Abstract
Sudden sensorineural hearing loss (SSNHL) is a common otological condition; however, its pathophysiology and prognostic factors remain unclear. We aimed to investigate the relationship between retinal microvascular changes and the severity and prognosis of SSNHL using optical coherence tomography angiography (OCTA), exploring the role of ocular microcirculation. A prospective cohort study was conducted on 88 patients with SSNHL between December 2019 and December 2022. All patients underwent OCTA on the affected side to assess retinal vasculature. Parameters analyzed included vessel density (VD), vessel length density (VLD), choriocapillaris flow deficit (CCFD), along with retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL) thickness. Hearing recovery was classified using the Modified American Academy of Otolaryngology-Head and Neck Surgery criteria, and correlations with OCTA findings were assessed. Patients with poor hearing recovery (Group 1) showed significantly thinner RNFL and GCL compared to those with improved hearing (Group 2). The superficial (SCP) and deep (DCP) capillary plexus VD had a significant negative correlation with initial hearing loss severity (rho = -0.272 and − 0.306, respectively; p < 0.05), suggesting that reduced ocular blood flow may be related to SSNHL severity. No significant correlations were observed for VLD and CCFD. Reduced RNFL and GCL thickness may serve as prognostic markers for SSNHL. The inverse correlation between SCP/DCP VD and hearing loss severity suggests that arterial hypoperfusion may contribute to SSNHL. Our results support the potential utility of OCTA as a noninvasive method for evaluating ocular blood flow in SSNHL patients.
Similar content being viewed by others
Introduction
Sudden sensorineural hearing loss (SSNHL) is defined as sudden hearing loss of at least 30 dB in hearing thresholds at three consecutive frequencies, occurring within 3 days1,2. The incidence of unilateral SSNHL is approximately 17.8/100,000, and the annual incidence increased substantially from 2011 to 20143. Despite the high prevalence of SSNHL, its pathophysiology remains unclear and is currently being investigated.
The chief complaint in patients with SSNHL is unilateral hearing disturbance, followed by sudden ear fullness, tinnitus, and occasional dizziness. The condition is usually diagnosed by comprehensive history taking, examination of the external auditory canal and tympanic membrane, and pure-tone audiometry (PTA). Identifying risk and prognostic factors for SSNHL is crucial, as it is closely related to the social burden of permanent sensorineural hearing loss and hearing rehabilitation. Multiple studies have investigated the nature of SSNHL to determine its risk and prognosis, identifying several potential underlying conditions, including microangiopathy (such as increased blood viscosity and microthrombosis), viral infection, and autoimmune disease4. To date, localized inflammation and compromised metabolic supply to the cochlea are considered central contributors. Corticosteroids, administered either systemically or via intratympanic injection, have conventionally served as the first-line therapy for SSNHL, as endorsed by both national and international clinical guidelines1. However, evidence supporting the specific mechanism is lacking. Microvascular dysfunction is considered an important cause of SSNHL5,6. The oxygen supply to the cochlea relies on diffusion through capillaries rather than direct vascular oxygenation7; therefore, mechanisms of ischemia and microcirculation are hypothesized to play a key role in SSNHL. Given that microvascular dysfunction associated with various systemic diseases can also lead to vascular changes in the retina, investigating retinal microcirculation may provide indirect insights into cochlear vascular status8,9. These retinal changes can be examined using various methods, including fundus examination, fluorescein angiography, and optical coherence tomography angiography (OCTA). OCTA has been developed for a more detailed evaluation of retinal structure and dynamic changes10.
A recent study using retinal fundus photography has reported that the presence of microvascular retinal signs is associated with hearing loss, suggesting that retinopathy could be an indicator of hearing loss11. A cross-sectional population-based study also indicated that loss of deep vascular density in the retina is associated with age-related auditory processing disorders12. However, the relationship between retinal microvascular dysfunction and SSNHL remains unclear, and its clinical significance is unexplored.
In the light of these findings, we hypothesized that microvascular changes may be present in patients with SSNHL, which cannot be confirmed by conventional tests. In the present study, we aimed to evaluate the relationships between changes in retinal vasculature and the severity and prognosis of SSNHL.
Results
After excluding five patients who met the exclusion criteria (three with diabetic retinopathy, one with a history of vitrectomy, and one with previous filtering surgery), 99 were included in the study. Six patients were subsequently excluded as they were lost to follow-up. Finally, 88 patients (38 males and 50 females) completed the study and their data were analyzed. Morbidity was reported in 38 (43.18%) right and 50 (56.81%) left ears, respectively. On the basis of the initial hearing level, 18 patients (20.45%) were diagnosed with mild (26 ~ 40 dB), 15 (17.05%) with moderate (41–55 dB), 19 (21.59%) with moderate-to-severe (56–70 dB), 21 (23.86%) with severe (71–90 dB), and 15 (17.05%) with profound (larger than 90 dB) hearing loss. The mean age was 57.71 ± 9.14 years for Group 1 patients with no improvement of hearing and 50.74 ± 14.01 years for Group 2 patients with complete recovery, partial recovery, and slight improvement of hearing (Table 1). Demographic characteristics, clinical manifestations, and initial hearing levels are shown in Table 2. No statistically significant differences were found between the two groups in baseline demographic characteristics, such as age, sex, side, delay before therapy, diabetes mellitus, hypertension, and initial hearing level, except for the final PTA results at 6 months after treatment (p < 0.001).
The OCT and OCTA parameters (CSMT, RNFL, GCL, CCFD, VD, and VLD) are shown in Table 3. These parameters were compared by dividing them into two groups according to the initial hearing threshold (initial severity) and degree of recovery. First, according to the initial hearing threshold (initial severity), SCP and DCP VP were significantly correlated with severity among the five subgroups (Spearman’s correlation coefficient rho − 0.272, p < 0.05, and − 0.306, p < 0.05, respectively) (Figs. 1 and 2). However, there were no significant differences in the VLD, CCFD, RNFL, and GCL thicknesses between the two groups. Second, according to the degree of recovery, RNFL and GCL thickness were 43 ± 3.2 and 65 ± 3.0 in Group 1, and 35 ± 8.1 and 59 ± 6.3 in Group 2, respectively. There were significant differences in the RNFL (p < 0.05) and GCL thicknesses (p < 0.05) between the two subgroups. However, when these parameters were compared by classifying them into five subgroups according to the initial hearing threshold (initial severity), there was no significant correlation among the five subgroups (mild-to-profound hearing loss) (Fig. 2).
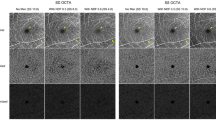
Representative cases with optical coherence tomography angiography images corresponding to the severity of sudden sensorineural hearing loss. The leftmost column shows baseline optical coherence tomography angiography (OCTA) images of a patient with stage 1 sudden sensorineural hearing loss (SSNHL). The middle column represents OCTA images of patients with stage 3 SSNHL, and the rightmost column shows OCTA images of patients with stage 5 SSNHL. (A–C) The first row represents superficial capillary plexus images in OCTA (D–F), while the second row denotes deep capillary plexus (DCP) images in OCTA. (G–I) Using these two images, it is possible to extract a binarized DCP image and calculate the DCP vascular density (VD) in the third row. As the severity of SSNHL worsens, the DCP VD decreases. (J–L) The parafoveal temporal regions (red dashed rectangles) with the foveal avascular zone (FAZ) of the DCP images are schematically depicted in the bottom row. At lower severity, the void spaces (red arrows) were scattered within the DCP. As the severity increased, the void spaces increased and contributed to the decreased DCP VD.
Spearman’s correlation analysis between OCTA parameters among the five subgroups of severity. (A) Superficial capillary plexus vessel density (SCP VD), (B) Superficial capillary plexus vessel length density (SCP VLD) (C) Deep capillary plexus vessel density (DCP VD), (D) Deep capillary plexus vessel length density (DCP VLD), (E) Retinal nerve fiber layer (RNFL) thickness, (F) Ganglion cell layer (GCL) thickness, (G) Central subfield macular thickness (CSMT), (H) Choriocapillaris flow deficit (CCFD).
Discussion
SSNHL is defined as an acute onset of sensorineural hearing loss of 30 dB or more in at least three consecutive frequencies, occurring within 3 days and of unknown cause1,2. The incidence of unilateral SSNHL has rapidly increased in recent years3. Several studies have been conducted to identify the risk and prognostic factors of SSNHL1,2,3,4, including studies reporting the presence of microvascular disorders as poor prognostic factors for SSNHL13,14. Hirano et al. also reported poor treatment outcomes for patients with microvascular diseases (including diabetes mellitus, hyperlipidemia, and hypertension) and assumed that the preexisting pathology of the auditory system is highly affected by aging6. The correlation between microvascular pathology and SSNHL has been reported using various test methods5,6,15. A prospective case-control study reported by Kocak et al. used videocapillaroscopic examination of the patients with SSNHL to measure capillary density (CD) and post-occlusive reactive hyperemia (PORH)5. Both the CD and PORH values were significantly reduced in patients with SSNHL. Patients with a significantly lower PORH showed a worse prognosis. Ju et al. used serological tests, including the neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and lymphocyte-monocyte ratio, in patients with diabetes and SSNHL15. All the ratios were higher than those of healthy participants. Among the different methods of assessing the microcirculation of blood flow, retinal signs are considered indicative of the cerebral microvasculature16. It serves not only as an early sensitive detector but also as a non-invasive method. Therefore, retinal examination can be added to detect microvascular changes in the hearing organs. A prospective cohort study reported the potential risk of abnormal retinal signs in age-related hearing loss11. Fundus photography was used to measure variables associated with the loss of vascular integrity and changes in the arteriolar wall. These findings suggest that retinopathy is associated with chronic hearing loss. Similar studies from Italy using OCTA reported an association between retinal vasculature and central auditory processing pathology in age-related hearing loss12. OCT and OCTA allowed for a more detailed evaluation of the retinal structures, perfusion length, and retinal area. The association between SSNHL and retinal vein occlusion (RVO) was reported in a recent study17. Using a retrospective nationwide cohort dataset, this study reported a significant development of RVO in SSNHL, particularly in older male patients. Therefore, in this study, we attempted to determine the relationship between the presence of retinopathy and the prognosis and severity of SSNHL using OCTA.
This study’s strength is that it yielded two main results. First, a significant difference was observed between the two subgroups (degree of recovery), particularly in the RNFL and GCL thicknesses (p < 0.05). Both parameters reflect the ocular microcirculation, including the peripapillary area and optic disc. Significantly lower vessel densities and blood flow indices in these areas have been observed in many patients with normal-tension glaucoma or ischemic optic neuropathy17,18,19,20,21,22. Therefore, if decreased blood flow is associated with the prognosis of SSNHL, RNFL and GCL thicknesses should be reduced in the aggravated group. In the present study, the mean values of both variables were significantly lower in the aggravation than in the improvement group. Therefore, decreased ocular blood flow can be considered a prognostic factor. Second, a significant negative correlation was observed between the SCP VD/DCP VD and SSNHL severity. SCP and DCP VD reflect slow blood flow in each layer of the retina and can vary under various conditions. Given that the retina contains a thin and highly resistant capillary network, VD decreases when perfusion to the arterial system is poor and increases when venous drainage is impaired23,24. Based on these results, we hypothesized that the vascular pathology of SSNHL is likely related to arterial hypoperfusion rather than to venous congestion (venous insufficiency). Notably, perfusion in the SCP and DCP has also been associated with many diseases, such as diabetic retinopathy, migraine, and COVID-1925,26,27,28,29,30. Therefore, measuring ocular blood flow to both the SCP and DCP is crucial. However, few examinations are capable of assessing SCP and DCP. Among them, OCTA is the only examination that can measure perfusion to the SCP and DCP noninvasively without using a contrast agent10,31. Considering the neuroanatomical context, it is important to note that partial decussation occurs in both the visual and auditory pathways. Although the optic chiasm allows crossing of nasal retinal fibers, retinal microvascular changes observed by OCTA are primarily local and not directly influenced by these post-retinal pathways32. Similarly, although the central auditory system involves bilateral projections and multiple decussations, SSNHL is typically a unilateral, peripheral condition33. Therefore, the observed ocular microvascular changes may reflect systemic vascular factors associated with the affected cochlea rather than effects mediated by central neural crossings. In this study, the findings imply a possible association between microvascular alterations and SSNHL, with retinal structural changes observed through OCTA potentially serving as prognostic indicators.
This study had some limitations. First, OCTA imaging was performed only on the ipsilateral (affected side) eye. Although SSNHL typically presents unilaterally, arterial hypoperfusion may affect both eyes. As a result, we were unable to compare retinal microvascular parameters between the affected and contralateral eyes, which limits our ability to determine whether the observed changes are specific to the affected side or reflect systemic vascular alterations. Future studies incorporating bilateral eye imaging are warranted to clarify this issue and strengthen the interpretation of retinal findings in the context of SSNHL. Second, we did not assess patients’ visual acuity. However, a recent study reported that the degree of myopia was closely related to the DCP VD29. To exclude other variables that could have affected the results, visual acuity testing should be conducted in future studies. Third, baseline and final visual acuity data for all included patients were absent. As the primary objective was to evaluate microvascular changes using OCTA parameters, visual acuity was not consistently recorded during follow-up, particularly in asymptomatic patients. Therefore, visual function could not be directly correlated with structural findings. Future prospective studies with standardized visual acuity measurements are warranted to strengthen the clinical relevance of OCTA-based metrics. And lastly, the imbalance in sample sizes between Group 1 and Group 2, may affect the robustness of statistical comparisons. As the data did not meet the assumption of normality, we applied the Mann-Whitney U test, a non-parametric method appropriate for unequal group sizes. Future studies with larger sample sizes and a more balanced distribution between groups are warranted to validate these findings.
Our findings suggest that ocular microvascular changes may serve as prognostic factors for SSNHL. RNFL and GCL thicknesses were significantly reduced in patients with poor recovery, indicating that impaired ocular perfusion may be linked to SSNHL. The negative correlation between SCP or DCP VD and the severity of hearing loss emphasizes that arterial hypoperfusion may play a key role in pathophysiology of SSNHL. These results highlight the importance of evaluating the ocular microcirculation in SSNHL using OCTA, a non-invasive method for assessing retinal blood flow.
Methods
Study design and participants
This prospective cohort study included patients who visited our clinic with SSNHL between December 2019 and 2022. All patients were diagnosed according to the criteria defined by the American Academy of Otolaryngology-Head and Neck Surgery, namely, hearing loss ≥ 30 dB affecting at least three consecutive frequencies with reference to the thresholds of the opposite ear within 72 h1. OCTA was performed on the day of SSNHL diagnosis. Patients without a history of ear surgery (including tympanoplasty and mastoidectomy), head trauma (particularly damage to the temporal bone area), diagnosis of various types of otitis media, or other inner ear diseases (e.g., Meniere’s disease, labyrinthitis, and autoimmune disease) were included. Patients with an identified etiology (including lesions of the internal auditory canal or cerebellopontine angle) or ocular disease were excluded. Patients who failed regular follow-ups for hearing loss or OCTA were also excluded. Records of patient age, sex, interval from onset to the start of treatment, presence of diabetes mellitus or hypertension, coincidence of vertigo, laboratory test findings, imaging data, initial and final PTA data and speech audiometry data were obtained. PTA was conducted at the initial visit and at follow-up visits after 1, 3, and 6 months. All patients were treated with systemic steroid (SS) and intratympanic steroids (ITS). The common SS regimen was as follows: oral methylprednisolone was administered at 0.8 mg/kg/d for 7 d, which was tapered for 5 d. For ITS treatment, 0.4–0.8 mL dexamethasone (dexamethasone phosphate; Jeil Pharmaceutical Co. Ltd., Republic of Korea) at a dose of 4 mg/mL was injected into the middle ear space. The patients were asked to remain in this position for 20 min without swallowing. ITS treatment was performed once daily for eight sessions. This study was approved by the Institutional Review Board of the Pusan National University Hospital (IRB No. 2212-020-122), and the study was conducted adhering to the Helsinki Declaration of 1975 as revised in 2013. Written informed consent was obtained from all patients.
OCTA measurements and data collection
In all patients, the ipsilateral eye (the affected side of SSNHL) was evaluated using OCTA. The patients were examined using SS-OCTA (PLEX Elite 9000; Carl Zeiss Meditec, Inc., Dublin, CA, USA) and AngioPlex software (ver. 2.0.1.47662; Carl Zeiss Meditec). The patients in each group were classified into the following five subgroups according to the severity of their initial hearing threshold: mild (26–40 dB), moderate (41–55 dB), moderate-to-severe (56–70 dB), severe (71–90 dB), and profound (> 90 dB). Hearing recovery was assessed according to the Modified American Academy of Otolaryngology-Head and Neck Surgery criteria based on the last PTA and speech audiometry 6 months after treatment (complete recovery, partial recovery, slight improvement, and no improvement) (Table 1)34.
Before obtaining OCTA images, 4 mg (5 mg/mL) of phenylephrine hydrochloride and 4 mg (5 mg/mL) of tropicamide (Tropherine; Hanmi Pharm, Seoul, Korea) were instilled into the eye three times at 10-min intervals. An experienced examiner performed the examination twice (at 5-min intervals) in the same position using SS-OCTA. All OCT data were acquired using SS-OCT and imaged with macular scans centered on the fovea based on the three-dimensional (3D) Macula 7 × 7 mm protocol. Only high-quality OCT volumetric data (TopQ Image Quality ≥ 45), without involuntary saccade or blinking artifacts, were used for the analysis35. Central subfield macular thickness (CSMT), average retinal nerve fiber layer (RNFL) thickness, and ganglion cell inner layer (GCL) thickness were extracted using the built-in software. The OCTA data included vessel density (VD, %) and vessel length density (VLD, %) of the superficial capillary plexus (SCP), deep capillary plexus (DCP), and choriocapillaris flow deficit (CCFD, %). After obtaining the OCTA images, these data were reprocessed using FIJI software (an expanded version of ImageJ, version 1.52u (National Institutes of Health, Bethesda, MD, USA)), and the results were acquired and analyzed.
The software setting allowed quantification of microvasculature images in five subfields (centered on the macula and four regions of the parafoveal ring (superior, inferior, nasal, and temporal)), only in the SCP, and included the CSMT, RNFL, and GCL9. OCTA data included the VD 3 mm center, S/N/I/T, VD ring (sum of S, N, I, and T), and foveal avascular zone (FAZ) area. The VLD in the SCP, DCP, and CCFD were obtained36. OCTA images with severe saccadic movements or blinking artifacts and poor image quality (signal strength ≤ 7/10) were excluded37.
Audiological test
PTA and speech audiometry assessments were performed using a clinical audiometer (Interacoustics AC40, Interacoustics A/S, Assens, Denmark) in a double-walled soundproof booth. Air conduction thresholds were measured at standard frequencies ranging from 250 to 8,000 Hz, and bone conduction thresholds were obtained from 250 to 4,000 Hz when necessary. Speech audiometry included measurement of speech reception thresholds (SRT) and speech discrimination scores (SDS) using standardized word lists delivered at suprathreshold levels. All tests were conducted by licensed audiologists following established clinical protocols.
Statistical analysis
Comparisons between the aggravated and recovered groups were performed using the Mann–Whitney, chi-square, and Fisher’s exact tests. Moreover, Mann–Whitney U test was used to compare OCTA measurements. Spearman’s correlation analysis was used to evaluate the correlation between parameters among the five severity subgroups. All statistical analyses were performed using SPSS (version 19.0; SPSS Inc., Chicago, IL, USA), and statistical significance was set at p < 0.05.
Data availability
The data that support the findings of this study are available from the authors but restrictions apply to the availability of these data, due to patient confidentiality issues and so are not publicly available. Someone wants to request the data from this study, please contact the corresponding author.
References
Chandrasekhar, S. S. et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol. Head Neck Surg. 161, S1–S45 (2019).
Cho, Y., Kim, J., Oh, S. J., Kong, S. K. & Choi, S. W. Clinical features and prognosis of severe-to-profound sudden sensorineural hearing loss. Am. J. Otolaryngol. 43, 103455 (2022).
Kim, S. H. et al. A trend in sudden sensorineural hearing loss: data from a population-based study. Audiol. Neurootol. 22, 311–316 (2017).
Kuhn, M., Heman-Ackah, S. E., Shaikh, J. A. & Roehm, P. C. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif. 15, 91–105 (2011).
Kocak, H. E. et al. Microvascular dysfunction affects the development and prognosis of sudden idiopathic hearing loss. Clin. Otolaryngol. 42, 602–607 (2017).
Hirano, K. et al. Prognosis of sudden deafness with special reference to risk factors of microvascular pathology. Auris Nasus Larynx. 26, 111–115 (1999).
Nagahara, K., Fisch, U. & Yagi, N. Perilymph oxygenation in sudden and progressive sensorineural hearing loss. Acta Otolaryngol. 96, 57–68 (1983).
Shin, Y. U. et al. Diabetic retinopathy and hearing loss: results from the fifth Korean National health and nutrition survey. J. Clin. Med. 10, 1–10 (2021).
Lee, W. H. et al. Retinal microvascular change in hypertension as measured by optical coherence tomography angiography. Sci. Rep. 9, 156 (2019).
Campbell, J. P. et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci. Rep. 7, 42201 (2017).
Kim, S. J. et al. Association between microvascular retinal signs and age-related hearing loss in the atherosclerosis risk in communities neurocognitive study (ARIC-NCS). JAMA Otolaryngol. Head Neck Surg. 146, 152–159 (2020).
Sardone, R. et al. Retinal vascular density on optical coherence tomography angiography and age-related central and peripheral hearing loss in a Southern Italian older population. J. Gerontol. Biol. Sci. Med. Sci. 76, 2169–2177 (2021).
Orita, S., Fukushima, K., Orita, Y. & Nishizaki, K. Sudden hearing impairment combined with diabetes mellitus or hyperlipidemia. Eur. Arch Otorhinolaryngol. 264, 359–362 (2007).
Sciancalepore, P. I., de Robertis, V., Sardone, R. & Quaranta, N. Sudden sensorineural hearing loss: what factors influence the response to therapy? Audiol. Res. 10, 234 (2020).
Ju, Y. R. et al. Clinical features and hearing outcomes of sudden sensorineural hearing loss in diabetic patients. J. Audiol. Otol. 25, 27–35 (2021).
Hanff, T. C. et al. Retinal microvascular abnormalities predict progression of brain microvascular disease: an atherosclerosis risk in communities magnetic resonance imaging study. Stroke 45, 1012–1017 (2014).
Kim, J. Y., Ko, I. & Kim, D. K. Sudden sensorineural hearing loss May increase the risk of retinal vein occlusion: a nationwide cohort study. Healthc. (Basel). 10, 1–10 (2022).
Kardon, R. H. Role of the macular optical coherence tomography scan in neuro-ophthalmology. J. Neuroophthalmol. 31, 353–361 (2011).
Papchenko, T. et al. Macular thickness predictive of visual field sensitivity in ischaemic optic neuropathy. Acta Ophthalmol. 90, e463–e469 (2012).
Fan, N., Wang, P., Tang, L. & Liu, X. Ocular blood flow and normal tension glaucoma. Biomed. Res. Int. 2015 308505 (2015).
Liang, Y. et al. Retinal neurovascular changes in patients with ischemic stroke investigated by optical coherence tomography angiography. Front. Aging Neurosci. 14, 834560 (2022).
Saks, D. et al. Quantification of localised vascular wedge-shaped defects in glaucoma. Clin. Exp. Ophthalmol. 50, 724–735 (2022).
Luo, X. et al. Ocular blood flow autoregulation mechanisms and methods. J. Ophthalmol. 2015 864871 (2015).
Arrigo, A. et al. Reduced vessel density in deep capillary plexus correlates with retinal layer thickness in choroideremia. Br. J. Ophthalmol. 105, 687–693 (2021).
Moon, B. G., Um, T., Lee, J. & Yoon, Y. H. Correlation between deep capillary plexus perfusion and long-term photoreceptor recovery after diabetic macular edema treatment. Ophthalmol Retina. 2, 235–243 (2018).
Zeng, Y. et al. Retinal vasculature-function correlation in non-proliferative diabetic retinopathy. Doc. Ophthalmol. 140, 129–138 (2020).
Abrishami, M. et al. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Can. J Ophthalmol. 56, 24–30 (2021).
Ke, W. et al. Analysis of macular microvasculature with optical coherence tomography angiography for migraine: a systematic review and meta-analysis. Front. Neurol. 13, 1001304 (2022).
Yao, H., Xin, D. & Li, Z. The deep vascular plexus density is closely related to myopic severity. Ophthalmic Res. 65, 455–463 (2022).
Dogan, M. et al. Cigarette smoking reduces deep retinal vascular density. Clin. Exp. Optom. 103, 838–842 (2020).
Carlo, L. S. B. et al. Vessel density of superficial, intermediate, and deep capillary plexuses using optical coherence tomography angiography. Retina 39, 247–258 (2019).
Schmehl, M. N. & Groh, J. M. Visual signals in the mammalian auditory system. Annu. Rev. Vis. Sci. 7, 201–223 (2021).
Imam, A. et al. The brain of the tree Pangolin (Manis tricuspis). VI. The brainstem and cerebellum. J. Comp. Neurol. 527, 2440–2473 (2019).
Chandrasekhar, S. S. et al. Clinical practice guideline: sudden hearing loss (update) executive summary. Otolaryngol. Head Neck Surg. 161, 195–210 (2019).
Munoz-Gallego, A. et al. Assessment of macular ganglion cell complex using optical coherence tomography: impact of a paediatric reference database in clinical practice. Clin. Exp. Ophthalmol. 47, 490–497 (2019).
Lee, J. J. et al. Impact of signal strength on quantitative retinal and choriocapillaris flow measurement from optical coherence tomography angiography. Sci. Rep. 12, 4692 (2022).
Coppe, A. M. et al. Superficial and deep capillary plexuses: potential biomarkers of focal retinal defects in eyes affected by macular idiopathic epiretinal membranes? A pilot study. Diagnostics (Basel). 12, 1–12 (2022).
Acknowledgements
Rest in Peace, Professor Han Jo Kwon, M.D., Ph.D.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF − 2022R1C1C1004183 and RS-2025-00555372). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Jia Kim and Sung-Won Choi had full access to all data in the study and was responsible for data integrity and accuracy of the data analysis. Jia Kim and Han Jo Kwon served as co–first authors with equal contributions to the manuscript. Study concept and design: All authorsAcquisition of data: All authors. Analysis and interpretation of data: Jia Kim and Han Jo Kwon.Drafting of the manuscript: Jia Kim, Han Jo Kwon and Sung-Won Choi.Analysis and interpretation of data: Jia Kim, Han Jo Kwon and Jae Jung Lee. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Jia Kim and Han Jo Kwon. Administrative, technical, or material support: All authors Supervision: Sung-Won Choi.Obtain funding: Sung-Won Choi.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, J., Kwon, H.J., Lee, J.J. et al. Optical coherence tomography angiography findings in patients with sudden sensorineural hearing loss. Sci Rep 15, 35469 (2025). https://doi.org/10.1038/s41598-025-19255-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19255-5