (0, 50, 100 mM) and NaHS (0, 75, 200 µM). Bars represent means ± standard deviations (n = 4). Lowercase letters (a–c) indicate significant differences among NaHS levels within each NaCl level, and uppercase letters (A–C) indicate significant differences among NaCl levels within each NaHS level, based on Tukey’s HSD test (p < 0.05).
Potassium content
Applied salinity stress significantly influenced K+ concentrations in roots (P ≤ 0.001) and leaves (P ≤ 0.05), whereas NaHS and its interaction with salinity was not significant (Supplementary Table S5). Exposure of seedlings to 50 and 100 mM salinity resulted in a decrease in root K+ content (≈ 23% and ≈ 75%, respectively) compared to the control, while leaf K+ content increased with rising salt concentrations (see Fig. 5, A and B). Specifically, exposure to 50 and 100 mM salinity caused leaf K+ content to rise by about 13% and 20%, respectively, compared to the control (Fig. 5, A and B).
Sodium content
Leaf and root Na+ content exhibited significant differences in carob seedlings treated with NaHS in combination with salinity (P ≤ 0.001) (Supplementary Table S5). The highest Na+ content in both leaves (≈ 8 mg g− 1 dry weight) and roots (14.5 mg g− 1 dry weight) were found in plants treated with 100 mM NaCl, that were statistically different than the controls (Fig. 6, A and B). Seedlings pretreatment resulted in a significant decrease in root and leaf Na+ contents of plants under 100 mM NaCl stress (Fig. 6, A and B).
Interaction effect of NaHS and salt stress on the concentration of Na+ in the leaves of carob seedlings treated with different levels of NaCl (0, 50, 100 mM) and NaHS (0, 75, 200 µM). Bars represent means ± standard deviations (n = 4). Lowercase letters (a–c) indicate significant differences among NaHS levels within each NaCl level, and uppercase letters (A–C) indicate significant differences among NaCl levels within each NaHS level, based on Tukey’s HSD test (p < 0.05).
Principal component analysis (PCA)
To investigate the combined effects of NaHS and NaCl treatments on the physiological and biochemical parameters of carob seedlings, a principal component analysis (PCA) was conducted (Fig. 7). Prior we tested the sampling adequacy by the overall Kaiser–Meyer–Olkin measure, which was larger than 0.5 (0.841), while the Batlett’s test was significant (p = 0.000). Communalities of all variables were larger than 0.5 so no variable was excluded. Considering the Kaiser test two principal components (PCs) were retained (for eigenvalues > 1). PC1 accounted for 75.1% while PC2 for 12.9% of the total variation. The PCA biplot revealed a clear separation among treatments. The effect of salt treatments was the most pronounced alongside PC1. NaHS applications at higher salt concentrations tended to be closer to lower-salt treatments, than non-treated higher salt concentrations. PC1 was primarily associated with the biochemical parameters, while growth traits and leaf K also contributed to PC2. Growth traits and root K showed negative loadings toward higher salt concentrations, whereas the remaining traits loaded positively.
PCA showing the effects of NaHS (µM) and NaCl (mM) on the measured physiological and biochemical traits: Shoot dry weight (DW), root dry weight and growth rate (seedling height), superoxide dismutase (SOD), guaiacol peroxidase (POD), and ascorbate peroxidase (APX) activities, and hydrogen peroxide (H2O2), malondialdehyde (MDA), leaf Na, root Na, leaf K and root K contents. PC1 and PC2 represent the proportion of variance explained by each axis. Arrows represent variable loadings, indicating the contribution of each parameter to the principal components.
Discussion
The impact of excessive soil salinity on growth suppression is a well-documented phenomenon, consistently reported in numerous studies14,32,33,34. The findings of the current study were in line with previous research, and as it was expected, high concentration (100 mM) salinity treatment hampers carob seedling growth, as manifested by the diminished biomass and the reduced growth of seedlings (Figs. 1 and 2). Multiple factors contribute to growth limitations under salt stress, including lower water uptake due to the altered water potential of the soil and plants, ionic interactions, and ion accumulation-induced toxicity17,32. The elevated Na+ concentration in the soil solution often leads to higher Na/K ratios, adversely affecting metabolism and consequently reducing plant growth35. Membrane permeability damage, indicated by increased MDA production in the present study (Fig. 3), is another factor contributing to the growth decline, aligning with previous research that highlights the suppressive effects of salinity on carob growth14.
We found that pre-treating plants with NaHS, significantly mitigated the loss in seedling biomass under salinity stress. The observed increase in biomass attributed to NaHS application, may be linked to its ability to reduce ROS production and help maintain redox balance36. NaHS pretreatment stimulated the accumulation of root and shoot biomass even under non-stress conditions, suggesting a potential role of H2S in biomass production in woody plants. Carter et al.37. observed that an H2S donor (GYY-4137) improved the biomass accumulation of lettuce and radish. Application of H2S at concentrations of 30–100 mg increased the weight of lettuce, sugar beet, and cotton by 47%, 69%, and 177%, respectively, indicating the beneficial role of H2S in enhancing plant biomass across various species37.
Based on plant biomass, our study reveals that carob seedlings demonstrated tolerance to 50 mM salinity after 45 days of exposure to salt stress, aligning with existing literature identifying carob as a salinity-tolerant plant species12. However, the decline in plant biomass under 100 mM salinity indicates the susceptibility of carob plants to higher salinity levels. Although the post-hoc test gave similar results, there was a more pronounced reduction in shoot weight compared to roots after 45 days of exposure to 100 mM salt stress, underscoring the shoots’ higher susceptibility to salinity. Leaf senescence and burn were observed in seedlings under 100 mM salinity after 45 days, while the 50 mM treatment and control showed no observable symptoms. A reduction in leaf number, including shedding, is a recognized adaptive response of carob plants to environmental stress, such as drought38,39, salinity14,37, nutritional deficiency40, and other abiotic stresses41.
The impact of salinity on plant responses varies at different stages of growth. Generally, it is believed that younger plants exhibit higher susceptibility, and prolonged exposure to salinity tends to result in more significant suppressions of vegetative growth42. Pretreatment with H2S reduced salinity-induced damage by lowering ROS content and increasing the activity of the enzymatic antioxidants (Fig. 3). ROS production can be indicated by H2O2 levels under stress conditions43, while MDA reflects membrane damage resulting from lipid peroxidation44. Previous studies have proposed that salinity-induced leaf tissue damage is primarily attributed to excessive ROS and MDA production38,45. Under normal conditions, ROS production is limited; however, their concentration significantly increases under salt stress, leading to oxidative stress, extensive damage to cellular membranes, chlorophyll destruction, inefficient photosynthesis, and cell death46.
In carob seedlings with elevated H2O2 levels, membrane peroxidation and an increase in MDA were observed. As there was no significant difference in the activity of leaf antioxidant enzymes (SOD, POD, APX) at the lowest salinity concentration (50 mM) when compared to the control, it can be stated that enzymatic antioxidants in carob seedlings are not being activated by low-level salinity stress (Fig. 4).
The maintenance of low levels of ROS in plants is achieved through the scavenging action of enzymes, with an upregulation of antioxidant enzymes activities in response to elevated ROS production under stress conditions47. In our study, salinity (100 mM) plus NaHS treatment significantly increased the activity of enzymatic antioxidants (SOD, POD, APX) compared to the high salinity (100 mM) treated control. This suggests that NaHS pretreatment enhances the seedlings’ ability to reduce H2O2 content. Similar findings have been reported in Cyclocarya paliurus (Batalin) Iljinsk plants48. The low values of MDA content resulting from NaHS pretreatment led to reduced lipid peroxidation, mitigating the adverse effects of salinity on membrane permeability. This reduction in lipid peroxidation, facilitated by H2S, has also been reported to be effective in strawberries49. In addition, previous studies have demonstrated that H2S facilitates the scavenging of free radicals by enzymes through post-translational modifications such as persulfidation50. Notably, no significant difference was observed in the activity of antioxidant enzymes between pretreated seedlings exposed to 0 and 50 mM salinity, suggesting that H2S may influence these enzymes more efficiently under more severe salt stress, i.e., 100 mM stress.
For most plants to survive under salinity stress, it is crucial to maintain low Na levels51. In the current study, Na levels were significantly higher in the leaves and roots of carob seedlings treated with 100 mM salinity compared to the control group (Fig. 6). This suggests a weakened efficiency in restraining Na+ entry into plant tissues within this concentration range. Under salt stress, ions typically accumulate in vacuoles to reduce water potential in cells52. The balance of Na+ and K+ in the cytoplasm can be disrupted under salt stress, and maintaining a low Na+/K+ ratio is a key feature of salinity tolerance53. The potential role of H2S in the regulation of K+ sequestration/uptake; ascorbate and glutathione redox homeostasis; and the cellular signaling (SOS) pathway were experimentally reported earlier54. As salinity stress intensified in our study, Na+ levels increased in carob leaves and roots. In contrast, there was a decrease in root K+ content (Fig. 5). This might be attributed to the influx of large amounts of Na+ during salt stress, interfering with root K+ uptake.
The NaHS pretreatment significantly reduced sodium content in the leaves and roots, although it had no effect on potassium (Figs. 5 and 6). This suggests that suppressing Na+ absorption, coupled with limiting its transfer to the shoots, are potential mechanisms by which H2S mitigates the negative effects of salinity on carob seedlings. Similar reductions in Na content were observed in wheat plants treated with H2S, indicating that H2S limited Na uptake55. In other studies, H2S has been shown to regulate Na quantity and enhance K entry by controlling H+ pump activity in root cells56. According to our findings H2S did not impact K accumulation but effectively suppressed Na uptake by the roots, hindering the transport of Na to the leaves and thereby reducing Na+ toxicity in carob leaves. Additionally, Wei et al.57 showed that H2S maintains Na+ and K+ balance in mangrove plants by influencing gene expression under salinity stress.
Potassium plays a crucial role in enhancing plant tolerance to stress conditions by regulating the osmotic balance within the plant cells and tissues, activating enzymes, and facilitating cell enlargement58. The toxicity of Na+ is significantly correlated with a plant’s ability to absorb, maintain, and distribute the ion59. In our study, when carob seedlings were exposed to 50 mM or 100 mM salt stress conditions, the K+ concentration increased in the leaves. This increase in leaf K+ content may be attributed to a decrease in osmotic potential, enhancing the plant’s ability to uptake water. The observed decrease in root K+ content under salt stress conditions is likely the result of the interaction between K+ and Na+, as they compete for uptake by the roots60. However, this decrease in root K content did not affect leaf K content, suggesting that the entry and transfer of K in the xylem were not significantly affected. Consistent with our findings, previous studies have reported the maintenance or increase in K+ levels in the leaves of barley58, poplar61, citrus32, and carob14. Therefore, it can be suggested that plants enhance their adaptation to salt stress by increasing leaf potassium content.
These findings were straightened by the PCA which highlighted that salt stress was the dominant factor shaping the response of carob seedlings (Fig. 7). The clustering of NaHS-treated seedlings at higher salt levels closer to those from lower-salt treatments indicates that hydrogen sulfide mitigated the negative effects of salinity, effectively buffering seedlings against stress. The overall positive loading of the biochemical parameters (except root K) supports the view that NaHS enhances protective biochemical adjustments, thereby helping seedlings maintain functional homeostasis under salt stress.
Conclusion
The augmentation of leaf K+ content facilitated the adaptation of carob seedlings to mild salt stress (50 mΜ), thereby alleviating the adverse effects of salinity. Even without NaHS treatment the seedlings tolerated mild salinity stress (50 mM NaCl treatment). However, under severe salt stress (100 mΜ), despite the increased K+ content and antioxidant enzyme activity, the deleterious impacts of salinity were evident in the carob seedlings. Notably, H2S played a pivotal role in maintaining cell membrane integrity by reducing Na+ concentration. Furthermore, H2S mitigated ROS production by activating antioxidant enzymes (SOD, APX, and POD). At its optimal concentration (75 µM), NaHS emerged as an effective tool to mitigate the adverse effect of salinity stress in carob seedlings, especially under high salinity concentration (100 mM NaCl treatment).
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Liu, C., Jiang, X. & Yuan, Z. Plant responses and adaptations to salt stress: a review Horticulturae 10 (11), 1221. https://doi.org/10.3390/horticulturae10111221 (2024).
RECROP COST. Integrative approaches to enhance reproductive resilience of crops for climate-proof agriculture. Plant. Stress. 15, 100704. https://doi.org/10.1016/j.stress.2024.100704 (2025).
Waheed, A. et al. Integrative mechanisms of plant salt tolerance: biological pathways, phytohormonal regulation, and technological innovations. Plant. Stress. 14, 100652. https://doi.org/10.1016/j.stress.2024.100652 (2024).
Gucci, R., Lombardini, L. & Tattini, M. Analysis of leaf water relations in leaves of two Olive (Olea europaea) cultivars differing in tolerance to salinity. Tree Physiol. 17, 13–21. https://doi.org/10.1093/treephys/17.1.13 (1997).
Ebert, G. Salinity problems in (Sub-) tropical fruit production. Acta Hortic. 531, 99–105. https://doi.org/10.17660/ActaHortic.2000.531.14 (2000).
Hasegawa, P. M., Bressan, R. A., Zhu, J. K. & Bohnert, H. J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant. Biol. 51, 463–499. https://doi.org/10.1146/annurev.arplant.51.1.463 (2000).
Arbona, V., Flors, V., Jacas, J., García-Agustín, P. & Gómez-Cadenas, A. Enzymatic and non-enzymatic antioxidant responses of Carrizo citrange, a salt-sensitive citrus rootstock, to different levels of salinity. Plant Cell. Physiol. 44, 388–394. https://doi.org/10.1093/pcp/pcg059 (2003).
Munns, R. Genes and salt tolerance: bringing them together. New. Phytol. 167, 645–663. https://doi.org/10.1111/j.1469-8137.2005.01487.x (2005).
Race, D., Curtis, A. & Booth, W. Carob agroforestry industry: an assessment of its potential for the low-medium rainfall Murray Valley region. Aust. J. Exp. Agric. 39, 325–334. https://doi.org/10.1071/EA97164 (1999).
Clodoveo, M. L., Crupi, P., Muraglia, M. & Corbo, F. Processing of Carob kernels to syrup by ultrasound-assisted extraction. Processes 10, 983. https://doi.org/10.3390/pr10050983 (2022).
Zannini, M. et al. Identification, bioaccessibility, and antioxidant properties of phenolic compounds in Carob syrup. Foods 13, 2196. https://doi.org/10.3390/foods13142196 (2024).
Batlle, I. & Tous, J. Promoting the Conservation and Use of Negelected and Underutilized Crops (eds. Heller, J. et al.) 7–69 (IPK and IPGRI, 1997).
Eshghi, S., Rostami, A. A. & Jamali, B. Carob tree: A suitable species for the future. Acta Hortic. 1190, 67–70. https://doi.org/10.17660/ActaHortic.2018.1190.11 (2018).
Correia, P. J., Gama, F., Pestana, M. & Martins-Loução, M. A. Tolerance of young (Ceratonia siliqua L.) Carob rootstock to NaCl. Agric. Water Manag. 97 (6), 910–916. https://doi.org/10.1016/j.agwat.2010.01.022 (2010).
El Kahkahi, R. et al. Morphological and physiological analysis of salinity stress response of Carob (Ceratonia siliqua L.) in Morocco. Sci. Int. 3, 73–81. https://doi.org/10.17311/sciintl.2015.73.81 (2015).
Aroca, A., Gotor, C. & Romero, L. C. Hydrogen sulfide signaling in plants: emerging roles of protein persulfidation. Front. Plant. Sci. 9, 1369. https://doi.org/10.3389/fpls.2018.01369 (2018).
Srivastava, V., Chowdhary, A. A., Verma, P. K., Mehrotra, S. & Mishra, S. Hydrogen sulfide-mediated mitigation and its integrated signaling crosstalk during salinity stress. Physiol. Plant. 174 (1), e13633. https://doi.org/10.1111/ppl.13633 (2022).
Yu, Y. et al. Hydrogen sulfide enhances cadmium tolerance in oilseed rape roots by augmenting glutathione-mediated antioxidant defense and ROS homeostasis. Ecotoxicol. Environ. Saf. 292, 118004 (2025).
Goyal, V., Jhanghel, D. & Mehrotra, S. Emerging warriors against salinity in plants: nitric oxide and hydrogen sulphide. Physiol. Plant. 171 (4), 896–908 (2021).
Dhindsa, R. S., Dhindsa, P. P. & Thorpe, T. A. Leaf senescence correlated with increased levels of membrane permeability and lipid-peroxidation and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 32, 93101. https://doi.org/10.1093/jxb/32.1.93 (1980).
Bradford, M. M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye biding. Anal. Biochem. 72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3 (1976).
Chance, B. & Maehly, A. C. Assay of catalase and peroxidase. Methods Enzymol. 2, 764–775. https://doi.org/10.1016/S0076-6879(55)02300-8 (1955).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 22, 867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232 (1981).
Junglee, S., Urban, L., Sallanon, H. & Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 5, 730–736. https://doi.org/10.4236/ajac.2014.511081 (2014).
Ali, M. B., Hahn, E. J. & Paek, K. Y. Effects of light intensities on antioxidant enzymes and malondialdehyde content during short-term acclimatization on micro propagated Phalaenopsis plantlet. Environ. Exp. Bot. 54, 109–120. https://doi.org/10.1016/j.envexpbot.2004.06.005 (2005).
Kalra, Y. P. Handbook of Reference Methods for Plant Analysis (CRC Press, 1998).
Wickham, H. & Bryan, J. readxl: Read Excel Files. R Package Version 1.4.3. https://CRAN.R-project.org/package=readxl (2023).
Lenth, R. V. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.8.9. https://CRAN.R-project.org/package=emmeans (2023).
Hothorn, T., Bretz, F. & Westfall, P. Simultaneous inference in general parametric models. *Biometrical Journal*. 50 (3), 346–363. https://doi.org/10.1002/bimj.200810425 (2008).
de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-7. https://CRAN.R-project.org/package=agricolae (2023).
Wickham, H. Programming with ggplot2. In Ggplot2: Elegant Graphics for Data Analysis 241–253 (Springer, 2016).
Ruiz, D., Martínez, V. & Cerdá, A. Citrus response to salinity: growth and nutrient uptake. Tree Physiol. 17, 141–150. https://doi.org/10.1093/treephys/17.3.141 (1997).
Kozlowski, T. T. Responses of Woody plants to flooding and salinity. Tree Physiol. 17, 490. https://doi.org/10.1093/treephys/17.7.490 (1997).
Sadat-Hosseini, M., Naeimi, A., Boroomand, N., Aalifar, M. & Farajpour, M. Alleviating the adverse effects of salinity on roselle plants by green synthesized nanoparticles. Sci. Rep. 12, 1–12. https://doi.org/10.1038/s41598-022-22903-9 (2022).
Grattan, S. R. & Grieve, C. M. Salinity–mineral nutrient relations in horticultural crops. Sci. Hortic. 78, 127–157. https://doi.org/10.1016/S0304-4238(98)00192-7 (1998).
Chen, Y. et al. Exogenous melatonin confers enhanced salinity tolerance in rice by blocking the ROS burst and improving Na+/K+ homeostasis. Environ. Exp. Bot. 189, 104530 (2021).
Carter, J. M. et al. Improved growth of pea, lettuce, and radish plants using the slow release of hydrogen sulfide from GYY-4137. PLoS One. 13 (12), e0208732. https://doi.org/10.1371/journal.pone.0208732 (2018).
Correia, P. J. & Martins-Loução, M. A. The use of macronutrients and water in marginal mediterranean areas: the case of carob-tree. Field Crops Res. 91, 1–6. https://doi.org/10.1016/j.fcr.2004.05.004 (2005).
Boutasknit, A., Baslam, M. & Ait-El-Mokhtar, M. Assemblage of Indigenous arbuscular mycorrhizal fungi and green waste compost enhance drought stress tolerance in Carob (Ceratonia siliqua L.) trees. Sci. Rep. 11, 22835. https://doi.org/10.1038/s41598-021-02018-3 (2021).
Correia, P. J., Pestana, M. & Martins-Loução, M. A. Nutrient deficiencies in Carob (Ceratonia siliqua L.) grown in solution culture. J. Hortic. Sci. 78, 847–852. https://doi.org/10.1080/14620316.2003.11511708 (2003).
Lips, S. H., Cruz, C. & Martins-Loucao, M. A. Growth and nutrition of Carob plants as affected by nitrogen sources. J. Plant. Nutr. 16, 1–15. https://doi.org/10.1080/01904169309364511 (1993).
Steppuhn, H., Van Genuchten, M. T. & Grieve, C. M. Root-zone salinity: II. Indices for tolerance in agricultural crops. Crop Sci. 45, 221–232 (2005).
You, J. & Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant. Sci. 6, 1092. https://doi.org/10.3389/fpls.2015.01092 (2015).
Golldack, D., Li, C., Mohan, H. & Probst, N. Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front. Plant. Sci. 5, 151. https://doi.org/10.3389/fpls.2014.00151 (2014).
Finkel, T. & Holbrook, N. J. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247. https://doi.org/10.1038/35041687 (2000).
Irato, P. & Santovito, G. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 10 (4), 579. https://doi.org/10.3390/antiox10040579 (2021).
Wakeel, A., Xu, M. & Gan, Y. Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int. J. Mol. Sci. 21 (3), 728. https://doi.org/10.3390/ijms21030728 (2020).
Chen, P., Yang, W., Wen, M., Jin, S. & Liu, Y. Hydrogen sulfide alleviates salinity stress in Cyclocarya Paliurus by maintaining chlorophyll fluorescence and regulating nitric oxide level and antioxidant capacity. Plant. Physiol. Biochem. 167, 738–747. https://doi.org/10.1016/j.plaphy.2021.09.004 (2021).
Bahmanbiglo, F. A. & Eshghi, S. The effect of hydrogen sulfide on growth, yield and biochemical responses of strawberry (Fragaria × ananassa cv. Paros) leaves under alkalinity stress. Sci. Hortic. 282, 10013. https://doi.org/10.1016/j.scienta.2021.110013 (2021).
Filipovic, M. R. & Jovanović, V. M. More than just an intermediate: hydrogen sulfide signaling in plants. J. Exp. Bot. 68, 4733–4736. https://doi.org/10.1093/jxb/erx352 (2017).
Munns, R. & Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant. Biol. 59, 651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911 (2008).
Munns, R. Comparative physiology of salt and water stress. Plant. Cell. Environ. 25 (2), 239–250. https://doi.org/10.1046/j.0016-8025.2001.00808.x (2002).
Zhu, J. K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant. Biol. 6, 441–445. https://doi.org/10.1016/S1369-5266(03)00085-2 (2003).
Christou, A., Manganaris, G. A., Papadopoulos, I. & Fotopoulos, V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 64 (7), 1953–1966. https://doi.org/10.1093/jxb/ert055 (2013).
Deng, Y. Q., Bao, J. & Yuan, F. Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing na++ content. Plant. Growth Regul. 79, 391–399. https://doi.org/10.1007/s10725-015-0143-x (2016).
Jiang, J. L. et al. H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating H2S metabolism and oxidative stress response. Front. Plant. Sci. 10, 678. https://doi.org/10.3389/fpls.2019.00678 (2019).
Wei, M. Y. et al.. Exogenous hydrogen sulfide mediates Na+ and K+ fluxes of salt gland in salt-secreting mangrove plant Avicennia marina. Tree Physiol. 42, 1812–1826 (2022).
Wu, H., Zhu, M., Shabala, L., Zhou, M. & Shabala, S. K+ retention in leaf mesophyll, an overlooked component of salinity tolerance mechanism: A case study for barley. J. Integr. Plant. Biol. 57, 171–185. https://doi.org/10.1111/jipb.12238 (2015).
Hu, Y. Drought and salinity: a comparison of their effects on mineral nutrition of plants. J. Plant. Nutr. Soil. Sci. 168, 541–549. https://doi.org/10.1002/jpln.200420516 (2005).
Hirsch, R. E., Lewis, B. D., Spalding, E. P. & Sussman, M. R. A role for the AKT1 potassium channel in plant nutrition. Science 280, 918–921. https://doi.org/10.1126/science.280.5365.918 (1998).
Sharmin, S., Lipka, U., Polle, A. & Eckert, C. The influence of transpiration on foliar accumulation of salt and nutrients under salinity in Poplar (Populus × canescens). PLoS One. 16 (6), e0253228. https://doi.org/10.1371/journal.pone.0253228 (2021).
Acknowledgements
The authors express their gratitude to Shiraz University for generously providing the necessary laboratory equipment and chemicals essential for this study.
Funding
This study was financially supported by Shiraz University and acknowledges the Research Excellence Programme of the Hungarian University of Agriculture and Life Sciences.
Author information
Authors and Affiliations
Contributions
SE and BJ played a key role, with FAB and SM contributing to both the experiments and the drafting of the manuscript. IM and DP provided joint supervision, overseeing the research and ensuring the integrity of the study. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Eshghi, S., Bahmanbiglo, F.A., Mirzaei, S. et al. Hydrogen sulfide pretreatment mitigates the adverse effects of salinity in young carob seedlings. Sci Rep 15, 34903 (2025). https://doi.org/10.1038/s41598-025-18801-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18801-5