Abstract
Micro-stratification in the context of visceral leishmaniasis (VL) was done for the first time in Nepal. Public health interventions and resources can be targeted to the specific needs of each stratum. The micro-stratification of VL risk was carried out to identify VL risk wards (smallest administrative unit) in the country. Ward wise number of VL cases for six years (2017–2022) were obtained from the Epidemiology and Disease Control Division to determine ward wise incidence of VL per 10,000 population. VL infection risk was assessed using receptivity measured through sandfly vector presence data, temperature, altitude, housing conditions, and vulnerability measured through migration data of the district. Among 6743 wards of 77 districts, there were 58 wards in 2017, 68 in 2018, 69 in 2019, 52 in 2020, 82 in 2021, and 84 in 2022 at high risk of VL. The number of high risk wards is increasing since 2020 and most of them are located in Karnali and Sudurpaschim provinces. We recommend strengthening health care facilities in high and moderate risk areas for early diagnosis and prompt case management, implementing active surveillance to respond to any VL cases, to prioritize and intensify indoor residual spraying for vector control, and conduct health education campaigns to increase VL awareness.
Similar content being viewed by others
Introduction
In 2023, 11,922 cases of visceral leishmaniasis (VL) were reported to the World Health Organization (WHO)1 although the annual estimates are around 50,000 to 90,000 cases worldwide2. India, Bangladesh and Nepal have contributed a significant number of VL cases to the total VL cases in the world. These three countries signed a memorandum of understanding (MoU) in 2005 to eliminate VL by 2015. In 2023, 6% of VL cases were from these countries1,2. Nepal achieved the elimination target by 2013 but could not sustain it after 20163. On 31 October 2023, Bangladesh was declared by the WHO as the first country of the world to have eliminated VL as a public health problem. The country achieved the elimination target of less than one case per 10,000 population at the sub-district (upazilla) level in 2017 and has since sustained it despite disruptions caused by the COVID-19 pandemic.
The number of VL cases has decreased by 90% in Bangladesh, India and Nepal compared to 20052. In 2023, VL cases were 170 in Nepal4, 524 in India5 and around 30 in Bangladesh (verbal communication). There is a changing epidemiology of VL during the consolidation phase of the elimination initiative. In Nepal, VL cases have now spread beyond the previous endemic areas6. Previous VL endemic villages have not reported VL cases for many years but new villages appear as new foci, similar to what has been reported from Bangladesh7. There are several determinants of VL transmission. The most significant of these are: the number of infected people (with or without clinical symptoms, and of post kala-azar dermal leishmaniasis (PKDL)), and the receptivity and vulnerability characteristics of an area8.
Micro-stratification of VL refers to the identification of level of risk of wards (villages) so that VL elimination interventions can be highly targeted. Traditional stratification considered only disease burden to stratify the level of risk of villages whereas micro-stratification of VL considers three critical factors for more robust stratification of villages considering associated risk factors for VL occurrence8. A ward in Nepal is the smallest administrative unit within a municipality. It serves as a local governance unit, typically made up of a group of villages or communities. Disease burden is measured in terms of cumulative incidence of confirmed VL cases (identified by passive surveillance, thus ignoring asymptomatic cases and PKDL) per 10,000 risk population at district level in Nepal during the last five years. Receptivity (ecology) is an environment which supports the vectors, household conditions, ecological/climatic conditions, vector behavior and bionomics that define relative transmission efficiency of the vector, and the duration of transmission (vectorial capacity). Vulnerability is measured in terms of population movement.
As per VL elimination strategy, the unit for elimination of VL has been defined as the total population of the district in Nepal. Analysis of VL information throughout the years from the district do not support the view that the total populations of the district are at the same risk since VL is a focal disease and is usually found in the poorest of the poor and marginalized communities. VL micro-stratification, including the geo-referencing, would provide the level of VL risk at the municipality and/or village level. This strategic information would be useful to the National VL Elimination Program to target interventions at community level. By analyzing risk factors and creating smaller, more defined strata, public health interventions and resources can be targeted to the specific needs of each subgroup. This approach is more efficient and cost-effective than implementing blanket interventions across an entire population. It allows for the customization of strategies to address the unique challenges faced by different groups in their efforts to control and prevent visceral leishmaniasis. VL micro-stratification would provide strategic information on the total area and the actual population at risk of VL. Therefore, micro-stratification of VL was conducted to determine the risk of VL at the ward level, to identify VL hotspots in VL endemic areas and new foci, and identify VL shifting patterns over the years.
Results
Province wise VL case reported districts, 2017–2022
The VL case reporting districts during 2017–2022 were highest in number in Koshi, Lumbini, Karnali and SudurPaschim provinces. The trends of VL cases was also increasing in Karnali and SudurPaschim provinces. VL affected districts were consistently and relatively fewer in Madhesh, Bagmati and Gandaki provinces. Kalikot district of Karnali province had an annual VL incidence of above 1 per 10,000 population in 2021 (1.38) and 2022 (2.0). (Table 1).
High VL risk wards in Nepal
Among 6743 wards of 77 districts in the country, there were 58 wards at high risk of VL in 2017, 68 wards in 2018, 69 wards in 2019, 52 wards in 2020, 82 wards in 2021, and 84 wards in 2022. It seems that the high risk wards are increasing after 2020 (Table 2).
Shifting of VL from East to West
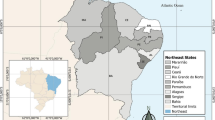
The risk Maps show that the high risk wards were distributed evenly from east to west in specific districts. However, it was found that after 2018, more high risk wards were observed towards the west, mainly in some districts of Karnali and Sudur Paschim province (Fig. 1).
VL risk wards of Nepal, 2017–2022 (The maps were generated using ArcGIS Desktop10.7.1(https://desktop.arcgis.com/). The maps were created using the base map layer data provided by the Survey Department of Nepal. The map layers (.shp files) were downloaded forfree from the Survey Department’s National Geo-Portal.(https://nationalgeoportal.gov.np/)
Province wise VL risk stratification
In Koshi province, there were 14 high risk wards in 2022 and 1118 wards in this province had a low risk of VL. The high risk wards were relatively fewer in the Covid-19 years of 2020 and higher in 2021. Although the districts of Madhesh province were more endemic for VL in the past, the recent Mapping showed that only 11 wards in 2022 and 10 wards in 2021 were at a high risk of VL. VL high risk wards in Bagmati province were only five in 2022 and four were at moderate risk. It seems that the number of high risk wards was consistently low in Bagmati province in six years compared to other provinces. VL high risk ward was only one in Gandaki province in 2022 and two in 2021. The high and moderate risk wards shifted more towards the south of the province.
In Lumbini province, VL high risk wards were 12 and 25 wards were at moderate risk in 2022. The time trend shows that this province is an emerging area of VL.
The number of VL high risk wards in Karnali province is increasing since 2017. It was found that 26 wards were at a high risk of VL in 2022 and 22 wards in 2021. Sudurpaschim province showed an increasing trend of high risk wards since 2019 as well. The high risk wards were nine in 2019, 15 in 2020 and 17 in 2021 and 15 in 2022 (Supplementary Fig. 1).
Discussion
Micro-stratification of VL in Nepal, the first of its kind in the country, was conducted using available data on morbidity, mortality, receptivity, and vulnerability. We used incidence of VL in the ward to measure the burden of the disease. This study utilized sandfly vector-related information from research conducted by the Public Health and Infectious Disease Research Center, BP Koirala Institute of Health Sciences, and ASCEND. The findings reveal significant changes in VL transmission and geographical distribution in recent years, with the disease spreading from the historically endemic Eastern and Madhesh Provinces to the Karnali and Sudur Paschim Provinces. The district Kalikot of Karnali province is constantly reporting VL incidence greater than 1 per 10,000 cases. Factors such as climate change, human movement, limited vector control measures, and the low socio-economic status of communities are believed to have contributed to this shift9,10. A study from India and Nepal to describe seasonal patterns of P. argentipes, the vector of VL, revealed that its density is positively associated with temperature and negatively associated with rainfall11. The new VL endemic areas, although located in hilly river basin, have temperature suitable for VL vector survival and breeding. Introduction of parasites to these new areas could be due to human movement to VL endemic areas thus establishing local transmission12. Although sandfly vector P. argentipes is susceptible to available insecticides13, very limited indoor residual spraying is being conducted in new VL endemic areas in Nepal. As VL continues to spread to new areas, the most affected communities are often those with limited resources to combat the disease. This highlights the urgent need for equitable public health interventions that prioritize the needs of the most at-risk populations, ensuring that efforts to control and eliminate VL are strengthened in these areas.
The rise in high-risk wards for visceral leishmaniasis (VL) in Nepal’s Karnali and Sudur Paschim provinces since 2020 can be attributed to both environmental and socio-economic factors. Further, disease prevention activities were severely affected by COVID-19 and that might fuel the spread of VL. Climate change has altered local conditions, making them more suitable for sandfly vectors, which are responsible for VL transmission. Additionally, increased human movement, especially migration from endemic areas, may have contributed to the spread of the disease. These provinces also face socio-economic challenges, including limited healthcare access, poor infrastructure, and high poverty levels, which exacerbate the risks and hinder effective disease control. The report of increased number of VL cases in Karnali and Sudur Paschim may also be attributed to a better reporting of cases, linked to greater population awareness and improved access to health services6. There is movement of people from these provinces to Himanchal Pradesh of India and they might bring infection to these places. Further, sandfly might get transported to these new areas because of recent expansion of road services facilitating the trade and travel through the vehicles. Provinces once considered low-risk have now become high-risk areas. This westward shift in VL endemicity to previously unaffected areas mirrors the pattern observed in other countries like Bangladesh and India, where VL has spread beyond traditional endemic regions7,8. An increased number of VL cases, including children, has been found in Nepal similar to the report from Kashmir, a sub-himalayan region of India14. This trend shows the indigenous transmission of VL in these new areas. The growing number of high-risk wards since 2020 despite ongoing efforts to control VL in Nepal underscores the need for continuous monitoring and adaptive control strategies.
The micro-stratification approach employed in this study points to three critical factors determining VL risk: disease burden, receptivity, and vulnerability. Disease burden was measured by VL incidence in the ward and high score was given for it as compared to other determinants. Receptivity, influenced by the presence of vectors and favorable environmental conditions such as temperature and altitude, plays a key role in sustaining VL transmission. Vulnerability driven by population movement and receptivity such as poor housing conditions, further exacerbates the risk of transmission. In this micro-stratification, more weight has been given to VL cases and the historical presence of vector in the region. VL case as a source of parasite is a necessary cause, and sandfly presence a sufficient cause for VL occurrence. Other risk factors are distant causes in increasing risk of VL and were given a lower weight. A similar approach has been used for micro-stratification of malaria and other vector borne diseases15,16,18. Stratifying VL risk wards based on these determinants allows for a more targeted and effective allocation of public health resources17,18. It can be utilized for intensifying elimination interventions in high-risk wards to achieve elimination targets at country level.
The implications for public health interventions are significant. Identifying high-risk wards enables targeted efforts such as strengthening healthcare facilities for early diagnosis, enhancing surveillance systems, implementing vector control measures, and conducting community awareness campaigns. This study suggests that localized strategies, informed by micro-stratification, may be more effective than blanket interventions across the entire population.
In alignment with the findings from the micro-stratification study, the publication by Baker et al. (2022) discusses the profound impact of climate change on the transmission and spread of infectious diseases, particularly vector-borne diseases (VBDs) such as VL19. As global temperatures rise, vectors like sandflies—responsible for transmitting VL—are expanding their geographical ranges and becoming active for longer periods6,20. This shift is particularly relevant to Nepal, where the micro-stratification of VL reveals changing epidemiology and the spread of the disease to new areas, notably in the Karnali and Sudur Paschim Provinces. Thomson and Stanberry (2022) highlight that climate change affects not only the vectors but also the ecosystems and human behaviors that influence disease transmission21. Increased temperatures and changing precipitation patterns create favorable conditions for vector breeding, particularly in areas previously considered low-risk for VL. In Nepal, these climatic changes22, combined with factors like human movement23 and inadequate vector control measures, have contributed to the westward shift in VL endemicity. The insights gained from the micro-stratification of VL in Nepal are vital for guiding targeted interventions and adapting public health strategies to the realities of a changing climate22,24,25.
Finally, the study raises concerns about the sustainability of VL elimination efforts in Nepal. Although the country achieved its elimination target by 2013, the re-emergence of high-risk wards after 2020 highlights ongoing challenges. Comparisons with Bangladesh, which has successfully maintained its elimination status, underline the importance of robust health systems, continuous surveillance, and community engagement. Nepal can learn from these experiences to strengthen its VL elimination efforts and address the evolving challenges posed by the disease.
There are notable strengths of this VL micro-stratification study. The study used important determinants of VL in micro-stratification and was able to show shifting of disease from east to west and south to north. The ward level information is useful to design specific and focused interventions for the hotspots. There are however some limitations. We used administrative boundaries for risk stratification, rather than geographical proximity of the wards. Data on climatic factors and vulnerability were not available at the ward level and we used assumptions for scoring these variables at ward level. Similarly, we used limited available survey data and projected for presence of vectors based on altitude of the wards. In addition, only the data on cases has been analyzed over time; data on altitude, vector, housing condition and vulnerability have been considered as constant over the years.
In conclusion, to address the existing challenges, it is essential to adopt a comprehensive approach that includes strengthening healthcare facilities in high and moderate-risk areas for prompt and effective diagnosis and case management. Enhanced surveillance systems are crucial for early detection and response to new VL cases, ensuring that the spread of the disease is promptly managed. Additionally, targeted vector control measures, informed by the micro-stratification findings, are necessary to reduce the presence of sandfly vectors in the most affected areas. The insights gained from the micro-stratification study provide a valuable framework for guiding targeted interventions and ensuring that resources are effectively allocated to areas where they are most needed. By aligning local strategies with broader climate and health considerations, Nepal can strengthen its capacity to manage and ultimately eliminate VL, despite the ongoing challenges posed by environmental and socio-economic changes.
Methods
Study design
This micro-stratification exercise was based on scoring given to pre-determined risk factors, combined together to give a final VL risk score. The assessment was based on secondary data available at the Epidemiology and Disease Control Division (EDCD) Nepal. VL burden data at ward/village level in the country, sandfly vector data at village level, migration history data available from line listing of VL cases and community collective housing characteristics were used for micro-stratification. Entomological data were collected from different research and academic institutions (see below). Stratification was done at the ward level within the district and the total score of VL risk was categorized as high, moderate and low endemic wards.
Questionnaire/checklist design
The study used forms specifically developed for collection of secondary data relevant to this study. Basically, ward (village) level information was collected using a format which had two parts. The first part contained demographic, geo-ecological, meteorological, socio-economic, and entomological information whereas the second part contained VL disease, diagnosis and treatment, severity/death, and containment information including vector control. Technical consultation meetings were organized with the Epidemiology and Disease Control Division (EDCD), WHO Country Office and other stakeholders to review the draft forms which were finalized incorporating their feedback. The checklist was reviewed by a technical working group (TWG) of VL experts and programme managers.
Study unit/mapping unit
Each ward in the municipality of the districts was included for stratification as high, moderate and low VL risk areas. This study defined the wards in Nepal as the unit for micro-stratification because this is the basic administrative unit of communities in Nepal. Ward is the smallest unit of local government of Nepal and administrative division of a municipality. Ward is used for providing basic health and administrative services, electoral purposes where the residents of each ward elect a representative to serve the local communities.
Collection of VL burden data
The study took place in 2023. Village wise VL mortality and morbidity data of all the districts were collected for six years (2017–2022) from the Epidemiology and Disease Control Division (EDCD). Missing information in datasets were completed through confirmation with the District Health Office and the reporting hospital. District and ward wise VL data were cross-checked and verified with HMIS and Early Warning and Reporting System (EWARS) data (Table 3). Ward wise incidence of VL per year was calculated per 10,000 population.
Housing, migration and environmental data
The housing characteristics of the wards of the districts published in 202126 was used for scoring (data available at ward level). Information on migration patterns was extracted from the International Organization for Migrants Migration Report, 2021. Information on climate and environmental situation of the villages was obtained from the Department of Meteorology (Table 3).
Data on vectors from entomological studies
Data on entomology were compiled from previously conducted entomological surveys in VL reported wards of 48 districts by Public Health and Infectious Disease Research Center, BP Koirala Institute of Health Sciences, and ASCEND Nepal during 2017–2023. The villages where the vector surveys were conducted employed CDC light traps and manual aspiration for collection of sandflies in and around six houses of index case household. P. argentipes sandflies were identified and Leishmania donovani present in the sandflies recorded. These data were used for receptivity calculation (Table 3). All of the VL cases reported wards were covered for sandfly surveillance. If the ward felt within the range of altitude where historically it had been reported P. argentipes vector, that was considered as positive for vector.
Data management and analysis
Line listing of the cases was reviewed, and cross checked against the VL cases register. Only cross-checked data, after data verification with line listings, were utilized for micro-stratification. The study used the software PostgreSQL for database management, Microsoft Excel for data cleaning/analysis/manipulation, ArcGIS 10.7 Prerelease for map generation and Microsoft PowerBI for data visualization.
The VL risk stratification has taken into account several key determinants of VL transmission. The determinants/variables have been given weights to stratify VL risk. Mapping was done at the ward level. Scores were assigned following the meeting of experts and programme managers held at the Epidemiology and Disease Control Division (Table 4). Scoring for malaria micro-stratification in Nepal was also taken as a reference.
Disease burden
For disease burden, 0.5 score was given, if ward had VL incidence > 0.5 per 10,000 population, 0.3 score was given if ward had VL incidence < 0.5, and zero score was given to wards if there were no VL cases.
Receptivity
The total score assigned for receptivity was 0.4.
The assigned weight for altitude was 0.010 and the score was assigned at ward level from 64 to 5884 m. An altitude ≤ 2000 m was considered a high risk level and was assigned the score of 0.010, altitude > 2000 and ≤ 2500 m was considered moderate risk level and assigned the score of 0.005, altitude > 2500 and ≤ 4000 m was considered low risk level and assigned the score of 0.0025 and altitude > 4000 m was considered no risk level and assigned the score of zero.
Similarly, VL cases were reported from wards with a temperature range of 15.3 to 36.3 °C. The total assigned score for temperature was 0.010. Temperature of ≥ 18 and ≤ 35 0 C was considered high risk level and assigned a score of 0.010, ≥ 16 and < 18 0 C was considered moderate risk level and assigned a score of 0.005, > 35 0 C was considered low risk level and assigned a score of 0.0025, and temperature of < 16 0 C was considered no risk and assigned a score of zero.
For housing structure data, “Number of households by type of materials used for outer walls of housing unit, NPHC 2021” was applied and further processed. This data contains eight categories of houses based on the type of materials of outer walls: Mud bonded bricks/stone, cement bonded bricks/stone, wood/planks, bamboo, unbaked bricks, galvanized sheet, prefabricated sheet, and other. For the purpose of this study, re-classification of housing structure was done primarily into two categories based on the previous research26. The new categories were modified as follows:
Housing structure | NPHC categories |
|---|---|
Permanent | 1. Cement bonded bricks/stone 2. Prefabricated sheet |
Temporary | 1. Mud bonded bricks/stone 2. Wood/planks 3. Bamboo 4. Unbaked bricks 5. Galvanized sheet 6. Other |
The analysis of the housing structure was done at the ward level and the total assigned score was 0.055. If the ratio of temporary housing structure to permanent housing structure in a ward was greater than one, it was considered at high risk and a score of 0.055 was assigned. If the ratio of temporary housing structure to permanent housing structure in a ward was less than or equal to one, it was considered low risk level and the score of 0.0275 was assigned.
The assigned score for historical vector presence at ward level or in ward of similar altitude was 0.325, if absent it scored zero.
Vulnerability
Based on the mobility of the people in the ward, a vulnerability score of 0.1 was assigned at ward level. It has been assumed that all 6743 wards are vulnerable to VL infection, thus 0.1 was assigned to all 6743 wards.
Total score of VL risk
The total score of VL risk was calculated as the sum of scores for each parameter. It was calculated for each ward and the total score of VL risk was categorized as > 0.66 as high risk for VL, ≤ 0.66 to > 0.33 as moderate risk and < 0.33 as low risk.
Data availability
“All data generated or analysed during this study are included in this manuscript and its Supplementary Information files.”
References
World Health Organization. Global leishmaniasis surveillance updates 2023. Weekly epidemiological record 99, 653–676. (2024). Available at: http://www.who.int/wer
World Health Organization. Leishmaniasis. (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
Banjara, M. R. & Joshi, A. B. Evidence for visceral leishmaniasis elimination in Nepal. Lancet Glob Health. 8 (2), e161–e162. https://doi.org/10.1016/S2214-109X(19)30538-8 (2020).
Department of Health Services, Nepal. Annual Health Report. Kathmandu, Nepal: Department of Health Services, Ministry of Health and Population (2024). (2079)/80.
National Center for Vector Borne Diseases Control, India. Kala-azar situation in India, 2014–2024. Available at: https://ncvbdc.mohfw.gov.in/index4.php?lang=1&level=0&linkid=467&lid=3750
Banjara, M. R. et al. Response to visceral leishmaniasis cases through active case detection and vector control in low-endemic hilly districts of Nepal. Am. J. Trop. Med. Hyg. 107 (2), 349–354. https://doi.org/10.4269/ajtmh.21-0766 (2022).
Dewan, A., Abdullah, A. Y. M., Shogib, M. R. I., Karim, R. & Rahman, M. M. Exploring Spatial and Temporal patterns of visceral leishmaniasis in endemic areas of Bangladesh. Trop. Med. Health. 45, 29. https://doi.org/10.1186/s41182-017-0069-2 (2017).
Picado, A. et al. Risk factors for visceral leishmaniasis and asymptomatic Leishmania donovani infection in India and Nepal. PLoS One. 9 (1). https://doi.org/10.1371/journal.pone.0087641 (2014). e87641.
Yapabandara, M. A. et al. Evidence-based malaria control in Timor Leste from 2006 to 2012. Malar. J. 14, 109. https://doi.org/10.1186/s12936-015-0614-6 (2015).
van den Berg, H. et al. Operational efficiency and sustainability of vector control of malaria and dengue: descriptive case studies from the Philippines. Malar. J. 11, 269. https://doi.org/10.1186/1475-2875-11-269 (2012).
Picado, A. et al. Phlebotomus argentipes seasonal patterns in India and Nepal. J. Med. Entomol. 47 (2), 283–286. https://doi.org/10.1603/me09175 (2010).
Martschew, E. et al. Visceral leishmaniasis in new foci areas of nepal: sources and extent of infection. J. Vector Borne Dis. 60 (4), 414–420. https://doi.org/10.4103/0972-9062.383637 (2023).
Chowdhury, R. et al. Susceptibility of field-collected Phlebotomus argentipes (Diptera: Psychodidae) sand flies from Bangladesh and Nepal to different insecticides. Parasites Vectors. 11 (1), 336. https://doi.org/10.1186/s13071-018-2913-6 (2018).
Basu, A. et al. Emerging pediatric visceral leishmaniasis in Kashmir valley: A report of three cases. Trop. Doct. 53 (2), 317–318. https://doi.org/10.1177/00494755231153244 (2023).
Djaskano, M. I. et al. Stratification and adaptation of malaria control interventions in Chad. Trop. Med. Infect. Dis. 8 (9), 450. https://doi.org/10.3390/tropicalmed8090450 (2023).
Ateba, F. F. et al. Spatio-temporal dynamics of malaria incidence: A comparison of two ecological zones in Mali. Int. J. Environ. Res. Public. Health. 17, 4698. https://doi.org/10.3390/ijerph17134698 (2020).
Okorie, P. N. et al. Lymphatic filariasis in nigeria; micro-stratification overlap mapping (MOM) as a prerequisite for cost-effective resource utilization in control and surveillance. PLoS Negl. Trop. Dis. 7 (9), e2416. https://doi.org/10.1371/journal.pntd.0002416 (2013).
Thawer, S. G. et al. Spatio-temporal modelling of routine health facility data for malaria risk micro-stratification in Mainland Tanzania. Sci. Rep. 13 (1), 10600. https://doi.org/10.1038/s41598-023-37669-x (2023).
Baker, R. E. et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 20 (4), 193–205. https://doi.org/10.1038/s41579-021-00639-z (2022).
Dhimal, M. et al. Climate change and its association with the expansion of vectors and vector borne diseases in the Hindu Kush Himalayan region: A systematic synthesis of the literature. Adv. Clim. Change Res. 12 (3), 421–429. https://doi.org/10.1016/j.accre.2021.05.003 (2021).
Thomson, M. C. & Stanberry, L. R. Climate change and vector borne diseases. N Engl. J. Med. 387 (21), 1969–1978. https://doi.org/10.1056/NEJMra2200092 (2022).
Wilson, A. L. et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 14 (1), e0007831. https://doi.org/10.1371/journal.pntd.0007831 (2020).
Watts, N. et al. The 2020 report of the lancet countdown on health and climate change: responding to converging crises. Lancet 397, 129–170. https://doi.org/10.1016/S0140-6736(20)32290-X (2021).
Caminade, C., McIntyre, K. M. & Jones, A. E. Impact of recent and future climate change on vector-borne diseases. Ann. N Y Acad. Sci. 1436, 157–173. https://doi.org/10.1111/nyas.13950 (2019).
Ma, J. et al. Climate change drives the transmission and spread of vector-borne diseases: an ecological perspective. Biology (Basel). 11 (11), 1628. https://doi.org/10.3390/biology11111628 (2022).
National Statistical Office. Housing and Household Dynamics in Nepal. National Population and Housing Census 2021 (National Statistical Office, 2024).
Acknowledgements
“This investigation received financial support from the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR - www.who.int/tdr), project P22-00851. We would like to acknowledge Epidemiology and Disease Control Division, Department of Health Services Nepal for providing necessary data on VL and discussion on scoring of variables.”
Author information
Authors and Affiliations
Contributions
“M.R.B., A.K., A.A., A.B.J. designed the study. A.B.J., M.R.B., U.R.P., G.D., K.R.P. collected data. A.B.J., M.R.B., G.R.P., D.J. analyzed data. M.R.B., A.B.J., C.H., A.K., A.A. drafted the manuscript. M.R.B., C.H., A.B.J., A.K., A.A. edited and finalized the manuscript. All authors have reviewed and approved the manuscript.”
Corresponding author
Ethics declarations
Competing interests
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated. The authors declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Banjara, M.R., Paneru, G.R., Joshi, D. et al. Utility of microstratification to identify hotspots for visceral leishmaniasis in Nepal. Sci Rep 15, 35204 (2025). https://doi.org/10.1038/s41598-025-19038-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19038-y