Abstract
The positive effect of plasma treatment on the germination, viability or decontamination of seeds is already known and has been widely explored. Typically, all measurements are carried out on the seeds immediately after plasma treatment. However, in agricultural practice, seeds are stored for a long time before sowing. Therefore, we analysed the durability of the plasma effect during the 6-month storage period, considering both the storage time and the storage temperature (4 °C or room temperature). As a model object, we used seeds of Pisum sativum (Saxon var.) treated by Diffuse Coplanar Surface Barrier Discharge (DCSBD) at atmospheric pressure in the ambient air. The plasma-induced increase in wettability and the formation of new polar functional groups on the seed coat remained stable during the 6-month storage period. In addition, the improved germination parameters promoted by plasma treatment were maintained during storage. Our results have shown that plasma-treated seeds can be stored for a longterm period before sowing without losing the benefits achieved by the plasma treatment.
Similar content being viewed by others
Introduction
The escalating global population presents significant challenges for agriculture today. Specifically, the cultivation of important crops and achieving high plant productivity depend on healthy seeds with high germination potential. While agricultural chemicals, such as fertilizers and pesticides, are employed to address these needs, their application poses a considerable environmental risk requiring a phased reduction and substitution with new environmentally friendly methods. Among the many alternative strategies to increase crop production and minimize the environmental impact of chemicals, plasma treatment emerges as a promising option1,2,3.
Plasma, as highly reactive matter full of active particles such as electrons, ions, metastables, reactive oxygen and nitrogen species (RONS) and UV radiation, etc. can elicit numerous beneficial effects in seeds and plants4,5. Under optimized conditions, plasma treatment of seeds can improve the germination and viability6,7 while also providing antimicrobial and antifungal effects8,9. Furthermore, as a mild stressor, plasma can induce an adaptive response, supporting seedling resistance to subsequent, more severe abiotic stresses10. Treatment of sensitive biological material such as seeds is preferably carried out using non-thermal plasma. When generated in air or other gas in contact with air, this type of plasma produces a large amount of RONS which are primarily responsible for plasma activation processes at atmospheric pressure11. The observed improvement of seed germination parameters and the higher biomass production induced by plasma are related to the triggering of important metabolic processes. Although plasma mainly affects the thin upper seed coat layer, it is also involved in these processes in the seed kernel. Despite extensive research in plasma agriculture over the last two decades, which has shown numerous correlations between plasma composition, operational parameters and different seed types, drawing definitive conclusions remain difficult due to the variability of plasma properties1,2.
Until now, different plasma sources have been applied to different seed types and their effects on the physico-chemical properties of the seed surfaces as well as the physiological properties have been investigated in detail12,13,14,15. Diffuse Coplanar Surface Barrier Discharge (DCSBD) is a robust planar plasma source with an active area of 160 cm2 which makes it possible to treat a large number of seeds at once, which is one of the main advantages of this plasma system. Depending on the seed size, we can treat tens to hundreds of seeds per batch, significantly exceeding the capacity of plasma jets12 corona discharge plasma13 RF plasma sources14 and various smaller DBD configurations15 which typically handle fewer than ten seeds of bigger sizes (e.g. pea, maize, wheat). While physico-chemical and physiological changes are frequently measured immediately after plasma treatment, the long-term stability of these changes has been investigated in only a few studies12,14,16. Hence, consideration of the temporal stability of plasma induced changes is important because the seeds need to be stored and distributed in different locations before sowing. The ageing effect is well-documented for plasma-treated substrates with different compositions (glass, metal, plastic)17,18 where surface properties can revert to their initial state over time, depending on treatment conditions and substrate nature. Limited research exists on the longevity of positive plasma effects on seed germination and surface properties. Recek et al.16 monitored the hydrophobic recovery of plasma-treated bean (Phaseolus vulgaris L.) for 1 month by water contact angle (WCA) measurements. Low-pressure oxygen RF plasma significantly increased wettability, with WCA values approaching 0°. The rate of hydrophobic recovery correlated with plasma exposure time, with longer exposures resulting in slower recovery. Similarly, Ahmed et al.14 used low-pressure RF plasma to treat Bambara seeds and investigated the hydrophobic recovery by monitoring WCA changes over 60 days. However, neither study considered the influence of storage time and plasma treatment conditions on germination parameters. The first article on the ageing effect of plasma-treated seeds, in which germination parameters were also observed, was published last year. Guragain et al.12 conducted a comprehensive study on the ageing effect of plasma-treated fenugreek seeds using a commercial argon plasma jet with a short treatment duration (10 and 20 s). The seeds, which were stored in the dark at room temperature for 3 months, retained the improved germination potential due to the plasma treatment, as the plasma-treated seeds still showed significantly higher germination parameters than the reference group.
Aside from the abovementioned study on fenugreek seeds, neither study considered the influence of storage time and conditions on the germination characteristics of agriculturally important crops like cereals, maize or legumes which naturally possess high germination potential. Our present study tries to contribute to this research direction. As model seeds, we chose the legume Pisum sativum (Saxon variety) treated with a non-thermal plasma generated by DCSBD under different plasma treatment conditions. The physico-chemical surface properties such as wettability, morphology and chemical changes of the plasma-treated seeds were determined by water contact angle (WCA) measurements, X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM). The physiological and genotoxicological characteristics of the plasma-treated pea seeds, such as germination, vigour indices and DNA damage (via comet assay), were carried out on the pea seeds stored in the dark at the room temperature (RT) and in the fridge (4 °C) every week after plasma treatment over a period of first 5 weeks and 6 months.
Materials and methods
Plant material
Dried pea seeds (Pisum sativum L., Saxon variety) used in the experiments were provided by the Selgen, Czech Republic in 2023. We monitored the effect of the plasma treatment on the germination of two types of pea seeds, with assigned germination values of 50% (poor germinating seeds) and 90% (well germinating seeds). Before experiments, the seeds were stored in a fridge at 4 °C in the dark. For the long-term investigation of the physico-chemical and physiological changes, the plasma-treated seeds were stored in the dark, at room temperature (RT) or in the cold (4 °C).
Plasma source and plasma treatment procedure
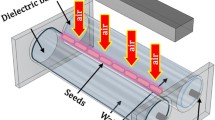
Plasma treatment of pea seeds was carried out with the Diffuse Coplanar Surface Barrier Discharge (DCSBD), an effective source of non-equilibrium plasma generated at atmospheric pressure in the ambient air. Detailed specifications and properties of DCSBD plasma source have already been published19,20. Briefly, DCSBD represents a planar electrode system including two parallel comb-like silver electrodes (one with 16 stripes) embedded in the alumina ceramics. On the surface of the ceramic plate with an active area of 20 × 8 cm2 is generated the visually diffused thin plasma layer (~ 0.3 mm). For our experiments, the electrode system was powered by alternating current with a high voltage of up to 20 kV (peak-to-peak) at about 15 kHz. Plasma treatment was performed at the input power of 400 W at atmospheric pressure in the ambient air. Due to the presence of thin plasma layer burning above the planar ceramic, the pea seeds were placed directly on the electrode and were constantly in the active plasma area during the treatment (Fig.1). Movement of the seeds along the ceramic and thus their homogeneous treatment was ensured by using the orbital shaker PSU-10i (BIOSAN) with the adjusted rotary speed of 330 rpm which carried the entire DCSBD plasma system. The different amounts of pea seeds for specific experiments (50 pcs – biological analysis, 120 pcs – WCA and its development over time; 300 pcs – biological analysis of ageing; 10 pcs – XPS, FTIR measurements) were treated in this experimental set-up. Considering the electrode area, not more than 150 pcs of seeds were treated in one batch. First, the DCSBD electrode was powered on, and the discharge was slowly set near 400 W. Then, the orbital shaker was turned on to enhance the homogeneous exposure of all seeds to plasma; the seeds were placed on the DCSBD ceramics and treated with diffused plasma for selected exposure times in the range of 5–120 s depending on the type of analysis.
Seed surface diagnostic methods
Water contact angle (WCA) measurements
The wettability of the pea surface was evaluated by measurement of water contact angle (WCA) by Drop Shape Analyzer DSA30 (KRŰSS, Hamburg, Germany) with software DSA3. The contact angle of one water droplet with a volume of 2 µL was measured on every seed. The resulting WCA values were calculated as the average contact angles of 10 droplets (10 seeds), whereby the highest and lowest values were removed before evaluation. To investigate the ageing process of plasma treated seeds, WCA was measured in the selected time intervals after the plasma treatment – immediately after treatment, after 1 day, 1 week and then every week up to next 5 weeks and after 6 months.
Imbibition
Dry seeds with a mass of around 30 g were plasma treated for 30 s by a DCSBD plasma source. Treated samples as well as REF samples separated into triplicates were placed in glass beakers and weighed on an analytical balance (KERN ABT220-4NM, KERN & SOHN GmbH, Germany) to quantify their starting weight (DW – dry weight). Then 50 ml of distilled water was poured into the beakers to let the seeds imbibe. In the selected time intervals (0.5, 1, 2, 4, 6, 7, 24 h) seeds were taken out from the water, dried using a paper towel and weighed (IW – imbibed weight). Imbibition was expressed as the mass increase of the sample in time according to the following equation:
Additionally, we also monitored the imbibition before the evaluation of germination and early growth parameters. In this case, to determine the imbibition of well-germinating (90% germ.) and poorly germinating (50% germ.) pea seeds treated with DCSBD plasma for 10, 20 and 30 s and for long-term plasma ageing experiments, dry seeds (50 pieces) were weighed before immersion in water and after 2 h. The resulting imbibition was expressed as weight of water per 1 h (mg H2O 1 h−1)21.
Scanning electron microscopy (SEM)
To monitor possible morphological changes on the surface of pea seeds, scanning electron microscope MIRA3 (Tescan, Brno, Czech Republic) with maximal resolution 1 nm was used. The detector of secondary electrons and an accelerating voltage of 5 kV was employed. To prevent any charging of the sample, samples were coated by 20 nm of Au/Pd composite layer.
X-ray photoelectron spectroscopy (XPS)
XPS measurements were carried out on an ESCALAB 250Xi X-ray Photoelectron Spectrometer (Thermo Fisher Scientific) at a take‐off angle of 90°. The system is equipped with a 500‐mm Rowland circle monochromator with a microfocused Al Kα X‐ray source. An X‐ray beam with a power of 200 W (650 μm spot size) was used. The survey spectra were acquired with a pass energy of 50 eV and an energy step of 1 eV. High‐resolution scans were acquired with a pass energy of 20 eV and an energy step of 0.1 eV. To counterbalance charges on the surface, an electron flood gun was used. The base pressure in the analysis chamber was in the 10-9 mbar range. After the acquisition, spectra were aligned to the lowest binding energy peak assigned to C–C/C–H, set at 284.8 eV. Spectral calibration, processing and fitting routines were carried out using Avantage software. XPS spectra were measured immediately after seed plasma treatment and then every week up to the next 5 weeks.
Germination and early growth parameters
For the assessment of germination and early growth parameters, the imbibed seeds were wrapped in wet, sterile filter paper and rolled into cylinders. These rolls were incubated in the dark at 24 ± 2 °C for 5 days. During this period, the number of germinated seeds was recorded daily for the first 3 days (at 24, 48, and 72 h). After the 5-day cultivation period, the length and fresh weight of the shoots and roots of the seedlings were measured. The collected data were used to calculate the germination percentage (%), seed vitality index, and seedling vitality index according to21. In the plasma ageing experiments, all the physiological parameters (imbibition, germination, seed vitality index, and seedling vitality index) were evaluated at specific time intervals: immediately after plasma treatment (0w), weekly for 5 weeks (1w–5w), and again after 6 months (6 m). In all experiments, seeds that were not treated with plasma were used as control samples (marked as reference, REF). In the long-term plasma ageing experiments, the reference samples stored at cold temperature (REF, C) refer to non-treated seeds stored at 4 °C (long-term storage), while the reference samples at room temperature (REF, RT) refer to non-treated seeds stored at room temperature for 6 months (6 m).
Genetic assays
DNA damage in pea seedlings, including various defects such as single-strand breaks, double-strand breaks, cross-links, and apyrimidinic and apurinic sites, was assessed using the alkaline comet assay as described in22.
Briefly, two roots from each sample were cut with a razor blade to release nucleoids into 150 µL of 0.4 M Tris–HCl buffer (pH 7.5), facilitated by mechanical disruption of the cell and nuclear walls. This process was carried out on ice and in the dark to prevent additional DNA damage. Next, 100 µL of the DNA-buffer suspension was mixed with 100 µL of 1% low melting point agarose. The mixture was then spread onto microscope slides pre-coated with 1% normal melting point agarose and covered with a coverslip. After 5 min, the coverslips were removed, and the slides were placed in an electrophoretic chamber filled with cold electrophoresis buffer (1 mM EDTA and 300 mM NaOH) for 8 min. Electrophoresis was then performed at 1.25 V/cm for 15 min at 4 °C. Following electrophoresis, the slides were neutralized three times with 0.4 M Tris–HCl buffer (pH 7.5) and stained with ethidium bromide (0.05 mM, 80 µL per slide) for 5 min. DNA damage was visualized using an OLYMPUS BX51 fluorescent microscope equipped with a UMWIG3 green excitation filter at 200×g.
Statistical analysis
Each data point was the mean of three replicates. All data obtained were subjected to a one-way analysis of variance (ANOVA), and the mean differences were compared by least significant difference (LSD) test. Comparisons with P < 0.05 were considered significantly different. In the figures, the spread of values is shown as error bars representing standard errors of the means.
Results and discussion
In the present study, well germinating (90% germ.) and poor germinating (50% germ.) seeds were exposed to plasma at the optimized operation conditions (input power 400 W, ambient air, rotation at 330 rpm) changing only the plasma exposure time as the exclusive parameter. Considering the size of the pea seed and previous experiments, we decided to investigate the exposure times in the range of 5–30 s on the physico-chemical properties of seed surface and germination potential. The first part of the study focused on the influence of the different plasma exposure times on wettability and chemical changes on the seed surface as well as on germination potential and DNA damage. Then, the optimized plasma exposure times were selected to test the persistence of plasma-induced changes in pea seeds during long-term storage (6 months) in cold (4 °C) and room temperature (RT) conditions.
Plasma effect on seed’s surface properties and germination parameters
Wettability and imbibition changes
The change in wettability after plasma treatment was measured by the water contact angle (WCA, Fig. 2a). The WCA reference value was 108 ± 5.8°, reflecting the hydrophobic character of the pea seed coat. The upper layer of the pea seed, which consists mainly of a subcuticular waxy layer and cellulosic microfibrils23 provides mechanical strength and represents a natural water barrier during seed dormancy. Accordingly, the presence of lipidic and aromatic substances (cutin, suberin, lignin) determines the water-repellent properties of the seed coat. Plasma treatment reduced the WCA from the original 108.2 ± 5.8° to 60.6 ± 3.2° after only 5 s of plasma treatment. A further extension of the plasma treatment time led to only a slight decrease in WCA, while the lowest value of 52.2 ± 7° was reached after 60 s for well germinating seeds (see Fig. 2a). Since we were curious whether the higher exposure times would lead to a further decrease of WCA, we added the 120 s treatment time to the study of the seed’s surface properties. Wettability increased and we achieved the WCA of 40.2° and 42.2° for well and poor germinating seeds, respectively. In general, only slight differences in WCA were monitored between the poor and well germinating seeds. The increase in wettability is driven by the oxidation of the hydrophobic molecules that form the seed coat, resulting in a higher hydrophilicity of the coat in general. This is usually associated with improved water permeability, which results in the absorption of a larger amount of water into the seed and, thus, faster germination. However, such behaviour was not confirmed in our study, as the imbibition of plasma-treated seeds was very similar to that of reference seeds, as can be seen in Fig. 2b.
Visualization of DCSBD plasma source. (a) Schematic representation of the configuration of the DCSBD plasma source and (b) image of the DCSBD discharge (top view) with placed pea seeds set up for plasma treatment, including the plastic barrier around the ceramic plate that prevents loss of the seeds.
Achieved water contact angles (WCA) after plasma treatment and imbibition development in time. (a) Dependence of WCA measured immediately after plasma treatment on the plasma exposure times compared to the reference value (REF); (b) Imbibition (%) of plasma treated seeds (exposure time 30 s) compared to reference seeds (REF) for well germinating (90% germ.) and poor germinating (50% germ.) pea seeds.
Surprisingly, the initially poor germinating seeds (50% germ.) absorbed water much faster during the first 4 h than the well germinating seeds (90% germ.). Similarly, the imbibition rate shown in Figure S1 (see Supplementary information), which was achieved after 1 h when the seeds were placed in water before germination experiments, was also higher for the poorly germinating seeds. The difference in the imbibition rate between the reference and plasmatreated seeds was insignificant.
However, the rate of imbibition and the amount of imbibed water by seeds are not directly proportional to the germination potential. Since imbibition has a physically driven mechanism during the initial stage, also the dead or damaged seeds can absorb water and even in a higher quantity than healthy seeds, which was monitored in the previous studies24,25. Another factor influencing the faster penetration of water across the seed coat is the presence of defects or cracks that may have been caused by the plasma treatment. Therefore, we looked at the morphology of the seed surface to assess possible cracks caused by the plasma treatment.
Morphological changes
Although the DCSBD plasma source is very effective, it is also very gentle on biological samples, hence, we did not detect any cracks or defects that would allow faster imbibition (Fig. 3). However, treatment times of more than 60 s resulted in abrasion, which damaged the natural structuring of the pea surface, which could contribute to a notable decrease in WCA after 120 s of plasma application. A similar abrasion of pea seeds along with formation of small cracks in the seed coat after plasma exposure were observed in the previous study, but not until 10 min of DCSBD plasma exposure26.
Chemical changes
Chemical changes occurring in the very thin layer of the seed surface after plasma treatment were monitored by XPS. The atomic composition before and after treatment is shown in the bar chart in Fig. 4. As already mentioned, the coat of the pea seeds consists of a wax layer and polysaccharides such as cellulose. Considering the original composition, one can generally expect a high proportion of hydrocarbon chains, representing long aliphatic chains in the lipids and aromatic groups in the lignin. However, as the polysaccharides consist of many OH groups and glycosidic bonds, a reasonable amount of oxygen can also be expected. The reference pea coat consists of 83% of carbon, 15% of oxygen and 2% of nitrogen. With increasing duration of plasma treatment, the O/C ratio increased from an initial 0.18 to 0.44 when it reached its maximum at 30 s of plasma treatment. Increasing the plasma treatment time to 60 s resulted in a return to the original chemical composition as the O/C ratio decreased to a value of 0.31 (see Table 1). The observed morphological changes after 60 s plasma treatment may be one reason for this behaviour. The plasma acts on the surfaces in several successive processes. At the beginning of the application, the plasma is able to remove impurities (cleaning), then plasma activation takes place, and polar functional groups are formed on the treated substrate. After prolonged exposure of the substrate to the plasma, these polar functional groups can be removed together with the treated material in the form of low molecular weight compounds. At this stage, etching occurs, and a thin layer of the bulk material is bare and can be activated again by plasma. From the SEM images in Fig. 3 it is obvious that 30 s of plasma treatment was also limiting for the morphological changes and after 60 s of exposure, the etching could cause the lowering of polar functional groups formed during the first 30 s.
Figure 5 shows the representative high-resolution spectra of the C1 peak of reference seeds compared to seeds treated for 30 s. The shape of the peak changes after plasma treatment; the part corresponding to the CC/CH bonds decreases and the part in the range of binding energies of 286–290 eV increases. This part of the spectra corresponds to polar functional groups C–O/C–N, which are assigned to hydroxyl or amino groups (286.2 eV), C=O/OCO appertaining to carbonyl, ether or amide groups (287.8 eV) and OC =O defining the carboxyl/ester groups (289.0 eV). Table 1 summarizes the relative percentage of chemical bonds from deconvoluted C1s spectrum. The higher increase in the ratio of polar functional groups to hydrocarbon bonds (C–X/C–C) was achieved after 30 s of treatment, which also correlates with atomic concentration data as well as etching monitored in the prolonged plasma treatment time.
Germination potential and DNA damage
The germination of pea seeds, which naturally germinate well, reached 100% in reference seeds and no negative effect of plasma treatment was observed (see Supplementary information: Figure S2). However, the early development of pea seedlings improved after plasma treatment. The seed vitality index was significantly higher in well-germinating seeds after 30 s of treatment (Fig. 6b), as was the vitality index of seedlings (Fig. 7b), although in this case the increase was not statistically significant at all exposure times. In poorly germinating seeds, germination improved significantly from 70% to almost 90% after 10 s and 20 s plasma treatment (see Supplementary information: Figure S2a). The vitality indices of the seeds and seedlings were also higher after 10–20 s of plasma treatment (significantly higher after 10 s) compared to the reference seeds (Figs. 6a and 7a). The effect of direct plasma application on pea seeds has been investigated in a few studies, mostly using surface dielectric barrier discharges with different configurations or pulsed corona plasma discharge15,27,28,29. A comparison of the results we obtained for the germination parameters with these studies shows that, usually the long treatment time in the range of minutes was necessary to achieve an improvement in the germination parameters27,28,29.
Seed vitality indices of pea seeds achieved in different plasma exposure times. Seed vitality index of (a) poor germinating (50% germ.) and (b) well germinating (90% germ.) plasma-treated (plasma exposure times 10, 20 and 30 s) and reference pea seeds (REF). Bars represent mean values from five independent experiments (each with 50 seeds per variant) ± standard deviation (SD). Different letters indicate statistically significant differences (P < 0.05) based on the LSD test.
Seedling vitality indices of pea seeds achieved in different plasma exposure times. Seeding vitality index of (a) poor germinating (50% germ.) and (b) well germinating (90% germ.) plasma-treated (plasma exposure times 10, 20 and 30 s) and reference pea seeds (REF). Bars represent mean values from five independent experiments (each with 50 seeds per variant) ± standard deviation (SD). Different letters indicate statistically significant differences (P < 0.05) based on the LSD test.
DNA damage in the roots of pea seedlings was assessed using the alkaline comet assay (see Supplementary Information: Figure S3). In seedlings from seeds with high germination rates (90%), plasma treatment resulted in a higher level of DNA damage compared to the untreated reference samples (Figure S3a). Although this increase was statistically significant, it was relatively small. The type of DNA damage detected by this method is primary and can typically be repaired by DNA repair mechanisms. In seedlings from poorly germinating seeds, no statistically significant increase in DNA damage was observed after plasma treatment (Figure S3b). This could be due to a higher initial level of DNA damage in the reference samples of these seeds compared to the reference samples of the well-germinating seeds. Overall, the increase in DNA damage was minimal in some samples, suggesting that non-thermal plasma treatment under these conditions had no detrimental effect on the DNA of pea seeds. These findings are consistent with previous studies investigating DNA damage in pea seedlings following NTP treatment of seeds. For example, NTP generated in ambient air did not cause DNA damage at shorter exposure times (15–60 s)25 and even at longer exposure times (60–300 s), no significant DNA damage was observed in pea seedlings (var. Prophet)10,24. Additionally, NTP generated in air did not induce DNA single- or double-strand breaks24. Furthermore, among various working gases tested, ambient air resulted in the lowest DNA damage in pea seedlings compared to oxygen, nitrogen, and their mixtures30.
Ageing behaviour of plasma-treated seeds
Based on the screening of the suitable plasma exposure time from the previous chapter considering the most improved germinating parameters, 15 s plasma exposure time was selected for poor germinating seeds (as an average value between 10 and 20 s, as these had similar positive effects) and 30 s for well germinating seeds as the optimal for further ageing studies.
Wettability changes
First the ageing behaviour was investigated by monitoring of WCA development in time. Due to negligible differences in the wetting properties of the well and poor germinating seeds, for the study of hydrophobic recovery by WCA measurements, we used only well germinating seeds. WCA development for 6 months storage is depicted in Fig. 8. Since the seeds are usually stored in a cold place, at first, we stored them in the fridge at 4 °C. Achieved WCA after 60 s of plasma treatment (52.2°) stayed stable during first 5 weeks and after 4 months of storage increased only negligible to 61.1°. Similar linear development was achieved also in the optimized plasma treatment time of 30 s as well as the lower-used treatment time of 5 s. After 6 months, the values of WCA of 5 s and 30 s plasma exposed seeds stabilized at the 76.7° and 67.6°, respectively. These values are still very far from the reference WCA value of 108.2° and reflect the retention of hydrophilic properties of the seed coat after long-term storage. We compared the WCA development of the seeds stored also at the room temperature, where the WCA values increased moderately more to values 83.7° and 72.4° for 5 and 30 s respectively. However, similarly as in the case of stored seeds in the cold, the hydrophilicity of the seed coat is still preserved.
Chemical changes
Monitoring the chemical changes revealed very stable surface chemistry over 5 weeks for the plasma exposure times of 5, 30 and 60 s investigated (see Supplementary Information: Figure S4, Table S1). Only for 5 s we achieved a slight fluctuation after 3 weeks, which may be caused by the short treatment time, during which the functional groups formed on the pea surface could not stabilize.
Germination potential monitored for 6 months
Both poor germinating (50% germ.) and well germinating (90% germ.) pea seeds showed no significant change in imbibition during the first weeks of storage when compared to nontreated reference seeds (Fig. 9). Surprisingly, after 6 months of storage both at cold and room temperature, plasma-treated seeds exhibited a significant increase in imbibition, with a more pronounced effect observed under cold storage conditions. This may relate to surface changes (increased lipid oxidation of the seed surfaces), though the observed changes in seed morphology and wettability were negligible. However, in combination with the time factor (length of storage), even slight abrasions caused by rotational movement during treatment could contribute to more efficient imbibition over time.
Monitoring of imbibition rate of plasma-treated pea seeds at the optimized exposure times during 6 months of storage. Imbibition rate (mg H2O h− 1 per seed) of (a) poor germinating (50% germ.) and (b) well germinating (90% germ.) pea seeds after 1 h of soaking. Variants: REF (C) – nontreated reference seeds stored at 4 °C; DCSBD 15/30s, 0w (C) to 5w (C) – seeds treated with DCSBD plasma for 15/30 s and stored for 0 to 5 weeks at 4 °C; DCSBD 15/30s, 6 m (C) – plasma-treated seeds (15/30s) stored for 6 months at 4 °C; DCSBD 15/30s, 6 m (RT) – plasma-treated seeds (15/30 s) stored for 6 months at room temperature; REF, 6 m (RT) – nontreated reference seeds stored for 6 months at room temperature. Bars represent mean values from five independent experiments (each with 50 seeds per variant, n = 250) ± standard deviation (SD). Different letters indicate statistically significant differences at P < 0.05 (ANOVA, LSD test).
As other results show, proper seed storage significantly affects subsequent germination and appropriately applied low-temperature plasma can partially mitigate the effects of poor storage over time. While many studies have reported immediate germination benefits of plasma in various crops28,29,31,32,33,34,35 few studies have focused on the long-term persistence of these positive effects12,36,37. Nicolau et al.36 found plasma treatment to be effective in breaking seed dormancy in Pityrocarpa moniliformis seeds immediately, and these positive effects persisted even after 2 years of storage, though to a lesser extent. The team of de Groot et al.37 concluded that low-temperature air plasma treatment can significantly increase imbibition and improve cotton seed germination under different storage conditions (RT or cold), with effects lasting at least 4 months.
In our experiments, the analysis of germination after the initial 24 h after imbibition revealed distinct responses in pea seeds subjected to plasma treatment and different storage conditions (Fig. 10). The germination of poorly germinating pea seeds (with 50% initial germination rate) following plasma treatment increased considerably after 6 months of storage at cold temperature (DCSBD 15s, C), compared to nontreated reference seeds (REF, C). In contrast, plasma-treated seeds stored at room temperature (DCSBD 15s, RT) showed a non-significant decline in germination compared to the corresponding reference seeds (REF, RT) likely due to high biological variability among the seeds. For well germinating pea seeds (with 90% initial germination rate), plasma treatment had a clearly positive effect on germination dynamics during short-term cold storage, and this beneficial effect persisted after 6 months of cold storage (DCSBD 30s, C). In contrast, when stored at room temperature (DCSBD 30s, RT), plasma-treated seeds showed no significant difference in germination performance compared to reference seeds (REF, C and REF, RT). These results suggest that plasma treatment has a beneficial effect on the initial onset of seed germination after long-term storage, and this effect is likely storage condition dependent.
Monitoring of seed germination of plasma-treated pea seeds at the optimized exposure times during 6 months of storage. Seed germination of (a) poor germinating (50% germ.) and (b) well germinating (90% germ.) pea seeds 24 h after imbibition. Variants: REF (C) – nontreated reference seeds stored at 4 °C; DCSBD 15/30s, 0w (C) to 5w (C) – seeds treated with DCSBD plasma for 15/30 s and stored for 0 to 5 weeks at 4 °C; DCSBD 15/30s, 6 m (C) – plasma-treated seeds (15/30s) stored for 6 months at 4 °C; DCSBD 15/30s, 6 m (RT) – plasma-treated seeds (15/30s) stored for 6 months at room temperature; REF, 6 m (RT) – nontreated reference seeds stored for 6 months at room temperature. Bars represent mean values from five independent experiments (each with 50 seeds per variant, n = 250) ± standard deviation (SD). Different letters indicate statistically significant differences at P < 0.05 (ANOVA, LSD test).
Our results also demonstrate that although plasma treatment influences the early phases of pea seed germination, the effect of plasma was transient; with germination rates convering by 72 h (see Supplementary information: Figure S5). This suggests that plasma treatment primarily influences the timing of germination initiation rather than the final germination capacity.
The seed vitality index and seedling vitality index of low-vigor seeds (50% initial germination) showed no significant response to plasma treatment during long-term cold storage but declined after 6-month storage at room temperature. In contrast, high-vigor seeds (90% initial germination) generally exhibited increased seed and seedling vitality indices following plasma treatment, when stored at cold, but this positive effect diminished after 6 months of room-temperature storage (Supplementary information: Figures S6–S9).
The positive effects of plasma treatment immediately after application, such as enhanced seed and grain vitality indices, improved seedling vitality, and increased mechanical strength of seedlings, have been documented in several studies24,25,32,33,38. To our knowledge, the available literature lacks data on the persistence of these effects during storage. In this context, our pilot study provides novel insights.
DNA damage monitored for 6 months
DNA damage in pea seedlings grown from seeds treated with non-thermal plasma showed minimal variation throughout the ageing experiment. In seeds with high germination rates (90%) treated with plasma for 30 s, DNA damage remained consistent with the reference sample from week 0 to 6 months of storage in the dark at 4 °C (Fig. 11a). Moreover, in well-germinating seeds stored at room temperature for 6 months, DNA damage levels in plasma-treated and reference samples, were comparable and similar to that in seeds stored at 4 °C. A similar trend was observed in seeds with lower germination rates (50%) treated with non-thermal plasma for 15 s. In these seedlings, DNA damage remained stable from 0 weeks to 6 months of storage, regardless of the storage temperature (Fig. 11b).
Monitoring of DNA damage of plasma-treated pea seeds at the optimized exposure times during 6 months of storage. DNA damage (analyzed by comet assay) of (a) well germinating and (b) poor germinating pea seeds after plasma treatment (plasma exposure time 30 s for seeds with 90% germination, and 15 s for seeds with 50% germination). Variants: REF (C) – nontreated reference seeds stored at 4 °C; DCSBD 15/30s, 0w (C) to 5w (C) – seeds treated with DCSBD.
Conclusion
It is of crucial importance whether the positive effect of the plasma, which causes an improvement in the germination parameters in the seeds, is permanent and whether different storage conditions lead to different results. The present article provides a detailed and comprehensive study on the preservation of the positive plasma-induced changes in the treated pea seeds during their long-term storage. The increased wettability of the seed coat and the number of new polar functional groups remained stable during the 6-month storage period and the stability was higher during storage in a dark cold place. In addition, the improved physiological parameters promoted by the plasma treatment were maintained or enhanced during long-term cold storage, even in the case of seeds with poor initial germination. Our results have shown that plasma treatment of seeds can be considered as a very effective, environmentally friendly and fast pre-sowing method that provides improved germination parameters to seeds which can be stored for a longer period of time before sowing without losing the benefits obtained by plasma treatment.
Data availability
All the evaluated data that support the findings presented in this study are available from the corresponding author, P.S.
References
Bilea, F. et al. Critical reviews in plant sciences non-thermal plasma as environmentally- friendly technology for agriculture: A review and roadmap. CRC Crit. Rev. Plant. Sci. 43, 428–486 (2024).
Waskow, A., Howling, A. & Furno, I. Mechanisms of plasma-seed treatments as a potential seed processing technology. Front. Phyiscs. 9, 1–23 (2021).
Randeniya, L. K. & de Groot, G. J. J. B. Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: A review. Plasma Process. Polym. 12, 608–623 (2015).
Ahmed, S. & Hayashi, N. Enhancement of antioxidative potential of mung bean by oxygen plasma irradiation of seeds. Sci. Rep. 14, 30465 (2024).
Pérez-Pizá, M. C. et al. Enhancement of soybean nodulation by seed treatment with non–thermal plasmas. Sci. Rep. 10, 4917 (2020).
Zahoranová, A. et al. Effect of cold atmospheric pressure plasma on the wheat seedlings Vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 36, 397–414 (2016).
Ďurčányová, S. et al. Efficacy comparison of three atmospheric pressure plasma sources for soybean seed treatment: Plasma characteristics, seed properties, germination. Plasma Chem. Plasma Process. 43, 1863–1885 (2023).
Mošovská, S. et al. Cold atmospheric pressure ambient air plasma Inhibition of pathogenic bacteria on the surface of black pepper. Food Res. Int. 106, 862–869 (2018).
Mošovská, S., Medvecká, V., Valík, Ľ., Mikulajová, A. & Zahoranová, A. Modelling of inactivation kinetics of Escherichia coli, Salmonella enteritidis and Bacillus subtilis treated with a multi-hollow surface dielectric barrier discharge plasma. Sci. Rep. 13, 1–10 (2023).
Kyzek, S. et al. Cold atmospheric pressure plasma can induce adaptive response in pea seeds. Plasma Chem. Plasma Process. 39, 475–486 (2019).
Bekeschus, S. Gas plasmas technology: From biomolecule redox research to medical therapy. Biochem. Soc. Trans. 51, 2071–2083 (2023).
Guragain, R. P. et al. Germination improvement of Fenugreek seeds with cold plasma: Exploring long-lasting effects of surface modification. Sci. Hortic. (Amsterdam). 324, 112619 (2024).
Srisonphan, S. Tuning surface wettability through hot carrier initiated impact ionization in cold plasma. ACS Appl. Mater. Interfaces. 10, 11297–11304 (2018).
Ahmed, N., Siow, K. S., Wee, M. F. M. R. & Patra, A. A study to examine the ageing behaviour of cold plasma-treated agricultural seeds. Sci. Rep. 13, 1675 (2023).
Slavíček, P. et al. The multi-hollow surface dielectric barrier discharge usage for the seeds’ treatment aimed to the dustiness decrease of free-floating particles from agrochemicals. Plasma Chem. Plasma Process. 43, 1887–1906 (2023).
Recek, N. et al. Germination of Phaseolus vulgaris L. Seeds after a short treatment with a powerful RF plasma. Int. J. Mol. Sci. 22, 6672 (2021).
Mortazavi, M. & Nosonovsky, M. A model for diffusion-driven hydrophobic recovery in plasma treated polymers. Appl. Surf. Sci. 258, 6876–6883 (2012).
Primc, G. & Mozetič, M. Hydrophobic recovery of plasma-hydrophilized polyethylene terephthalate polymers. Polymers (Basel) 14, 2496 (2022).
Černák, M., Černáková, L., Hudec, I., Kováčik, D. & Zahoranová, A. Diffuse coplanar surface barrier discharge and its applications for in-line processing of low-added-value materials. Eur. Phys. J. Appl. Phys. 47, 22806 (2009).
Černák, M. et al. Generation of a high-density highly non-equilibrium air plasma for high-speed large-area flat surface processing. Plasma Phys. Control Fusion 53, 124031 (2011).
Abdul-Baki, A. A. & Anderson, J. D. Vigor determination in soybean seed by multiple criteria 1. Crop Sci. 13, 630–633 (1973).
Gichner, T., Patková, Z., Száková, J., Žnidar, I. & Mukherjee, A. DNA damage in potato plants induced by cadmium, Ethyl methanesulphonate and γ-rays. Environ. Exp. Bot. 62, 113–119 (2008).
Janská, A., Pecková, E., Sczepaniak, B., Smýkal, P. & Soukup, A. The role of the testa during the establishment of physical dormancy in the pea seed. Ann. Bot. 123, 815–829 (2019).
Švubová, R. et al. Novel insight at the effect of cold atmospheric pressure plasma on the activity of enzymes essential for the germination of pea (Pisum sativum L. Cv. Prophet) seeds. Plasma Chem. Plasma Process. 40, 1221–1240 (2020).
Švubová, R. et al. Enhanced in situ activity of peroxidases and lignification of root tissues after exposure to non-thermal plasma increases the resistance of pea seedlings. Plasma Chem. Plasma Process. 41, 903–922 (2021).
Stolárik, T. et al. Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum sativum L). Plasma Chem. Plasma Process. 35, 659–676 (2015).
Bußler, S., Steins, V., Ehlbeck, J. & Schlüter, O. Impact of thermal treatment versus cold atmospheric plasma processing on the techno-functional protein properties from Pisum sativum ‘salamanca’. J. Food Eng. 167, 166–174 (2015).
Abeysingha, D. N., Dinesh, S., Roopesh, M. S., Warkentin, T. D. & Thilakarathna, M. S. The effect of cold plasma seed treatments on nodulation and plant growth in pea (Pisum sativum) and lentil (Lens culinaris). Plasma Process. Polym. 21, 1–12 (2024).
Yemeli, G. B. N., Janda, M. & Machala, Z. Non-thermal plasma as a priming tool to improve the yield of pea in outdoor conditions. Plasma Chem. Plasma Process. 42, 1143–1168 (2022).
Tomeková, J., Kyzek, S., Medvecká, V., Gálová, E. & Zahoranová, A. Influence of cold atmospheric pressure plasma on pea seeds: DNA damage of seedlings and optical diagnostics of plasma. Plasma Chem. Plasma Process. 40, 1571–1584 (2020).
Zahoranová, A., Hoppanová, L., Šimončicová, J. & Tučeková, Z. Effect of cold atmospheric pressure plasma on maize seeds: Enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem. Plasma Process. 38, 969–988 (2018).
Holubová, Ľ. et al. Cold atmospheric pressure plasma treatment of maize Grains—Induction of growth, enzyme activities and heat shock proteins. Int. J. Mol. Sci. 22, 8509 (2021).
Peťková, M. et al. The effects of cold atmospheric pressure plasma on germination parameters, enzyme activities and induction of DNA damage in barley. Int. J. Mol. Sci. 22, 2833 (2021).
Ji, S. H. et al. Effects of high voltage nanosecond pulsed plasma and micro DBD plasma on seed germination, growth development and physiological activities in spinach. Arch. Biochem. Biophys. 605, 117–128 (2016).
Nishime, T. M. C., Wannicke, N., Horn, S., Weltmann, K. D. & Brust, H. A coaxial dielectric barrier discharge reactor for treatment of winter wheat seeds. Appl. Sci. 10, 7133 (2020).
Nicolau, J. P. B. et al. Cold plasma is effective at overcoming dormancy and maintaining germination in pityrocarpa moniliformis after storage. ACTA Paul Enferm. 56, 0–5 (2025).
de Groot, G. J. J. B., Hundt, A., Murphy, A. B., Bange, M. P. & Mai-Prochnow, A. Cold plasma treatment for cotton seed germination improvement. Sci. Rep. 8, 1–10 (2018).
Švubová, R. et al. Evaluation of the impact of cold atmospheric pressure plasma on soybean seed germination. Plants 10, 177 (2021).
Acknowledgements
The authors acknowledge R&D centre CEPLANT supported by MEYS CR (LM2023039) for XPS measurement and access to SEM equipment.
Funding
This work was supported by the Slovak Research and Development Agency under the Contract no. APVV-21-0147. Funded by the EU NextGenerationEU through the Recovery and Resilience Plan for Slovakia under the project No. 09I03-03-V04-00143.
Author information
Authors and Affiliations
Contributions
A.Z. and E.G. designed the experiment, P.S., A.Z. and S.D. performed all plasma treatments, P.S. measured WCA, SEM and imbibition, M.S. performed the XPS measurements, D.K., M.B. and R.S. performed all germination studies and statistical evaluation, S.K. performed comet assay and evaluated DNA damage with statistics, P.S., D.K., S.K., R.S., M.B. interpreted data and wrote the manuscript. A.Z and E.G. had oversight of the whole experiment and contributed to the revision of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Šrámková, P., Kostoláni, D., Kyzek, S. et al. Extending shelf life: cold plasma as a tool to preserve long-term germination potential of pea seeds. Sci Rep 15, 35001 (2025). https://doi.org/10.1038/s41598-025-18952-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18952-5