Abstract
To improve the damage performance of melt-cast explosives, one effective approach is to increase the proportion of solid-phase high-energy components. However, elevating the solid-phase content May alter the crystallization behavior of the 2,4-dinitroanisole (DNAN) melt or suspension, which can impact defect formation and overall quality and ultimately affecting the safety and detonation performance of the cast explosives. This study focuses on DNAN casting process with the addition of octogen (HMX) solid form, particularly examining its crystallization thermodynamics and kinetics. The effects of the content and particle size of the solid-phase additive HMX on the crystallization and solidification behaviors of DNAN are investigated using microcalorimetry, and the thermodynamic and kinetic parameters of DNAN crystallization are determined. The results indicate that incorporating HMX to DNAN increases the degree of undercooling; however, excessive HMX content damages the forming quality. Optimal forming quality and solidification rate are both achieved at a 43% content of HMX. Additionally, coarse HMX particles may impede the nucleation and crystallization of liquid-phase DNAN, making it difficult to meet the undercooling requirements.
Similar content being viewed by others
Introduction
Melt-casting explosives are a mixed explosive material that involves the incorporation of high-energy solid components (such as octogen (HMX) and cyclonite) and some auxiliary reagents into a liquid-phase molten carrier (such as trinitrotoluene (TNT)) for casting. The casting process of explosives is characterized by its low cost, the excellent ability to be mechanized and automated and not restricted by the shape and size of the projectile, making it the primary method for filling conventional munitions1,2,3. Since the introduction of TNT, TNT-based melt-casting explosives have been a primary combat explosive used in weapons and ammunition by various nations. Nonetheless, conventional TNT-based melt-casting explosives present numerous shortcomings: (1) susceptibility to defects such as shrinkage porosity, voids, and cracks; (2) exhibiting low energy levels; (3) producing toxic waste. Consequently, in order to meet the demand for high-energy insensitivity in munitions, nations worldwide are focused on discovering novel insensitive casting liquid-phase carriers with suitable physicochemical properties as alternatives to TNT4.
Recently, 2,4-dinitroanisole (DNAN) has received considerable attention due to its excellent safety performance and low toxicity5. When comparing equivalent energy outputs, DNAN-based melt-casting explosives exhibit superior safety and mechanical performance than TNT-based melt-casting explosives. Notably, DNAN is the only compound that has been evaluated through low vulnerability experiments among various casting carriers. Currently, DNAN serves as an alternative to TNT, and melt-casting explosives utilizing DNAN as a carrier can effectively mitigate safety concerns. These explosives have found applications in relevant arms and ammunition both domestically and internationally, including the PAX series3,6, ARX-40277,8, IMX-1019,10, RBUL-211, MCX series12,13,14,15, and RD-116. To enhance the damage performance of cast explosives, one effective approach is to increase the content of solid high-energy components17. However, the increase in solid content may alter the rheological properties and crystallization behavior of slurries or suspensions, which can affect the formation of defects in the explosive column and the quality of the final product, and ultimately influencing the safety and detonation performance of cast explosives. Research indicates that, in addition to the components and process parameters of melt-cast explosives, crystallization thermodynamics (undercooling, solidification temperature, exothermic heat of crystallization, etc.) and kinetics (crystallinity, crystallization rate, etc.) are closely linked to defect formation. The heat released during crystallization may affect the viscosity and temperature distribution of the melt, thereby altering the crystallization and solidification processes and influencing the quality of the final product18. Huang et al.19,20,21 reported that by applying pressure to reduce the undercooling of the explosive, the latent heat of phase transition can be minimized, which lowers the temperature difference inside and outside the charge, leading to a higher quality filling. Shao et al.22,23,24 discovered that the addition of AP can raise the solidification temperature of DNAN by 20–30 °C, reducing the undercooling and consequently shortening the solidification time. Zhang et al.25 employed differential scanning calorimetry (DSC) and optical microscopy to investigate the effects of different solid components (AP, GN, NQ, and HMX) on the solidification and temperature of DNAN. The results indicated that, compared to the other three solid components, AP significantly increases the solidification temperature of DNAN with the lowest solidification rate. In contrast, the influence of the other solid components on the solidification temperature is not significant. Previously, researchers utilized DSC to cool the melt at a specific cooling rate (~°C/min) that is much higher than the rate of natural cooling (~°C/h), asserting that microcalorimetry is more suitable for examining the thermal effects during the solidification process. As far as we know, there is no microcalorimetry method used to study the solidification behavior and crystallization process of melt-cast explosives. Although numerical simulation can now simulate the solidification and crystallization process of melt-cast explosives, acquire variations in parameters like melt or suspension viscosity and temperature, and analyze the defect formation process and charge shaping quality. However, the accuracy of numerical simulation needs to be further enhanced to better predict and explain related experimental phenomena, which relies on the acquisition of basic information such as the physical parameters, crystallization thermodynamics and kinetics of melt-cast explosives. Although the quality of formation can be improved through enhancements in molding techniques and process optimizations, such as pressurization19,20, vacuum vibration21,26, temperature control with hot core rod27, insulation with risers28, and layered pouring29, there is still a significant gap in systematic research on the physical mechanisms involved in the cooling and solidification process, especially concerning crystallization thermodynamics and kinetics.
This study investigates the DNAN casting matrix, employing microcalorimetry to analyze the effects of the content and particle size of the solid additive HMX on the crystallization and solidification behaviors of DNAN. Thus, this research can not only provide fundamental data related to crystallization thermodynamics and kinetics for numerical simulation of the solidification behavior of melt-cast explosives, but also offer multi-dimensional data references for a deeper understanding of defect formation and shaping quality in melt-cast explosives.
Materials and methods
Microcalorimetry
The microcalorimetric method is employed to determine the thermodynamic and kinetic parameters of actual processes by detecting minute heat changes associated with physical and chemical variations. This method is characterized by its high sensitivity and extended monitoring capabilities, making it one of the most effective techniques for investigating subtle thermal changes and slow reaction dynamics. A microcalorimeter can measure both the heat released by the system during a process and the rate at which this heat is evolved. During the solidification of energetic materials, an exothermic process usually occurs. The temperature control program of the microcalorimeter facilitates experiments at varying heating and cooling rates while simultaneously recording the correlation between heat flow, time, and temperature. This approach allows for the analysis of solidification behavior in samples with different compositions, enabling the determination of the thermodynamic and kinetic parameters of the solidification process.
Experimental procedure
The composition ratios of the experimental samples are shown in Table 1. The raw Materials used in the experiment, DNAN and HMX, were supplied by the Institute of Chemical Materials, Chinese Academy of Engineering Physics. DNAN and the additive HMX are weighed according to the proportions specified in the table, placed in a weighing bottle, and combined with an undesirable solvent, petroleum ether, followed by ultrasound mixing for 10 min. The combined experimental samples are placed in a ventilated area until the solvent has completely evaporated, at which point microcalorimetric testing is performed. Concurrently, to examine the influence of varying HMX particle sizes on the melting and solidification processes of DNAN, two HMX Materials with different particle sizes are chosen, 431 μm (labeled as HMX (I)) and 5.3 μm (labeled as HMX (II)). The microcalorimeter is employed to evaluate the melting and solidification processes of each sample group, Maintaining a total sample Mass of 300 mg for each experiment. During the microcalorimetric tests, the temperature control program is set as shown in Table 2. Considering the impact of the molten liquid temperature on undercooling, the temperature is increased to 115 °C and held for 1 h to mitigate the influence of thermal history on the solidification process. During the cooling stage, the cooling rates of 10 °C/h, 5 °C/h, and natural cooling are employed, while the changes in heat flow with respect to time and temperature are recorded. Once the temperature has returned to room temperature, the sample chamber is removed, and the sample column is extracted for structural characterization analysis.
Apparatus
Microcalorimeter: UT-310, manufactured by Mianyang Phanaly Technology Co., Ltd., operating temperature: room temperature to 300 °C; temperature accuracy: 0.1 °C; heating rate: 0.1 to 1 °C/min; heat flow conversion coefficient: 30 V/mW @ 50 °C.
X-ray diffraction (XRD): X’Pert PRO X-ray diffractometer from Malvern Panalytical Ltd, CuKα radiation with wavelength of 1.5406 Å, measurement method: step scanning, step angle 0.03°, tube voltage 40 kV, tube current 40 mA, scan range 2θ is 5° to 80°.
Results and discussions
Structural characterization of Raw materials
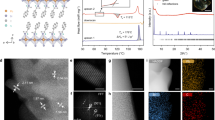
Initially, the crystal structures of the two HMX raw materials HMX (I) and HMX (II) were characterized using XRD, with the results displayed in Fig. 1. It can be observed that the XRD patterns of the two HMX samples exhibit peaks at almost the same diffraction angles, indicating that they belong to the same crystal type(β-HMX). However, there is a significant difference in the peak intensities between them, primarily due to : (1) According to the Scherrer equation, smaller particle sizes induce greater lattice strain, resulting in peak broadening; (2) Smaller particles potentially exhibit impaired crystal perfection, lowering crystallinity and weakening diffraction intensity; (3) Prolonged growth of larger HMX(I) crystals enables external conditions to induce preferred orientation by selectively exposing certain crystal planes. Such orientation preference intensifies diffraction from particular facets, resulting in comparatively stronger peak signals. This work also exclusively examines the effect of the most stable HMX crystal form (β-phase) under ambient conditions on DNAN crystallization and solidification. Additionally, as shown in Fig. 1, X-ray diffraction patterns of the solidified DNAN/HMX composites showed no evidence of HMX polymorphic transformation.
Optical microscopy was employed to observe HMX (I) and HMX (II), with the images shown in Fig. 2. It presents a significant difference in particle size between the two HMX materials at the same magnification, where HMX (I) has a much larger particle size than HMX (II) and retains a nearly perfect crystal structure. To further quantify the differences in particle size between the two HMX materials, a laser particle size analyzer was used to measure the particle sizes of HMX (I) and HMX (II). The results indicated that the D50 values for HMX (I) and HMX (II) were 431 μm and 5.3 μm, respectively.
Structural characterization of the solidified samples
Figure 3 presents the XRD patterns for different mass ratios of DNAN/HMX (II). It can be observed that as the DNAN content increases, the intensity of the diffraction peaks at corresponding diffraction angles also increases. Meanwhile, as the HMX content increases, the intensity of the diffraction peaks for HMX also increases. Compared to the raw materials HMX and DNAN, no new peaks were observed in the cast explosive DNAN/HMX, indicating that no polymorphic transformation occurred for HMX and DNAN during the entire melting and solidification process. Simultaneously, compared to raw HMX and DNAN materials, the XRD patterns of the DNAN/HMX mixture show neither new peaks nor disappearance of existing peaks. Consequently, the incorporation of HMX induces no polymorphic transition.
Melting and crystallization behaviors of DNAN
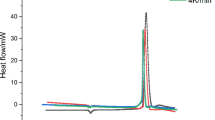
The thermal effects of the melting and solidification processes of DNAN were analyzed using a microcalorimeter, with the results shown in Fig. 4, and the heat flow curves for the melting and solidification processes are plotted in Fig. 4a and b, respectively. The heat flow during the melting process indicates that the melting point of DNAN is 96.15 °C, which is consistent with the literature reported28, with the melting endothermic peak occurring at 97.65 °C. It can be seen from Fig. 4b that the liquefied DNAN starts to crystallize at 80.94 °C, resulting in an undercooling degree of 15.21 °C. In the early stage of crystallization, a large number of nuclei begin to form, releasing a small amount of heat. Once the nuclei appear, the crystallization rate of DNAN increases, leading to a heightened exothermic rate, which results in a gradual increase in the heat flow, as illustrated in Fig. 5. As the crystallization process reaches its final stage, the remaining molten liquid decreases, the exothermic process slows down, and the crystallization rate begins to decrease until the sample is completely solidified.
Effects of HMX on the melting and crystallization behaviors of DNAN
Effect of HMX content on the melting behavior of DNAN
To examine the impact of HMX content on the melting behavior of DNAN, mixed samples with different mass ratios of DNAN/HMX (II) were prepared, and each group was subjected to microcalorimetric testing. The temperature range for the melting process was set between 80 and 115 °C, with a heating rate of 10 °C·h⁻¹. The results are shown in Fig. 6, where the curves of heat flow as a function of temperature are plotted in Fig. 6a and those of heat flow with time in Fig. 6b. The melting point of pure HMX is approximately 278–281 °C, therefore, HMX remains solid during the melting test. As shown in Fig. 6a, when the HMX content is low, the heat flow value of the endothermic peak is at its lowest, indicating the highest heat absorption. This occurs because the sample Mass is fixed at 300 mg, and thus lower HMX content corresponds to higher DNAN content, leading to a more significant thermal effect during DNAN melting. As the HMX content increases, the amount of DNAN particles in the sample decreases, resulting in a more dispersed distribution. In this case, HMX functions primarily to conduct heat without melting, which would lower the surrounding temperature. Furthermore, the heat required for lower DNAN content decreases, resulting in a gradual reduction in the initial melting temperature of the sample. According to Fig. 6b, there are considerable differences in the onset times of the endothermic peaks for DNAN/HMX at different Mass ratios, but the duration of the melting process is generally consistent. This can be attributed to the higher thermal conductivity of HMX compared to that of DNAN. As the HMX content increases, the thermal conductivity of the cast explosive system rises, leading to lower heat absorption in the systems with a higher HMX content. Consequently, the melting process occurs solely within DNAN, leading to similar melting durations across different HMX content levels in the cast explosive system. The melting point of pure HMX is approximately 278–281 ℃; thus, during the melting experiment (heated to 115 ℃), HMX remains in solid form. Therefore, in the DNAN/HMX mixed system, only DNAN melts while HMX remains unmelted. Varying HMX content primarily affects the overall heat transfer process, thereby influencing the timing of DNAN’s melting peak without altering its endothermic peak profile.
During the melting of DNAN, kinetic equations can be formulated using the heat flow data. The melting conversion rate α at any given time t or temperature T can be represented as the ratio of the thermal effect to the total thermal effect:
where H denotes the accumulated thermal effect of the melting reaction, with a unit of kJ•mol⁻¹; H0 represents the total thermal effect of the melting reaction; \(S^{\prime}\) and S are the area enclosed by the baseline and the heat flow curves at time t and the entire melting process, respectively. Based on this equation, curves representing the relationship between the melting conversion rate of different mass ratios of DNAN/HMX mixtures and temperature or time were plotted, as shown in Fig. 7. Specifically, Fig. 7a and b illustrate the variations of conversion rate with temperature and time, respectively.The results demonstrate that increasing temperature enhances the melting conversion rate of DNAN. After DNAN melting, higher HMX content reduces DNAN’s melting temperature while increasing its conversion rate. This effect is most significant at temperatures under 100 °C. Yet at approximately 100 °C, the HMX concentration exhibits negligible influence on conversion rate as complete melting is essentially achieved. Extended processing time leads to improved melting conversion, although HMX content displays no clear pattern in affecting melting time. Different DNAN/HMX compositions ultimately require comparable time to achieve complete melting.
Effect of HMX content on the crystallization behavior of DNAN
To investigate the effect of HMX content on the crystallization behavior of the energetic compound DNAN, mixed samples with different mass ratios of DNAN/HMX were prepared, and each group of mixed samples underwent microcalorimetric testing. The sample Mass was 300 mg, and the temperature range for the solidification process was 90–45 °C, with a cooling rate of 5 °C•h⁻¹. The test results are shown in Fig. 8, where the variations of heat flow with temperature and time are plotted in Fig. 8a and b, respectively. As observed in Fig. 8, the solidification temperatures of DNAN/HMX mixtures with varying mass ratios do not show a distinct pattern, yet al.l are below the solidification temperature of DNAN (80.94 °C). According to the uniform nucleation theory30, when the temperature drops below the melting point, short-range ordered atomic clusters (groups) may develop into “seed” nuclei that further grow into crystallites, initiating solidification. Due to the solubility of HMX in DNAN, the presence of dissolved HMX hinders the formation of short-range ordered clusters in molten DNAN, resulting in the need for a higher degree of undercooling to initiate crystallization. Thus, the addition of HMX increases the undercooling of DNAN. When the HMX content is 43%, the DNAN reaches its lowest undercooling in the mixed systems. This may be due to the limited solubility of HMX in DNAN at this concentration, reducing the hindrance effect of HMX on DNAN nucleation, resulting in the lowest undercooling. Meanwhile, DNAN can crystallize at higher temperatures, resulting in a reduced temperature difference between the inside and outside of the charge, which may lead to superior forming quality. At specific HMX concentrations (60% and 37.5%), HMX may be more evenly distributed in DNAN, which enhances thermal conductivity and accelerates solidification rates, thereby increasing the undercooling. Simultaneously, a greater degree of undercooling can reduce the critical nucleus size, thereby facilitating nucleation. This leads to an enhanced nucleation rate and a more rapid solidification rate30. (as illustrated in Fig. 8b). However, if the undercooling continues to increase, the lower crystallization temperature will hinder atomic migration, leading to a decrease in the nucleation rate. Furthermore, excessively high or low HMX content causes uneven distribution of HMX in DNAN, which affects its thermal conductivity and solidification rate. In mixed samples with low DNAN content, the total amount of DNAN is low, resulting in a lower exothermic peak during crystallization. As the DNAN content increases, the exothermic peak gradually rises. Therefore, to ensure both solidification rate and forming quality, the degree of undercooling must meet specific criteria, and a 43% content of HMX can yield superior forming quality and solidification rate.
The crystallization behavior is governed by crystallization kinetics. The amount of heat released during the crystallization process of the melt is related to the crystallinity φ as:
where ΔH denotes the crystallization heat at a specific temperature T or time t, while ΔHc represents the total crystallization heat for the process. By integrating the heat flow data, we can obtain the crystallinity curves versus temperature and time, as illustrated in Fig. 9a and b, respectively. In the initial stage of crystallization, a small amount of DNAN precipitates with the aid of the medium HMX, releasing a small amount of heat. As the temperature decreases, the precipitation rate of DNAN gradually accelerates. By the end of the crystallization process, the remaining molten liquid decreases, the heat release process slows down, and the crystallization rate begins to decelerate until the sample is completely precipitated. The results plotted in Fig. 9a indicate that, compared to the other systems, the presence of 43% HMX allows DNAN to complete crystallization at a higher temperature. Additionally, Fig. 9b shows that when the HMX content is 43%, the crystallization time of DNAN is the longest and the crystallization rate is the slowest, which is consistent with the aforementioned conclusion regarding the lower undercooling of this system. Taking into account the influence of HMX contents on the undercooling of DNAN during the solidification process, along with the correlation between crystallinity and temperature or time, it is evident that an HMX content of 43% results in the lowest undercooling (at least among the tested contents) of DNAN and a suitable crystallization rate, potentially yielding excellent quality of cast explosives.
In addition, the mechanical properties of the DNAN/HMX system were calculated according to the method in previous research31. As shown in Table 3, the results revealed that relative to pure HMX, all Cii elastic constants were reduced whereas other elastic constants showed differential changes, demonstrating decreased anisotropy in the melt-cast explosives and consequently improved isotropy relative to pure HMX. Notably, the 43% HMX composition showed the most significant Cii reduction, implying maximal isotropy and likely enhanced mechanical performance in this specific formulation. Computational analysis of elastic moduli indicates decreased bulk and shear moduli in the DNAN/HMX composite relative to pure HMX, leading to superior plastic deformability and enhanced elastic response in the composite. Moreover, blending with DNAN substantially elevates the Poisson’s ratio of DNAN/HMX, providing additional evidence for the composite’s enhanced plasticity compared to pure HMX. Crucially, the 43% HMX formulation exhibits the most significant decreases in bulk and shear moduli, coupled with an elevated Poisson’s ratio, demonstrating exceptional elastic-plastic properties. Additionally, the maximal B/G ratio in this system correlates with enhanced toughness, while its positive Cauchy pressure (C12-C44) confirms improved ductility versus brittle HMX in the 57% DNAN composite. These findings are consistent with microcalorimetric data demonstrating superior formability and solidification rate at 43% HMX loading.
Effect of particle sizes of HMX on the melting and crystallization behaviors of DNAN
To investigate the effects of particle sizes of HMX on the melting and crystallization behaviors of DNAN, the heat flow of the mixed systems with different HMX particle sizes was analyzed, and the results are plotted in Figs. 10 and 11. In Fig. 10, the dashed and solid lines correspond to the heat flow curves of the melting processes for DNAN/HMX (II) and DNAN/HMX (I) samples, respectively. Figure 11a and b depict the heat flow curves for the solidification processes of DNAN/HMX (II) and DNAN/HMX (I) samples, respectively. As seen in Fig. 10, the melting temperatures during the melting processes of DNAN/HMX (II) and DNAN/HMX (I) samples are quite similar, and the endothermic peak temperatures are also nearly identical, suggesting that the two particle sizes of HMX exert a slight impact on the melting behavior of DNAN. It can be seen from Fig. 11 that the solidification temperatures of DNAN in the two different particle sizes of HMX media do not exhibit a distinct relationship with the DNAN content. From a heat transfer perspective, smaller HMX particles possess larger specific surface areas and greater contact area with the DNAN matrix. During heat transfer, finer particles disperse more uniformly in the matrix, creating additional heat conduction pathways that facilitate rapid heat transfer and diffusion. Regarding solidification mechanisms, smaller HMX particles more readily interact with DNAN molecules, serving as heterogeneous nucleation sites that induce orderly arrangement of DNAN molecules. The high surface area and homogeneous dispersion of fine HMX particles provide abundant nucleation sites, thereby promoting DNAN nucleation and crystallization. Consequently, compared to coarse HMX particles, fine HMX particles markedly enhance DNAN’s nucleation efficiency, accelerating crystallization rate and improving crystallinity, manifested in thermal curves as higher solidification onset temperature and reduced undercooling. The heat flow curve for the fine particle DNAN/HMX (II) is consistent with the previously discussed trend regarding the influence of HMX content on the solidification process of DNAN. Specifically, when the mass ratios of DNAN/HMX are 40/60 and 62.5/37.5, the crystallization temperatures are both lower, resulting in a higher undercooling for the DNAN samples. When the mass ratios of DNAN/HMX are 20/80 and 80/20, the crystallization temperatures are higher, and the undercooling is lower. However, the heat flow curve for the coarse particle DNAN/HMX (I) varies with HMX content in a manner inconsistent with that of the fine particles. When the mass ratio of DNAN/HMX is 57/43, the crystallization temperature is the lowest, and the undercooling of the DNAN sample is the highest, which is in direct contrast to the situation with fine particle HMX. This may be due to DNAN being surrounded by larger particles of HMX or adsorbed onto their surfaces, which further hinders the nucleation and crystallization of the liquid phase DNAN.
Conclusions
This study investigated the melting and solidification behaviors of the DNAN/HMX system, and the effects of HMX content and its particle size on the melting behavior and crystallization thermodynamics and kinetics of DNAN were found. The conclusions are summarized as follows:
(1) The results indicate that the effect of HMX on the melting process of DNAN is not significant. Adding HMX to DNAN increases the degree of undercooling.
(2) The effect of varying HMX content on the degree of undercooling of DNAN does not follow a consistent pattern.
(3) To achieve a balance between solidification rate and forming quality, the degree of undercooling must satisfy specific requirements. A composition containing 43% HMX, results in excellent forming quality and solidification rate.
(4) Coarse HMX particles may impede the nucleation and crystallization of liquid-phase DNAN, making it difficult to achieve the necessary undercooling conditions.
Data availability
All data supporting the findings of this study are available within the paper.
References
Chen, F., Liu, Y., Wand, Y. & Zhang, Q. Review on Melt-cast carrier explosives. Chin. J. Energ. Mater. 28, 1109–1119 (2020).
Ma, Q. et al. Strategies for constructing melt-castable energetic materials: A critical review. Energ. Mater. Front. 2, 69–85 (2021).
Ravi, P., Badgujar, D., Gore, G., Tewari, S. & Sikder, A. Review on melt cast explosives. Propellants, Explos., Pyrotech. 36, 393–403 (2011).
Zhu, D. L., Zhou, L., Zhang, X. R. & Xing, R. T. Comparison of comprehensive properties for DNAN and TNT-Based Melt-cast explosives. Chin. J. Energ. Mater. 27, 923–930 (2019).
Meng, J., Zhou, L., Cao, T. T. & Wang, Q. Research progress of 2,4-Dinitroanisole-based Melt-cast explosives. Chin. J. Energ. Mater. 28, 13–24 (2020).
Niles, J. & Doll, D. Development of a practical reduced sensitivity composition B replacement. Energ. Mater. Karlsruhe, Germany. 28 – 21. (2001).
Davies, P. & Provatas, A. D. N. A. N. A replacement for TNT in Melt-cast formulations. DNIA Insensitive Munitions and Energetic Materials Technology Symposium, Orlando, Bristol, United Kingdom, (2006).
Provatas, A. & Wall, C. Thermal testing of 2, 4-dinitroanisole (DNAN) as a TNT replacement for melt-cast explosives. 42th International Annual Conference of ICT. Karlsruhe, (2011).
Davies, P. & Provatas, A. Characterisation of 2, 4-dinitroanisole: an ingredient for use in low sensitivity melt cast formulations. Weapons Systems Division, Defense Science and Technology Organization, (2006).
Taylor, S., Park, E., Bullion, K. & Dontsova, K. Dissolution of three insensitive munitions formulations. Chemosphere 119, 342–348 (2015).
Wang, C., Wei, M., Liu, X. & Liu, Y. Charging technology application of high power insensitive melt–pour explosive based on DNAN. Ordnance Ind. Autom. 32, 42–45 (2013).
Johansen, Ø. et al. Insensitive munitions-development and qualification of new melt-cast formulations. DNIA Insensitive Munitions and Energetic Materials Technology Symposium, Nashville, TN, USA, (2016).
Gunnar, O. Determination of Detonation Velocity and Pressure for MCX-6100. FFI-rapport 2015/02323 (Norwegian Defence Research Establishment, 2015).
Nevstad, G. O. Characterization of MCX-8100. FFI-rapport 2015/02448, Norwegian Defence Research Establishment, Kjeller,Norwegian, (2015).
Nevstad, G., Prytz, A. K., Odegardstuen, G. & Heiberg, O. M. IM assessment for a state of the art 155 mm HE round. Insensitive Munitions & Energetic Materials Technology Symposium, (2015).
Niu, G. T., Jin, D. Y., Wang, Q. H., Huang, W. B. & Niu, L. Effect of charge structure on charge quality of large size melt–cast explosive. Initiators & Pyrotechnics, 1, 30–33 (2015).
Meng, J., Zhou, L., Cao, S. & Wang, Q. Rheological properties of DNAN/HMX Melt-cast explosives. Chin. J. Energ. Mater. 26, 677–685 (2018).
Ma, S., Yuan, J., Liu, Y., Chang, S. & Wang, J. H. Experiment and numerical simulation of DNAN solidification process. Chin. J. Energ. Mater. 22, 240–244 (2014).
Huang, Y., Zheng, B., Xie, Z. & Wang, D. Pressured solidification process of melt-cast explosive. Chin. J. Energ. Mater. 21, 25–29 (2013).
Luo, Y. M., Zhang, M. M., Yang, F. & Li, B. B. Effect of pressure on DNAN solidification process. Sci. Technol. Eng. 20, 3048–3052 (2020).
Meng, J., Zhou, L., Jin, D., Niu, G. & Wang, Q. H. Effect of forming process on casting quality of 2, 4-dinitroanisole-based casting explosive. Acta Armamentarii. 39, 1719–1726 (2018).
Shao, Y., Ren, X., Liu, Z., Wang, X. & Zhang, G. DNAN and its non-isothermal crystallizations in RDX and AP. Chem. Anal. Meterage. 20, 22–26 (2011).
Wang, H., Jiang, F., Wang, H., Luo, Y. & Gao, J. Non-isothermal crystallization kinetics of DNAN in RDX. Chin. J. Energ. Mater. 20, 423–426 (2012).
Zhang, M., Luo, Y., Li, B., Fang, X. & Yang, F. Influence on mechanical and micro solidification of DNAN by solid additives and polymer. Chin. J. Energ. Mater. 29, 345–351 (2021).
Zhang, M., Wang, H., Li, B., Lv, D. & Luo, Y. Solidification process and solidification temperature of DNAN containing NHn(n = 0–4) compounds and its mechanism. Chin. J. Energ. Mater. 30, 130–137 (2022).
Peng, H. et al. Study on molding process of melt-casting explosive based on non-TNT with high solid content and density. Ordnance Ind. Autom. 38, 84–87 (2019).
Yuan, X. et al. Process of improved hot mandrel for large Length-Diameter ratio warhead melting cast. Chin. J. High. Press. Phys. 35, 015302 (2021).
Yuan, J. et al. Monitoring experiment and simulation on solidification of DNAN based melt-cast explosive with FBG sensors. Propellants, Explos., Pyrotech. 49, e202300232 (2024).
Zhang, P. et al. Research progress on solidification process and On-line monitoring technique of Melt-cast explosive. Chin. J. Energ. Mater. 32, 1242–1256 (2024).
Kurz, W., Fisher, D. & Rappaz, M. Fundamentals of Solidification 5th Edition. Trans Tech Publications Limited, (2023).
Gao, F., Liu, J., Huang, Q., Xia, H. & Shi, H. Molecular dynamics simulations for component interaction in DNAN/NTO melt-cast explosives. Mater. Today Commun. 38, 108282 (2024).
Acknowledgements
The authors thank the support from the Special Project for the Construction of High-level Talent Teams in Hebei Province (244A7603D), and the National Natural Science Foundation of China (No. 52105504).
Author information
Authors and Affiliations
Contributions
Conceptualization, J.L, H.X. and X.A.; Data curation, F.G. and H.S.; Formal analysis, F.G. and H.S.; Funding acquisition, X.A. and J.L.; Investigation, F.G., H.S, H.X. and X.A.; Methodology, F.G. and H.S.; Project administration, J.L., H.X. and X.A.; Resources, J.L.; Software, F.G. and H.S.; Supervision, J.L. and H.X.; Visualization, F.G. and H.S.; Writing—original draft, F.G.; Writing—review & editing, H.X. and X.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, F., Shi, H., Liu, J. et al. Effects of octogen on the crystallization and solidification behaviors of DNAN through microcalorimetry. Sci Rep 15, 33266 (2025). https://doi.org/10.1038/s41598-025-18856-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18856-4