Abstract
Cancer-related fatigue (CRF) is a multifaceted and subjective phenomenon that significantly impacts patients on physical, emotional, and mental levels. This study aims to identify specific subtypes of Cancer-related fatigue (CRF) in patients with Hepatocellular Carcinoma (HCC) and to explore the factors influencing each subtype. This cross-sectional study enrolled 220 participants from a tertiary cancer hospital. Latent Profile Analysis (LPA) and multinomial logistic regression were conducted to identify distinct fatigue profiles and to explore the influencing factors for different categories of CRF among the patients. The analysis revealed three potential categories of CRF severity: Physical balance -Low fatigue (20.1%); Physical imbalance -Moderate fatigue (69.6%); and Physical prominent -High fatigue (10.2%). It was found that the severe insomnia the greater the probability of patients belonging to the Physical prominent -High fatigue (OR = 1.299, 95%CI: 1.188–1.419). Has partner (OR = 5.171, 95%CI: 1.739–15.377), the severe financial stress (OR = 2.570, 95%CI: 1.209–5.463) and the moderate ISI (OR = 1.212, 95%CI: 1.136–1.292) were associated with the Physical imbalance - Moderate fatigue group. Protective factors for the Physical balance - Low fatigue group included higher scores in the physical activity Index (OR = 0.930, 95%CI: 0.870–0.995), Hope Index (OR = 0.647, 95%CI: 0.552–0.758), General self-efficacy (OR = 0.874, 95%CI: 0.793–0.965), Body Mass Index (OR = 0.799, 95%CI: 0.552–0.758), and Child-Pugh A classification (OR = 0.310, 95%CI: 0.119–0.808). CRF in patients with HCC demonstrates significant heterogeneity. It is conducive to the clinical identification of CRF risk characteristics and the design of personalized intervention measures.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) ranks among the most prevalent Malignancies of the digestive system, with its incidence rate persistently increasing. Projections indicate that by 2040, liver cancer will become the third leading cause of cancer-related mortality1,2. The Majority of patients receive a diagnosis during the intermediate to advanced stages of the disease, with conventional treatment yielding a median survival duration of approximately 10 months3. The integration of interventional therapies with systemic treatments, including radiotherapy, chemotherapy, targeted therapy, and immunotherapy, constitutes a pivotal focus and prospective direction in the treatment strategies of patients with advanced liver cancer4. As defined by the National Comprehensive Cancer Network (NCCN), cancer-related fatigue (CRF) is “a multifactorial syndrome characterized by a distressing, persistent sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment”5. Current analyses reveal an overall pooled prevalence of CRF at 52%, with 21–52% of survivors continuing to experience CRF for up to three years following diagnosis6,7,8. CRF significantly diminishes the quality of life for patients9,10 and is linked to increased mortality rates among cancer patients11. In the context of HCC, the severity and duration of CRF are exacerbated due to the specific characteristics of liver cancer and the complexity of its treatment regimens12,13.
Therefore, understanding the risk factors associated with CRF is crucial for healthcare professionals to develop effective interventions and management strategies for affected patients. Previous research has identified several factors contributing to CRF, including chemoradiotherapy, female sex, pain, insomnia, psychological symptoms, perceived social support, and physical activity (PA)7,14,15,16. It is important to note that the majority of studies have concentrated on cancers with lower tumor invasiveness and symptom burden, such as breast and prostate cancer17,18, while more aggressive cancers with shorter survival times, such as pancreatic and advanced liver cancer, have received less attention. However, most prior studies have focused on the whole population, assessing the severity of CRF symptoms by categorizing total scores from relevant scales into distinct critical values to determine individual CRF levels. Empirical research indicates that fatigue characteristics are not merely dichotomous (present or absent)19; even individuals classified similarly in terms of CRF may exhibit qualitative differences. This suggests that individuals with the same CRF level might differ in their responses to specific measurement tools. Consequently, existing research findings may not provide precise interventions for each patient, lacking specificity in guiding clinical individualized practice. This shortcoming could lead to suboptimal intervention outcomes and potentially result in significant wastage of medical resources.
Recently, Latent Profile Analysis (LPA) has gained prominence as an individual-centered statistical method in latent variable modeling. LPA facilitates the homogeneous grouping of continuous data by segmenting individuals with similar symptoms into subgroups, allowing for more nuanced analysis of these distinct subgroups. It aims to reveal the intricate associations between external continuous variables through latent class variables. LPA provides a more objective, rational, and precise methodology for clustering, with extensive applications across various domains, including the identification of symptom clusters in oncology20,21. Similarly, LPA has been applied in behavioral research to identify differentiated intervention effects, offering a person-centered approach that enhances the interpretability of subgroup analyses and improves the precision of intervention strategies22.
The aim of our study was to investigate the distinct profiles of CRF experienced by patients with liver cancer undergoing interventional therapy. We sought to identify the characteristics and differences among patients in various groups and to assess how these subgroups differ demographically in clinical characteristics and modifiable factors such as PA, hope index, general self-efficacy, and insomnia.
Methods
This research employs a cross-sectional study design and rigorously adheres to the ethical standards outlined in the Declaration of Helsinki, following the ethical principles for medical research involving human subjects as established by the World Medical Association. The study protocol received approval from the Ethics Committee for Clinical Trials of Hunan Cancer Hospital (Approval No. SBQLL-2021-174) and complies with the guidelines for strengthening the reporting of observational studies in epidemiology.
Participants
We purposive sampling to recruit HCC patients from the Department of Tumor Intervention at a tertiary cancer hospital in Hunan Province between April 2022 and December 2022. A trained research nurse conducted the screening and enrollment of patients based on predefined inclusion and exclusion criteria. Prior to participation, all patients received a study information leaflet detailing the research’s purpose and procedures, and written informed consent was obtained from each participant.
Inclusion criteria were as follows: (1) pathologically confirmed diagnosis of HCC based on histopathological examination of tissue obtained via biopsy or surgical resection. Diagnosis was established by expert hepato-pathologists according to WHO criteria, relying on characteristic architectural (e.g., trabecular, pseudo-glandular) and cytological features (e.g., bile production, cytological atypia). Immunohistochemical staining (including GPC3, HSP70, Glutamine Synthetase, and Arg-1) was utilized in diagnostically challenging cases; (2) advanced HCC with no eligibility for surgical intervention; (3) currently undergoing interventional therapy or scheduled to undergo interventional therapy following consultation with the medical team; (4) age ≥ 18 years; and (5) conscious and capable of oral communication.
Exclusion criteria included: (1) a history of other malignant tumors or serious complications, such as active malignancies, severe cardiac arrhythmias, or systemic infections; and (2) a history of psychiatric disorders.
Sample size
Based on correlation analysis in cross-sectional studies, we estimated the required sample size as 10–20 times the number of variables. The study included 20 independent variables, and we expanded the calculated sample size by 20% to control for potential missing data, resulting in 250 participants as the minimum number required.
Patient-reported assessments
Following the participants’ admission, we approached and invited those who met the eligibility criteria to participate in this study. All participants were thoroughly informed about the study’s objectives, as well as any potential benefits or risks involved. A research assistant, who had undergone training in data collection techniques, administered paper-based questionnaires within the hospital setting and provided assistance to participants who encountered difficulties completing the survey independently. Upon completion of the questionnaires by the patients, the investigator meticulously reviewed the responses to ensure all items were properly completed and that no data were missing.
Measures
Cancer-related fatigue
The Cancer Fatigue Scale (CFS), developed by Okuyama in 200023, was utilized to assess cancer-related fatigue (CRF) symptoms in patients with cancer. The scale consists of 15 items divided into three subscales: physical (7 items, total score of 28 points), cognitive (4 items, total score of 16 points), and emotional (4 items, total score of 16 points). Each item is rated on a 5-point Likert scale ranging from “nothing at all” to “very much,” with scores from 0 to 4, resulting in a total score range of 0 to 60. Higher scores indicate greater levels of fatigue, and a total CFS score of 18 or above is classified as “clinical fatigue”. Cronbach’s alpha coefficients for this scale were 0.87 (physical), 0.81 (affective), 0.76 (cognitive), and 0.84 (total), In this study, the CFS exhibited satisfactory internal consistency, with a Cronbach’s alpha of 0.759.
Godin leisure-time exercise questionnaire (GLTEQ)
The Physical Activity Index (PA-Index) was evaluated utilizing the Leisure Score Index derived from the Godin-Leisure Time Exercise Questionnaire (GLTEQ), a tool extensively employed among cancer patients24. The GLTEQ assesses the frequency of leisure-time physical activities over the past week, categorized into vigorous (characterized by a rapid increase in heart rate, such as vigorous swimming and playing basketball), moderate (characterized by a slight increase in heart rate, such as brisk walking and square dancing), and light activities (characterized by normal heart rates, such as walking, yoga, and Tai Chi). The PA-Index, which quantifies the total volume of exercise, is computed using the formula: PA-Index = (9 × frequency of vigorous exercise/week) + (5 × frequency of moderate-intensity exercise/week) + (3 × frequency of light-intensity exercise/week). Patients are classified as active (PA- Index ≥ 24) or insufficiently active (PA- Index < 24) according to the 2010 release of the American College of Sports Medicine Exercise Guidelines for patients with cancer25.
Herth hope index (HHI) scale
This study employed the Chinese version of the Herth Hope Index (HHI) scale, revised by Zhao26, originally developed by Herth27. The questionnaire comprises 12 items distributed across three dimensions: positive attitudes toward reality and the future (4 items), positive actions (4 items), and close relationships with others (4 items). Each item is rated on a four-point Likert scale, with scores ranging from 1 to 4, corresponding to “strongly disagree,” “disagree,” “agree,” and “strongly agree,” respectively. Higher scores on this scale are indicative of elevated levels of hope, with scores ranging from 12 to 23 representing low levels of hope, 24 to 35 indicating moderate levels, and 36 to 48 signifying high levels. The overall Cronbach’s coefficient for the scale is 0.969, and 0.842 for this sample.
General self-efficacy (GSES)
The General Self-Efficacy Scale (GSES), originally developed by Schwarzer et al. and subsequently translated and revised by Wang et al.28,29, is a unidimensional instrument comprising 10 items evaluated on a 4-point Likert scale: 1 (completely wrong), 2 (basically right), 3 (almost right), and 4 (absolutely right). Higher scores on this scale reflect greater general self-efficacy. In this study, the GSES exhibited satisfactory internal consistency, with a Cronbach’s alpha of 0.863.
The insomnia severity index (ISI)
The Insomnia Severity Index (ISI) is a scale designed to assess insomnia symptoms, consisting of 7 items with a total score range of 0 to 28. This scale is both reliable and sensitive to clinical patient responses30. According to the results, a score of 0–7 indicates no clinically significant insomnia, 8–14 suggests mild insomnia, 15–21 denotes moderate insomnia, and 22–28 indicates severe insomnia. For this sample, the Cronbach’s alpha coefficient was 0.767.
Sociodemographic, clinical variables, health-related and behavioral factors
Sociodemographic characteristics were systematically documented, encompassing variables such as age, gender, educational attainment, marital status, social support, and economic stress. Disease-related data included parameters such as disease duration, stage, treatment modality, presence of comorbidities, liver function score, pain levels, and hemoglobin concentration. Health-related factors were assessed through Body Mass Index (BMI) and the prognostic nutritional index (PNI, PNI = serum albumin (g/L) + 5× lymphocyte count (×109/L)).
Statistical methods
Latent profile analysis
Mplus 8.3 software was employed to construct a latent profile model of CRF levels in patients with HCC, utilizing the three-dimensional scores of the CFS as observed indicators. Profiles ranging from one to five were systematically selected for analysis. The model’s optimal categorization was determined using a combination of the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), sample size-adjusted BIC (aBIC), Lo-Mendell-Rubin test (LMRT), Bootstrapped Likelihood Ratio Test (BLRT), and entropy metrics. A higher entropy value indicates greater classification accuracy of the model, with an entropy value of ≥ 0.8 corresponding to a classification accuracy exceeding 90%. A significant BLRT and LMRT (P <.05) suggests that the model represents a substantial improvement over the preceding model.
Single factor and multi-factor analysis
Upon determining the optimal latent profile model, version 4.4.2 of the R software was utilized to compare demographic and psychosocial factors among HCC patients across different categories. The continuous variables in this study did not conform to a normal distribution and were therefore described using the median and interquartile range [M (Q₁, Q₃)]. Group comparisons were conducted using the Kruskal-Wallis H test. Categorical data were presented as frequencies and proportions, with group comparisons performed using either the chi-squared test or Fisher’s exact test. To explore the influencing factors for different latent categories of CRF in advanced HCC patients, unordered multinomial logistic regression was conducted using SPSS version 26.0. This analysis considered the CRF latent categories as the dependent variable and factors showing significant differences in the univariate analysis as independent variables. Statistical significance was determined at a p-value of less than 0.05. In this study, missing values were < 5%, and missing data were treated as a new category.
Results
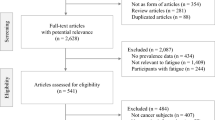
A total of 275 eligible patients with HCC cancer, 35 patients declined participation, 12 patients discontinued filling out the questionnaire midway, and 8 patients selected the same option for all questionnaire items. Consequently, 220 patients were ultimately included in the data analysis (effective response rate of 80%). The average score of CFS was 31.30 (SD = 6.160, range 6–57, 95% CI: 30.20–32.40), with a prevalence rate of 94.5% (208/220) for clinical fatigue (CFS score ≥ 18). The PA-index of the participants was 9 (0, 15), with the highest being 63, significantly correlated with CRF (r =.214 p =.002). The sociodemographic and clinical characteristics of the patients in this study are provided in Supplementary File, Table S1.
Analysis of latent fatigue level profiles in HCC patients
The three dimensions of the CFS scale-emotional, somatic, and cognitive-were employed as explicit indicators, encompassing a total of 1 to 5 categorical models. Ultimately, the 3-profile model was identified as the optimal fit (Table 1). Although both the 3-profile and 4-profile models exhibited relatively low values for AIC, BIC, and aBIC, a comparative analysis revealed that the minimum class probability in the 4-profile model was 2.6% (< 10%). Conversely, the BLRT and MLRT test for the 3-profile model was statistically significant, with an entropy value exceeding 0.8, thereby indicating the superiority of the 3-profile model over the 4-profile model. The three dimensions for the three latent fatigue level categories are depicted in Fig. 1.
In summary, patients in the first category (n = 43; 20.1%) demonstrated a low level of fatigue with more balanced three-dimensional scores, which we term the “Physical Balance - Low Fatigue” group. Patients in the second category (n = 22; 10.2%) exhibited elevated scores, with physical fatigue significantly higher than other dimensions, defined as the “Physical Prominent - High Fatigue” group. Patients in the third category (n = 155; 69.6%) had scores between the first and second categories, with physical fatigue scores higher than other dimensions, termed the “Physical Imbalance - Moderate Fatigue” group (Fig. 1).
Characteristics of the different classes
The results of the single-factor analysis revealed significant differences in marital status (p <.05), economic burden (p <.05), Child–Pugh score (p <.001), cancer staging (BCLC) (p <.05), pain (p <.05), PA–Index (p <.001), HHI (p <.001), GSEG (p <.001), ISI (p <.001), BMI (p <.001), and PNI (p <.05). The first group exhibited the highest levels of PA–Index 15 (9, 21), HHI 37 (34, 41), GSES 31 (29, 38), and BMI 24.44 (22.36, 25.45). The second group showed the highest levels of ISI 17 (10.50, 20.75) (Table 2).
Influencing factors of CRF level in different groups of patients
In this study, three potential profiles were utilized as dependent variables, with “Physical balance - Low fatigue” serving as the reference group. Independent variables were selected based on their statistical significance in univariate analysis. The results of the parallel line test, with a P-value of 0.003, indicated that the conditions for employing an ordered logistic regression model (P >.05) were not met. Consequently, a multinomial logistic regression model was chosen for multivariate analysis. This model inherently compares categorical data, eliminating the need for dummy variables31. The results of the multivariate analysis indicated that severe ISI was significantly associated with an increased probability of patients being categorized under the “Physical prominent - High fatigue” profile (OR = 1.299, 95% CI: 1.188–1.419). Additionally, having a partner (OR = 5.171, 95% CI: 1.739–15.377), experiencing severe financial stress (OR = 2.570, 95% CI: 1.209–5.463), and a moderate ISI (OR = 1.212, 95% CI: 1.136–1.292) were linked to a higher probability of patients falling into the “Physical imbalance - Moderate fatigue” category. Conversely, a higher PA-Index (OR = 0.930, 95% CI: 0.870–0.995), greater GSES scores (OR = 0.874, 95% CI: 0.793–0.965), higher Herth Hope Index (HHI) scores (OR = 0.647, 95% CI: 0.552–0.758), increased BMI (OR = 0.799, 95% CI: 0.552–0.758), and a Child-Pugh A classification for liver function (OR = 0.310, 95% CI: 0.119–0.808) were associated with a higher probability of patients being classified under the “Physical balance - Low fatigue” category (Table 3).
Discussion
To the best of our knowledge, this study was the first to utilize LPA to identify subgroups of CRF in patients with HCC during the peri-interventional period. We identified three distinct subgroups of CRF based on the three dimensions of the CFS: emotional, somatic, and cognitive. The characteristics of patients within these subgroups were documented. These findings supported the application of person-oriented methodologies in symptomatology research.
Our study revealed that CRF was highly prevalent among advanced HCC patients during the peri-interventional period, with a mean CFS score of 31.3 and a clinical fatigue incidence rate of 94.5%. The fatigue scores observed in this sample align with those reported by Yang XM in HCC patients undergoing transcatheter arterial embolization12. Notably, the incidence and severity of CRF in our study exceed those reported in colorectal and breast cancer studies, where clinical fatigue incidences were 69.4% and 43%, respectively32,33. This discrepancy may be attributed to the fact that most liver cancer patients present at advanced stages, and the cumulative side effects of combination therapies contribute to a substantial symptom burden. Secondly, faced with the reality of short survival, the psychological burden of patients is heavier than that of colorectal and breast cancers. In conclusion, given the high prevalence and severity of per-interventional CRF in HCC patients, there is a critical need for healthcare providers to enhance the assessment and management of CRF.
Three distinct subtypes of CRF were identified through LPA: Physical balance - Low fatigue (20.1%), Physical imbalance- Moderate fatigue (69.6%), and Physical prominence- High fatigue (10.2%). Consistent with the findings of a meta-analysis, the prevalence of moderate CRF was higher than that of mild or severe CRF7. This classification underscores the heterogeneity of CRF among patients with HCC, particularly highlighting the predominance of physical fatigue across all subtypes. This observation aligns with previous research, which has consistently reported the highest scores in the physical fatigue dimension33,34,35. Several factors contribute to this phenomenon: firstly, loss of appetite, the main clinical manifestations, exacerbated by the combined effects of chemotherapy and immune-targeted therapies, leads to inadequate nutritional intake; secondly, the liver’s critical role in the metabolism of most substances, coupled with limitations in glycogen storage and protein synthesis, insufficient energy supply of the organism results in diminished physical function in HCC patients. Thirdly, impaired liver function may directly exert neurotoxic effects through the imbalance of blood ammonia metabolism and indirectly affect the fatigue symptoms of liver cancer patients by influencing other metabolic pathways. For instance, blood ammonia inhibits the tricarboxylic acid cycle (TCA) and Adenosine triphosphate synthesis in the brain, leading to insufficient central energy supply and a decrease in exercise tolerance, thereby exacerbating fatigue36. Consequently, physical fatigue in HCC patients may manifest earlier and more intensely than cognitive and emotional fatigue dimensions. It is suggested that we should recognize the difference of nursing needs in different categories of liver cancer patients, pay attention to their physical management, and formulate targeted nursing intervention plans.
Although the “Physical prominent - High fatigue” subtype represented the smallest proportion of patients (10.2%), the severity of fatigue in this group was significantly greater than in other subtypes. This subtype also exhibited more pronounced insomnia symptoms, with an odds ratio of 1.299. The relationship between CRF and insomnia is intricate and multifaceted, as several studies have documented their co-occurrence and potential shared mechanisms, indicating a strong association between CRF and insomnia symptoms7,15,37. It is suggested that patients with severe insomnia may experience heightened fatigue, and thus, addressing insomnia should be prioritized to alleviate fatigue. Cognitive behavioral Therapy (CBT-I) has the strongest evidence base among psychotherapeutic interventions for improving insomnia in patients with cancer38,39. CBT-I can significantly improve sleep efficiency, reduce the time to fall asleep and the number of nocturnal awakenings40,41. Unlike pharmacological options, CBT-I has no known adverse interactions with HCC treatments (e.g., sorafenib, immunotherapy) and avoids the risk of hepatotoxicity, which is a critical consideration for this patient population with compromised liver function. Given the shared mechanisms of insomnia across cancer types, and safety of CBT-I in oncology settings, CBT-I represents a promising, non-pharmacological intervention worthy of future investigation in targeted RCTs for HCC patients. Until such population-specific evidence is available, CBT-I may be considered a rational clinical option.
For the “Physical balance - Low fatigue” subtype, higher scores in the PA-Index, HHI, GSES, BMI, and Child-Pugh A classification were identified as protective factors. This implies that increased fatigue levels are associated with reduced PA, poorer liver function, diminished hope, and lower self-efficacy. According to the American College of Sports Medicine guidelines for exercise in cancer, published in 2019, engaging in moderate-intensity aerobic or resistance training, or a combination thereof, can significantly reduce CRF42. It is worth noting that GLTEQ only focuses on sports activities during leisure time and does not include those related to career, family or transportation. This limitation may cause the associations observed in this study to be underestimated or overestimated: (1) If a patient consumes a large amount of physical strength in daily occupational or household activities (such as engaging in physical labor or standing for long periods of time), GLTEQ will systematically underestimate the total activity level. These “unmeasured activities” may lead to patients with high physical load being wrongly classified as the “low-exercise group”, and the actual existing exercise-induced fatigue relief effect is diluted (underestimated). (2) If a patient reduces their occupational or household activities due to severe fatigue (such as taking leave for rest), but maintains recreational exercise, GLTEQ overestimates the proportion of exercise in total energy expenditure and may exaggerate the independent impact of leisure exercise on fatigue (overestimation). However, GLTEQ remains a validated and widely used tool in the tumor population, providing practical advantages for clinical assessment. We emphasize that our research results should be interpreted in the context of leisure activities. Hope, regarded as an intrinsic source for overcoming illness and restoring confidence in cancer patients, alongside self-efficacy, which underscores an individual’s belief in their capabilities, are manifestations of optimism. While prior research has predominantly concentrated on negative emotions such as anxiety and depression as contributors to CRF7,14,16, optimism has been demonstrated to exert a direct negative impact on CRF and serves as a crucial pathway affecting fatigue43. Saito M also posits that self-efficacy significantly influences CRF and impacts health-related quality of life in young survivors of childhood cancer44. Furthermore, studies indicate that even suboptimal levels of PA can alleviate depressive symptoms by enhancing self-efficacy45,46 and can improve sleep disorders47. Therefore, interventions that foster hope and self-efficacy through PA may represent a promising strategy for reducing CRF. Additionally, the better of nutritional status, as measured by BMI, the more likely to be the low fatigue group in this study. These findings align with prior research indicating a high prevalence of nutritional risk among preoperative HCC patients, which is significantly associated with CRF48. Child-Pugh A was strongly correlated with low fatigue (OR = 0.310), emphasizing the critical role of liver function status in CRF. In clinical practice, the early evaluation of fatigue and limitations in physical activity among liver cancer patients is crucial. Enhancing exercise behavior intervention management strategies, fostering positive psychological outlooks, reinforcing nutritional support, and improving liver function may contribute to alleviating fatigue in liver cancer patients. Furthermore, interventions focusing on protective factors such as increased physical activity, enhanced hope, and self-efficacy could be advantageous for all patient subtypes.
Among peri-interventional HCC patients, factors such as spousal support (OR = 5.171), financial stress (OR = 2.570), and insomnia (OR = 1.212) were more likely to categorize patients into the “Physical imbalance -Moderate fatigue” subtype. Supporting evidence from other studies suggests that HCC patients with lower economic status exhibited higher levels of CRF compared to those with better economic conditions7,16. Finance stress may exacerbate fatigue by increasing psychological burden and restricting access to medical resources, while insomnia may directly initiate a vicious cycle of fatigue. Consequently, interventions targeting this subtype should integrate financial assistance with sleep management strategies. Compared to patients with low fatigue level, married patients were more likely to report moderate fatigue than those without a spouse, which contrasts with previous studies on transcatheter arterial embolization in patients with HCC12. One possible explanation is that the majority of subjects in this study were from rural areas. Patients lacking spousal support often had to engage in more household tasks to sustain their livelihood, and the increase in physical activity may have reduced the incidence of fatigue. Additionally, according to the family stress coping theory, married cancer patients undertake multiple roles in the family, such as spouse, parent, financial supporter, etc. The responsibilities and expectations of these roles may increase the psychological burden of patients during the illness, leading to an increase in fatigue. The spouses of cancer patients also face psychological and physical health challenges, which may have an indirect impact on the patients’ fatigue49. Therefore, healthcare providers should consider the unique challenges faced by married HCC patients when developing strategies for managing CRF.
Strengths and limitations
The strength of this study is that the heterogeneous research approach brings new opportunities. By identifying population subtypes and conducting in-depth studies on their characteristics, it helps to discover new pathogenesis and intervention targets, providing a more solid theoretical basis for personalized intervention management for HCC patients. However, this study has several limitations. First, it was a cross-sectional study relying on self-reported data, which may be subject to recall bias and the causal relationship cannot be determined. The observed relationships (e.g., between CRF and PA) could plausibly operate in either direction. CRF may lead to reduced PA, just as reduced PA may exacerbate CRF. The cross-sectional design prevents us from determining temporal precedence. Second, selection bias may have occurred, as patients with severe fatigue may have been less likely to participate, while those without fatigue may have been less motivated to join the study. Thirdly, a limitation of LPA is the variability in results that can arise from different model selection criteria. Finally, the samples come from a single center, which may limit the universality of the results. Future studies can further verify the results through longitudinal design, objective measurement and multi-center samples, and explore more characteristic factors of each potential profile.
Conclusion
In conclusion, this study revealed the heterogeneity of CRF in patients with liver cancer and identified the characteristics of different subtypes. These findings provide important evidence for individualized intervention of different subtypes of fatigue in clinical practice, and emphasize the importance of improving PA, cultivating positive emotions, enhancing sleep quality, optimizing nutritional status and managing liver function as practical strategies to reduce the risk of CRF. Future intervention studies can be based on the classification results of this study to design targeted multi-dimensional management strategies to ensure that liver cancer patients receive the highest level of support and personalized assistance throughout the treatment process.
Data availability
Due to the risk of patient/participant re-identification, the datasets used and analyzed during the current study can be reasonably requested by the corresponding author for data.
Abbreviations
- CRF:
-
Cancer-related fatigue
- HCC:
-
Hepatocellular carcinoma
- HHI:
-
Herth Hope Index
- ISI:
-
The Insomnia Severity Index
- GSES:
-
General self-efficacy
- PA:
-
Physical activity
- BMI:
-
Body-mass index(kg/m2)
- PNI:
-
Prognostic Nutrition Index
- BCLC:
-
Barcelona Clinic Liver Cancer
- GLTEQ:
-
Godin Leisure-Time exercise Questionnaire
- CFS:
-
Cancer Fatigue Scale
References
Cao, W., Chen, H. D., Yu, Y. W., Li, N. & Chen, W. Q. Changing profiles of cancer burden worldwide and in china: a secondary analysis of the global cancer statistics 2020. Chin. Med. J. (Engl). 134 (7), 783–791 (2021).
Rahib, L., Wehner, M. R., Matrisian, L. M. & Nead, K. T. Estimated projection of US cancer incidence and death to 2040. JAMA New. Open. 4 (4), e214708 (2021).
Zheng, R. S. et al. Analysis of the prevalence of malignant tumors in China in 2015. Chin. J. Oncol. 41(1), 19–28 (2019).
Zhou, J. et al. Guidelines for the diagnosis and treatment of primary liver cancer (2022 Edition). Liver Cancer. 12 (5), 405–444 (2023).
NCCN clinical practice guidelines in oncology. Cancer-related fatigue version 1.2019. www.Most current guidelines on CRF. nccn.org. Accessed 11 Jul 2019 (2019).
Roila, F. et al. Prevalence, characteristics, and treatment of fatigue in oncological cancer patients in italy: a cross-sectional study of the Italian network for supportive care in cancer (NICSO). Support Care Cancer. 27 (3), 1041–1047 (2019).
Ma, Y. et al. Prevalence and risk factors of cancer-related fatigue: A systematic review and meta-analysis. Int. J. Nurs. Stud. 111, 103707 (2020).
Thong, M. S. Y., van Noorden, C. J. F., Steindorf, K. & Arndt, V. Cancer-related fatigue: Causes and current treatment options. Curr Treat Options Oncol. 21(2), 17. https://doi.org/10.1007/s11864-020-0707-5 (2020) (published correction appears in Curr Treat Options Oncol 2022 23(3):450–451).
Fontvieille, A. et al. Effects of a mixed exercise program on cancer related-fatigue and health-related quality of life in oncogeriatric patients: A feasibility study [Journal of Geriatric Oncology 12 Issue 6 (2021) 915–921]. J Geriatr Oncol. 13(7), 1070 (2021).
Tucker, K., Staley, S. A., Clark, L. H. & Soper, J. T. Physical activity: impact on survival in gynecologic cancer. Obstet. Gynecol. Surv. 74 (11), 679–692 (2019).
Behringer, K. et al. Cancer-related fatigue in patients with and survivors of hodgkin lymphoma: the impact on treatment outcome and social reintegration. J. Clin. Oncol. 342016, 4329–4337 (2016).
Yang, X. M., Yang, X. Y., Wang, X. Y. & Gu, Y. X. Influence of transcatheter arterial embolization on symptom distress and fatigue in liver cancer patients. World J. Gastrointest. Oncol. 16 (3), 810–818 (2024).
Wu, X. J. Effect of shenmai injection on TCM pattern score in patients with carcinogenic fatigue and its mechanism. Liaoning J. Traditional Chin. Med. 41 (6), 1171–1173 (2014).
Brownstein, C. G. et al. Physiological and psychosocial correlates of cancer-related fatigue. J. Cancer Surviv. 16 (6), 1339–1354 (2022).
Huang, S. T. et al. Risk factors for cancer-related fatigue in patients with colorectal cancer: a systematic review and meta-analysis. Support Care Cancer. 30 (12), 10311–10322 (2022).
Chen, C. Y. et al. Persistent fatigue in patients with hepatocellular carcinoma receiving radiotherapy. J. Nurs. Res. 32 (2), e319 (2024).
Lundt, A. & Jentschke, E. Long-Term changes of symptoms of anxiety, depression, and fatigue in cancer patients 6 months after the end of yoga therapy. Integr. Cancer Ther. 18, 1534735418822096 (2019).
Huang, H. P. et al. The effect of a 12-week home-based walking program on reducing fatigue in women with breast cancer undergoing chemotherapy: A randomized controlled study. Int. J. Nurs. Stud. 99, 103376 (2019).
Huang, Q., Geng, Z., Fang, Q., Stinson, J. & Yuan, C. Identification of distinct profiles of cancer-Related fatigue and associated risk factors for breast cancer patients undergoing chemotherapy: A latent class analysis. Cancer Nurs. 44 (6), E404–E413 (2021).
Park, J. H. et al. Latent profile analysis for assessing symptom clusters in women with breast cancer. J. Cancer Surviv (2024).
Fox, R. S. et al. Sleep disturbance and cancer-related fatigue symptom cluster in breast cancer patients undergoing chemotherapy. Support Care Cancer. 28 (2), 845–855 (2020).
Yang Qing, Z. et al. Latent profile/class analysis identifying differentiated intervention effects. Nurs. Res. (2022).
Okuyama, T. et al. Factors correlated with fatigue in disease-free breast cancer patients: application of the cancer fatigue scale. Support Care Cancer. 8 (3), 215–222 (2000).
Godin, G. & Shephard, R. J. A simple method to assess exercise behavior in the community. Can. J. Appl. Sport Sci. 10 (3), 141–146 (1985). PMID: 4053261.
Schmitz, K. H. et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 42 (7), 1409–1426 (2010).
Zhao, H. P. & Wang, J. Social support and hope for hemodialysis patients. Chin. Nurs. J. 35(05), 49–51 (2000).
Herth, K. Enhancing hope in people with a first recurrence of cancer. J. Adv. Nurs. 32 (6), 1431–1441 (2000).
Schwarzer, R., Baßler, J., Kwiatek, P., Schroder, K. & Zhang, J. X. The assessment of Optimistic Self-beliefs: comparison of the German, Spanish, and Chinese versions of the general Self-efficacy scale. Appl. Psych 46(1) (1997).
Wang, C., Hu, Z. & Liu, Y. Evidences for reliability and validity of the Chinese version of general self efficacy scale. Chin. J. Appl. Psych (01):37–40. (2001).
Morin, C. M., Belleville, G., Bélanger, L. & Ivers, H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34(5), 601–608 (2011).
Zhang, X. et al. Fear of recurrence in cancer patients: a latent profile analysis. Chin. J. Nurs. 58 (6), 662–669 (2023).
Álvarez-Bustos, A. et al. Prevalence and correlates of cancer-related fatigue in breast cancer survivors. Support Care Cancer. 29 (11), 6523–6534 (2021).
Nowe, E. et al. Cancer-Related fatigue and associated factors in young adult cancer patients. J. Adolesc. Young Adult Oncol. 8 (3), 297–303 (2019).
Xian, X., Zhu, C., Chen, Y., Huang, B. & Xu, D. A longitudinal analysis of fatigue in colorectal cancer patients during chemotherapy. Support Care Cancer. 29 (9), 5245–5252 (2021).
Ying, M. et al. Analysis of carcinogenic fatigue level and influencing factors in young and middle-aged patients with primary liver cancer after hepatic arterial chemoembolization. Nurs. Pract. Res. 20 (07), 958–964 (2023).
Feng, L. R., Barb, J. J., Regan, J. & Saligan, L. N. Plasma metabolomic profile associated with fatigue in cancer patients. Cancer Med. 10 (5), 1623–1633 (2021).
Crowder, S. L. et al. Relationships among physical activity, sleep, and Cancer-related fatigue: results from the international Colo care study. Ann. Behav. Med. 58 (3), 156–166 (2024).
Ma, Y. et al. Efficacy of cognitive behavioral therapy for insomnia in breast cancer: A meta-analysis. Sleep. Med. Rev. 55, 101376 (2021).
Garland, S. N. et al. Randomized controlled trial of virtually delivered cognitive behavioral therapy for insomnia to address perceived cancer-Related cognitive impairment in cancer survivors. J. Clin. Oncol. 42 (17), 2094–2104 (2024).
Thakral, M., Von Korff, M., McCurry, S. M., Morin, C. M. & Vitiello, M. V. Changes in dysfunctional beliefs about sleep after cognitive behavioral therapy for insomnia: A systematic literature review and meta-analysis. Sleep. Med. Rev. 49, 101230 (2020).
Carroll, J. E. et al. Remission of insomnia in older adults treated with cognitive behavioral therapy for insomnia (CBT-I) reduces p16INK4a gene expression in peripheral blood: secondary outcome analysis from a randomized clinical trial. Geroscience 45 (4), 2325–2335 (2023).
Campbell, K. L. et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 51 (11), 2375–2390 (2019).
Zeng, H. J. et al. Influencing factors and path analysis of self-regulation fatigue in maintenance hemodialysis patients. Chin. J. Nurs. 59(02), 156–164 (2024).
Saito, M., Hiramoto, I., Yano, M., Watanabe, A. & Kodama, H. Influence of Self-Efficacy on cancer-Related fatigue and Health-Related quality of life in young survivors of childhood cancer. Int. J. Environ. Res. Public. Health. 19 (3), 1467 (2022).
Sejbuk, M., Mirończuk-Chodakowska, I. & Witkowska, A. M. Sleep quality: A narrative review on nutrition, stimulants, and physical activity as important factors. Nutrients 14 (9), 1912 (2022).
Pearce, M. et al. Association between physical activity and risk of depression: A systematic review and Meta-analysis. JAMA Psychiatry. 79 (6), 550–559 (2022).
Noetel, M. et al. Effect of exercise for depression: systematic review and network meta-analysis of randomised controlled trials. BMJ (Clinical research 384, e075847 (2024).
Zhu, X. J. et al. Correlation and influencing factors between nutritional risk and cancer-induced fatigue in preoperative patients with primary liver cancer. Mod. Practical Med. 34 (01), 34–36 (2022).
Ding, Z. et al. A dyadic analysis of family adaptation among breast cancer patients and their spouses based on the framework of family stress coping theory. Front. Public. Health. 12, 1453830 (2024).
Acknowledgements
This work was supported by the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University/Hunan Cancer Hospital.
Funding
This study was supported by Hunan Provincial Nature Science Foundation of China [2025JJ80865].
Author information
Authors and Affiliations
Contributions
Zhen Liang and Juan Li contributed to the conception and design of the study. Zhengdi She collected the data from the patients in the hospital. Wen Lu and Sha Lin entered and checked the data. Zhen Liang and Juan Li analyzed and interpreted the data. Juan Li was the major contributor in writing the manuscript. Zhen Liang and Yan Tan critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Hunan Cancer Hospital (Date: 2021-8-25/No. SBQLL-2021-174).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Lin, S., She, Z. et al. Exploring the heterogeneity of cancer-related fatigue in advanced hepatocellular carcinoma patients via latent profiles and influencing factors. Sci Rep 15, 35151 (2025). https://doi.org/10.1038/s41598-025-19135-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19135-y