Abstract
PLN-R14del is a pathogenic Phospholamban (PLN) gene variant, characterized by ventricular arrhythmias and dilated cardiomyopathy in heterozygous carriers. Disease development is highly heterogeneous, indicating involvement of additional disease triggers, influencing both the onset and severity of the disease. A heterozygous PLN-R14del mouse model (R14Δ/+) was used to investigate whether cardiac pressure induced by transverse aortic constriction (TAC), could accelerate disease onset.
Wild-type littermates and sham operated animals were used as controls. surgery. At 6-weeks, both TAC groups exhibited increased in left ventricular wall thickness, ventricular and atrial weights, and reduced ejection fraction, with comparable hypertrophic and fibrotic responses. Furthermore, differential gene expression showed comparable activation of cardiac remodeling and stress pathways, and changes in metabolic genes expression. Importantly, TAC did not induce sarco-endoplasmic reticulum malformation in R14Δ/+ mice, suggesting that general cardiac stress and remodeling pathways are insufficient to trigger PLN-R14del cardiomyopathy .
In conclusion, TAC induced pressure overload provoked robust cardiac remodeling with activation of common stress pathways in young adult WT and R14Δ/+ mice. It did not trigger PLN-R14del-specific sarco-endoplasmic malformation or accelerate disease progression. These findings imply that activation of common cardiac stress pathways alone may be insufficient to accelerate the onset of PLN-R14del cardiomyopathy in early adulthood.
Similar content being viewed by others
Introduction
Phospholamban (PLN) is a small protein that regulates the major Sarco/Endoplasmic Reticulum Calcium ATPase (SERCA2a), thereby controlling the rate of Ca2+ uptake into the sarcoplasmic reticulum (SR)1. PLN therefore acts as a modulator of the cardiomyocyte contraction-relaxation cycle. Numerous PLN pathogenic variants have been identified and PLN-R14del, which lacks the codon for arginine residue 14, is the most prevalent known pathogenic variant and has been extensively studied2. Heterozygous carriers of PLN-R14del can develop arrhythmogenic cardiomyopathy (ACM) and/or dilated cardiomyopathy (DCM), which can lead to severe heart failure (HF)3,4. Although it was originally hypothesized that this pathogenic variant would cause super-inhibition of SERCA, resulting in cytosolic calcium overload, more recent results suggest that this may not be the case5. Mouse PLN-R14del studies, revealed that abnormal S/ER malformation with strong perinuclear localization of PLN positive S/ER clusters was associated with cardiomyocyte cell death and consequent heart failure6. This strong perinuclear PLN localization has been shown to be a hallmark of this disease both in patients and PLN-R14del mice and has not been observed in other forms of ACM/DCM7.
PLN-R14del cardiomyopathy shows a high variability in disease onset and disease severity. Although, on average, disease develops around middle age, some carriers already develop severe disease at early adulthood, whereas others only develop mild symptoms at advanced age. This heterogeneity is even present between family members and is poorly understood8,9. Exercise, which can be a risk factor in other inherited cardiomyopathies, was not associated with PLN-R14del cardiomyopathy10. Similarly psychological stress or personality traits do not appear to be associated with PLN-R14del disease. Investigation of polymorphisms as secondary disease drivers has so far also not been promising, but this could also be a consequence of the relatively small cohorts11,12.
We previously generated a heterozygous mouse model (R14Δ/+) that shows key features of human PLN-R14del cardiomyopathy, which allows to investigate potential secondary disease drivers13. This mouse model develops characteristic cardiomyocyte perinuclear PLN clustering at one year of age with subsequent decrease in cardiac function, cardiomyocyte hypertrophy, fibrotic tissue replacement, and cardiac dilatation13. This disease onset corresponds with the disease onset in middle aged humans and together with the relative slow disease progression makes it an ideal model to investigate potential modifying factors that could enhance disease onset and severity. In a recent study, using a transgenic PLN mouse model unable to form naturally occurring PLN pentamers, it was shown that cardiac pressure overload induced by transverse aortic constriction (TAC), decreased survival in these mice. This was attributed to the narrower range of SERCA2a activity modulation in these transgenic mice causing more pronounced cardiac dysfunction after TAC14. Recent data indicate that also in PLN-R14del cardiomyopathy altered balances between PLN complexes may exist, resulting in a less dynamic behavior15. It is therefore tempting to believe that the TAC induced stress pathways may also trigger PLN-R14del cardiomyopathy. Pressure overload induced cardiac stress has also been shown to accelerate disease development in several other genetic mouse models probably by activating pathways involving Ca2+ homeostasis, myofibrillar protein16oxidative stress17 and mitochondrial autophagy18amongst others. Cardiomyocyte hypertrophy and fibrotic remodeling are features observed both in TAC and PLN-R14del cardiomyopathy and activation by TAC may lower the threshold for subsequent PLN-R14del cardiomyopathy development6. With the hypothesis that elevated cardiac stress could accelerate the course of PLN-R14del cardiomyopathy, we conducted TAC in R14Δ/+ mice and investigated cardiac remodeling and function as well as abnormal PLN-R14del clustering.
Method
Study design and animal model
At the age of 10 weeks, baseline echocardiography was performed and mice were randomly divided for sham or TAC operation. This resulted in four groups with 7 mice in the sham-WT group, 6 in the sham-R14del group, 10 in the TAC-WT group, and 13 in the TAC-R14del group. To enhance the consistency of the procedure and minimize variability introduced by operators, we used a refined TAC procedure by implementing rubber O-rings with a fixed inner diameter19. General appearance and body weight was monitored weekly. Mice were sacrificed 6 weeks post-surgery, after which organs, tissue and plasma were collected for subsequent histological and molecular analyses. All in vivo procedures were conducted under sustained anesthesia with 2-2.5% isoflurane in oxygen. This study was approved by the Dutch Central Committee for animal experiments (CCD; license number AVD1050020199105) and the animal ethical committee of the University of Groningen (permit numbers IVD199105-02-004) and followed the guidelines set out in the Directive 2010/63/EU of the European Parliament for using animals in research. It also followed the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for reporting on experiments done with animals20. All methods were performed in accordance with the relevant guidelines and regulations. The minimum number of animals required for each experiment was calculated based on G*power (3.1.9.7) analyses, setting the significance level at 5% and the power at 80%. PLN-R14del cardiomyopathy is a monogenic disease caused by a pathogenic variant located on an autosomal chromosome and it affects males and females similarly5. Previous studies have demonstrated comparable clinical phenotypes in both male and female patients and also cardiac imaging revealed similar pathological changes, but males with low-voltage ECGs showed somewhat worse prognostic value21,22. Also, in PLN-R14del mice, male and female characteristics have been investigated and revealed comparable pathological phenotypes6,13,23. Since the effect of TAC is dependent on the aortic diameter, which is related to rodent age and weight, it is essential to use a similar group of mice24. Combining male and female mice, that have different weights, would complicate this study by generating more variability necessitating much larger group numbers19. Since this is ethically not desired and the pathology of this monogenic disorder is similar in males and females we decided to use male mice only.
Echocardiography
M-mode and 2-dimensional echocardiography were performed before surgery and 6 weeks after TAC/Sham procedure, using a Vevo 3100 preclinical imaging system with a 40-MHz MXX550D linear array transducer (FUJIFILM VisualSonics, Canada) to monitor LV morphology and function as described previously6. Follow-up hemodynamic assessment was evaluated by Vevo LAB software version 5.5.0 (FUJIFILM VisualSonics). The data acquisition and analysis followed the guidelines provided by the European Society of Cardiology Working Group on Myocardial Function25 and were performed in a blinded manner.
To verify the effectiveness and consistency of TAC, pressure gradient doppler echocardiography was performed 1-week after surgery. Using the suprasternal view, pulsed-wave doppler recordings were obtained at the site of the aortic arch to measure the peak velocity of blood flow across the constriction. The pressure gradient was calculated using the modified Bernoulli equation.
Histological staining
Transverse bi-ventricular slices were fixated in 4% formaldehyde solution (4% formalin; Klinipath, the Netherlands) for a minimum of 24 h. Subsequently, the fixed hearts were dehydrated in an automated system (Leica TP1020; Leica Microsystems, Germany) embedded in paraffin (Klinipath) and sliced into 4-µm sections for histological staining. The slides underwent deparaffinization prior to advancing to the subsequent staining procedure.
Masson trichrome staining was performed to identify collagen deposition, adhering to the standard protocol. Subsequently, the entire stained sections were scanned using a Nanozoomer 2.0-HT digital slide scanner (Hamamatsu Photonics, Japan). Quantification of fibrosis in each section was accomplished by analyzing the percentage of the total section area occupied by fibrotic tissue using the positive Pixel Count v9 algorithm of Aperio’s ImageScope software (version 12.4.0; Leica Microsystem).
To assess the size of cardiomyocytes, ventricular tissue was stained with fluorescein isothiocyanate (FITC)-conjugated wheat germ agglutinin (WGA, Sigma-Aldrich, MO, USA) and DAPI (4’,6-diamidino-2 phenylindole, DAPI, blue) to visualized the extracellular matrix, and nuclei. 30–50 transversally cut cardiomyocytes of each animal were measured for size quantification. The whole stained sections were imaged using an Olympus VS200 ASW digital fluorescent slide scanner (Olympus- Life Science, Japen) at 40x magnification. QuPath Open Software for Bioimage Analysis (version 0.4.3) was used to measure the surface area. Cardiomyocytes area is displayed as a µm2.
To reveal the localization of PLN in mouse cardiac tissue samples, the 2D12 anti-PLN antibody (Invitrogen, CA, USA) were used. Subsequently incubated with secondary antibody donkey anti-rabbit Alexa FluorTM 555 (A31572, Invitrogen). In addition, DAPI (4′,6-diamidino-2-phenylindole; Invitrogen, CA, USA), and WGA (Sigma-Aldrich, MO, USA) were co-stained to visualize the cell nuclei and boundary.
Quantitative polymerase chain reaction (q-PCR)
Total RNA was extracted from snap frozen left ventricular tissue using TRI Reagent (Sigma-Aldrich, MO, USA) according to the manufacturer’s protocol. cDNA synthesis was conducted utilizing the QuantiTect reverse transcription kit (Qiagen, Germany). q-PCR was performed using iQ SYBR green supermix (Bio-Rad, CA, USA) and C1000 Thermal cycler CFX 384 Real-Time PCR Detection System (Bio-Rad).
Gene expression levels were determined by qPCR analysis using iQ SYBR green supermix (BioRad) and, C1000 Thermal cycler CFX 384 Real-Time PCR Detection system (Bio-Rad) with the manufacturer’s instructions and recommended protocol. Gene expression data was normalized to the reference gene values from one of the components of the large 60 S ribosomal subunit (Rplp0, encoding 36B4) using CFX Manager software (version 3.0; Bio-Rad). The resulting values were then expressed relative to the wild-type (WT) sham group. Primer sequences are listed in Table S1.
Protein isolation and Immunoblotting
Total proteins were extracted from snap frozen left ventricles (LVs) using Radioimmunoprecipitation assay lysis buffer as described previously13. Protein concentration was measured using a Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, MA, USA). The protein was then adjusted to a consistent concentration for subsequent Western blot analysis. Samples were heated to 95 °C for 5 min, followed by loading onto a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) system. Load 5ug of protein per well for PLN gel and 20 ug of protein for SERCA gel. Proteins separated by SDS-PAGE were then transferred to an Immun-Blot polyvinylidene fluoride (PVDF) membrane using a semi-dry transfer system (Amersham Biosciences, UK). Revert 700 total protein stain (LI-COR # 926-11021) was applied for total protein detection and subsequent western blot normalization. Following staining, the membranes were blocked with blocking buffer, then incubated overnight at 4 °C with a primary antibody, followed by a one-hour incubation at room temperature with a secondary antibody. Detection was accomplished through enhanced chemiluminescence using Western Lightning Ultra Chemiluminescent Substrate (204-22101) and Cytiva Amersham ImageQuant 800. To prepare 1X working solution, equal volumes Reagent A and Reagent B were combined. Targeting protein quantification is performed using Image Studio Lite Software Ver.5.2. The primary and secondary antibodies that were used are presented in Table S2.
RNA sequencing
RNA isolation was performed as mentioned above. RNA Library preparation and sequencing of 5 RNA samples from each group of animals was perfomed by Lexogen NGS services (Vienna, Austria). The QuantSeq reads (fastq files) were mapped to the Ensembl Mus Musculus genome (GRCh38). Data filtering, normalization, principal component (PC) analysis and differential expression analysis were performed using the DESeq package in R. Differentially expressed genes (DEGs) were defined by a Benjamini-Hochberg adjusted P value of < 0.1 and an absolute fold change of > 0.5. Pathway analysis of DEGs was performed using g: Profiler (https://biit.cs.ut.ee/gprofiler/gost).
Statistical analyses
Data are presented as means ± standard error of the mean (SEM). Because of the low variability observed in the sham groups, a smaller number of mice were included in the sham group. Due to the small group sizes, nonparametric comparisons were conducted between groups. The Kruskal-Wallis test followed by Dunn’s multiple comparisons test was employed for multigroup comparisons. Statistical analysis of DEGs was done in R version 4.3.1. All statistical analyses were performed using the GraphPad Prism (version 10.1.2). Statistical significance was considered at a level of P < 0.05.
Results
TAC induced cardiac hypertrophy and cardiac dysfunction in both WT and R14Δ/+ mice
Echocardiographic assessment of trans-TAC pressure gradients demonstrated a clear increased higher value in TAC-operated groups compared to the sham group and no difference between the TAC groups (Fig.S1). At six weeks post-TAC surgery, revealed a significant decrease in the ejection fraction (%LVEF) and increase in the thickness of diastolic left ventricular anterior wall (LVAWd), and diastolic left ventricular posterior wall (LVPWd) after TAC in both the R14Δ/+ and WT groups (Fig. 1A-C). However, more variation was apparent in %LVEF and not all mice showed a similar decline in %LVEF, indicating that at this stage mice had either compensated or decompensated heart failure (Fig. 1A). The increase in echo determined ventricular wall thickness was corroborated by a significant higher LV mass in all TAC mice (Fig. 1E). Moreover, atria weights were also strongly increased (Fig. 1F) indicative for a hypertrophic stress response in both LV and atria, which is common to pressure overload. There was, however, no difference between the wildtype and the R14Δ/+ group in these responses. Together, R14Δ/+ mice did show similar cardiac hypertrophy and cardiac dysfunction as compared to WT mice, indicating that pressure overload does not accelerate or aggravate cardiac dysfunction in PLN-R14del cardiomyopathy.
TAC induced cardiac hypertrophy and cardiac dysfunction to a similar extend in WT and R14del mice. (A) Ejection fraction (EF%) of individual mice in each group, (B) diastolic left ventricular anterior wall thickness (LVAWd), and (C) diastolic left ventricular posterior wall thickness (LVPWd). (D) Representative cardiac echo graphic images of mice in sham and TAC groups. (E) The ratio of ventricle/tibia and (F) atria/tibia. The data significance was analyzed using Kruskal-Wallis tests, followed by Dunn’s multiple comparisons test. **p < 0.01, ***p < 0.001.
TAC induced cardiac remodeling to a similar extend in WT and R14Δ/+ mice
In response to cardiac pressure overload, the heart undergoes both structural and functional remodeling. WGA staining of LV histological slices revealed significant cardiomyocyte hypertrophy in the TAC groups as compared to their sham groups (Fig. 2A-B). This aligns with the increased LV wall thickness and cardiac weights (Fig. 1). In addition, Masson trichrome staining of midventricular sections demonstrated an excessive extracellular matrix deposition within the ventricles in both WT and R14Δ/+ mice TAC groups as compared with the sham groups (Fig. 2C-D). These cardiac remodeling features were also reflected by changes in gene expression. The relative mRNA expression of NPPA, encoding Atrial Natriuretic Peptide (ANP), a common cardiac wall stress and hypertrophy marker was elevated by more than 20-fold in both TAC groups. Similarly, increased expression of fibrotic genes, including Tissue Inhibitor of Metalloproteinases 1 (Timp1) and collagen type I alpha 1 (Col1a1), corresponded with the histological staining data (Fig. 2E-G). Again, there was no difference in the response to TAC in both groups and cardiac pressure overload therefore induced a similar extent of cardiac remodeling in both genotypes.
Cardiomyocytes hypertrophy and cardiac fibrosis are induced to a similar extend in both WT and PLN-R14del mice post TAC. (A) Representative images of extracellular Matrix (wheat germ agglutinin, WGA, green) and nuclei (4’,6-diamidino-2 phenylindole, DAPI, blue) staining on left ventricular (LV) sections and (B) cardiomyocytes size measurement on transversally cut cardiomyocytes (scale bar 20 μm). (C) Representative (average based) Masson trichrome-staining on LV sections (scale bar 20 μm), and (D) quantification of fibrosis area. Relative mRNA level of (E) cardiac stress (NPPA) and (F, G) fibrosis-related genes (col1a1 and Timp). The data significance was analyzed using Kruskal-Wallis tests, followed by Dunn’s multiple comparisons test. *p < 0.05, ***p < 0.001, ****p < 0.0001.
TAC did not imbalance the PLN and SERCA mRNA and protein level
The SERCA2a/PLN ratio has been suggested to be important for cardiac function and to be altered in heart failure26. We therefore also analyzed the level of SERCA2a, PLN and their ratio in WT and R14Δ/+ mice (Fig. 3A-E). There is a mild, but statistically non-significant, decrease in the expression levels of SERCA2a in TAC surgery group compared to the sham mice (Fig. 3A, C), while the expression levels of PLN protein remained unchanged (Fig. 3B, D). Moreover, no marked differences were found in the SERCA2a/PLN ratios could be observed (Fig. 3E). This indicates that TAC did not disturb the ratio between PLN and SERCA2a levels and that this remains similar between WT and R14Δ/+ mice.
PLN and SERCA level remain similar in both WT and PLN-R14del mice. (A, B) Representative Western blot images of total protein, SERCA protein, and PLN protein levels in LVs. (C, D) SERCA and PLN protein quantification corrected by total protein. (E) SERCA/PLN protein ratio. The data significance was analyzed using Kruskal-Wallis tests, followed by Dunn’s multiple comparisons test. PVDF (polyvinylidene fluoride) membranes were sectioned according to the molecular weights of the target proteins and incubated with the corresponding antibodies. Full-length, uncropped Western blot images are included in the supplemental material for reference.
TAC did not induce abnormal PLN-R14del SER clustering
Malformation of the cardiac SER, with characteristic perinuclear PLN localization, is a hallmark of PLN-R14del cardiomyopathy. This has been observed in human patient biopsies, and in cardiac tissue of R14Δ/+ mice and homozygous R14Δ/Δ mice13. We were wondering if cardiac stress induced by TAC would be able to induce and accelerate this abnormal SER clustering in R14Δ/+. Histological staining with anti-PLN antibody together with WGA and DAPI staining did not reveal any abnormal SER clusters in 14-week-old R14Δ/+mice, which is in accordance with our earlier observation that these appear only after 12 month in these mice. Importantly, cardiac stress induced by TAC did also not induce abnormal SER cluster formation in any R14Δ/+ mice at this age (Fig. 4A). PLN staining in these R14del mice was indistinguishable from that observed in WT mice. As a control we also performed immunofluorescence staining in LV tissue of 21 months PLN-R14Δ/+ mice, confirming earlier observations of abnormal SER clusters in cardiomyocytes in these old mice (Fig.S2). Previously, we showed that P62 is elevated together with SER cluster formation and this has recently been attributed to impaired autophagy23,27. Although P62 Western blotting revealed a significant increase in p62 in WT group after TAC, this was not additionally elevated in R14Δ/+ (Fig. S3) confirming that TAC was not able to further induce this process and trigger R14Δ/+ specific disease processes, including abnormal SER formation.
Whole genome gene expression profiling reveals similar pathway changes between R14Δ/+ and WT after TAC
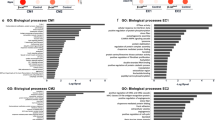
To further investigate potential cardiac differences between WT and R14Δ/+ mice and the effect of TAC induced cardiac stress on signaling pathways in these mice total RNA-sequencing on LV tissues was performed on 5 mice of each group. Principal component analysis revealed a strong clustering of the sham groups on one side and the TAC groups on the other side (Fig. 5A). However, no separation between WT TAC vs. R14Δ/+ TAC and WT sham vs. R14Δ/+ sham were observed (Fig.S4). We subsequently conducted functional enrichment analysis to discern gene programs exhibiting differential regulation between disease states and their respective controls. Examining the most significantly enriched gene ontology (GO) terms for biological processes revealed common terms for both the WT and R14Δ/+ TAC groups. The shared biological processes between WT TAC vs. WT Sham and R14Δ/+ TAC vs. R14Δ/+ Sham were investigated. Common positive enriched biological processes included developmental, extracellular matrix, morphogenesis and response to stimulus terms and reflect the cardiac remodeling process observed after TAC (Fig. 5B). Not surprisingly, the genes enriched in these biological pathways are predominantly associated with hypertrophy (e.g., NPPA, NPPB, and MYH7) and fibrosis (e.g., COL4A3, COL8A1, TIMP1, MMP2). In addition, immune response-related genes are enriched (IL4RA, and Ccl21a) (Table S3). Negative enriched biological processes are dominated by metabolism and ion-transport related genes, including genes for mitochondrial processes (ATP5pf, PINK1, MT-ND5, SLC25A4), amino acid metabolism (MTR and GPT), fatty acid and lipid metabolism (ACADVL, HADHA, and ACSS1) (Table S4). Again, the gene expression profiles after TAC were equally altered in WT and PLN-R14Δ/+ mice, yet these did not influence the progression of PLN-R14del cardiomyopathy.
RNA sequencing identified common biological processes activated in WT and R14del mice in response to TAC. (A) Principal component (PC) analysis plot of RNA sequencing results on mice LV tissue (n = 5 per group). Genes contributing to the variance in PC1, represent 66.09% of the total variance, and genes contributing to the PC2 is 7.87% of the total variance. (B) Top 20 most positive enriched gene biological processes in WT mice and R14Δ/+ TAC mice. Top 15 most negative enriched gene biological processes in WT and R14Δ/+ TAC mice.
Discussion
The onset and severity of disease manifestation in human PLN-R14del carriers exhibit considerable variability, but underlying mechanisms explaining this variation have not been identified. In this study, we investigated whether cardiac stress could accelerate disease onset and exacerbate disease progression in a R14Δ/+ mouse model, which displays similar disease characteristics to those observed in human patients. Although, transverse aortic constriction induced cardiac wall stress and activated numerous cardiac remodeling pathways, it did not accelerate PLN-R14del disease progression in heterozygous R14Δ/+ mice. Importantly, it also did not provoke abnormal sarcoplasmic reticulum (SER) formation in R14Δ/+ mice, a key characteristic of PLN-R14del cardiomyopathy in both humans and mice. These results suggest that cardiac wall stress and concomitant activation of common cardiac stress and remodeling pathways are unlikely to act as common triggers for PLN-R14del cardiomyopathy.
The appearance of sporadic abnormal SER clusters and subsequent disease development in heterozygous PLN-R14del mouse model does not start before one year of age and cardiomyopathy features become visible by 18 months of age13. Also clinical studies indicate a potential link with aging, as symptoms typically manifest in the fifth decade of life21. However, disease development is rather heterogeneous and it is unclear which factors are involved. Accumulating cellular stress is a common factor during ageing and cardiomyocytes, are also affected by age related factors, like hypertension, oxidative stress, genomic stress, metabolic stress and reduction of protein quality controls systems. Applying cardiac wall stress to the heart can stimulate stress pathways and often trigger or accelerate disease. This has been shown in numerous genetic animal models that by themselves do not directly show disease phenotypes, but only after stress induced by cardiac pressure overload16,17,18. This was also shown in a transgenic mouse strain in which a mutant PLN protein was expressed that had lost the ability to form pentamers (TgAFA-PLN) and in PLN-KO mice14. These mice showed decreased survival rate after TAC (within 4 weeks) and increased myocardial fibrosis. It was suggested that the diminished range of SERCA2a mediated calcium handling in TgAFA-PLN and in PLN-KO mice was responsible for enhanced disease development under pressure overload. We did not observe accelerated disease development in PLN-R14del mice, suggesting that altered calcium handling may not be the driving force in this disease. This is in agreement with recent finding indicating that in PLN-R14del abnormal SER formation is most likely the main disease driver for this cardiomyopathy6. Our observation that pressure overload was not able to induce abnormal SER formation in PLN-R14del mice, is in line with the absence of disease acceleration in mice after TAC. This further supports the notion that PLN-R14del cardiomyopathy is not just a calcium related disease, but involves other pathological processes, like abnormal SER formation.
Cardiac remodeling pathways were similarly activated by cardiac pressure overload in WT and PLN-R14del mice. This includes hypertrophic response and alteration in metabolic gene expression profiles, indicative for the well know switch to fetal gene expression and a shift from fatty acid to glucose metabolism28. PLN-R14del cardiomyopathy has been linked to metabolic alterations2but our observation reveals that a change into a fetal-like metabolic state does not trigger PLN-R14del disease. Furthermore, the activation of cardiac remodeling pathways after TAC did not provoke specific features of PLN-R14del cardiomyopathy in these mice. Therefore cardiac remodeling, which also occurs upon ageing29does not appear to be a trigger factor for this disease.
A few mouse studies did report about induction of ER stress pathways after TAC30,31but we were not able to confirm this and did not observe potent activation of these pathways. Since ER stress was not clearly triggered in the experiment, the study doesn’t provide enough evidence to understand its involvement in this specific heart condition (Fig.S5). This will require further investigation in other disease models, since activation of the ER stress unfolded protein response (UPR) is a common feature of PLN-R14del cardiomyopathy in human and mice32. So far, we do not know if UPR activation is contributing to disease or is a protective response to PLN-R14del induced ER malformation. At least in vitro, using human iPSC derived cardiomyocytes, was shown that drug induced ER chaperone BiP expression was able to improve PLN-R14del cardiomyocyte function, suggestive for a protective action32. Since ER malformation is a hallmark of PLN-R14del cardiomyopathy, this clearly needs further attention and will require in vivo studies with specific ER stress activators.
While this study demonstrates that TAC induced pressure overload in young adult PLN-R14del mice does not accelerate disease onset, it is important to acknowledge that the manifestation of PLN-R14del cardiomyopathy may be influenced by multiple converging factors beyond mechanical stress alone. The lack of phenotype induction under TAC may reflect the need for additional or alternative disease triggers that more closely mimic the clinical complexity observed in human mutation carriers. Such as applying TAC at an older age, when age-associated stressors are more pronounced, could offer a more sensitive condition for phenotype induction. Moreover, other physiological or pharmacological stressors may serve as relevant accelerants. These include chronic β-adrenergic stimulation via isoproterenol-loaded osmotic mini-pumps, intense physical exertion through forced swimming or treadmill model, or nutrient deprivation models to induce autophagy. This would not only improve our understanding of PLN-R14del cardiomyopathy but may also guide the development of targeted intervention strategies tailored to specific disease modifiers.
This study also has some limitations. First, only males were included in this study and potential sex-specific effects cannot be excluded. Although no obvious sex differences have been observed in this PLN-R14del mouse model, so far23male sex has been suggested to influence heart failure outcomes in human phospholamban mutation carriers33. Therefore, the use of male mice was considered justified for this study, but future research should aim to address potential sex-related differences more specifically. Secondly, we cannot exclude that activation of pressure overload stress pathways at a later age may provoke PLN-R14del cardiomyopathy features and accelerate disease. If so, it would mean that additional age-related priming factors are required. So far, we have no evidence for this. Finally, we did only investigate DCM related processes and cannot exclude that ACM related features may be affected in these mice upon pressure overload. This would, however, require ex vivo investigations after TAC, since, so far arrythmia could not be detected in vivo in these mice.
In conclusion, cardiac stress induced cardiac remodeling pathways do not trigger PLN-R14del cardiomyopathy features in a mouse model with this pathogenic variant. It therefore remains elusive which factors may promote this cardiomyopathy and this may require investigation of other ageing and stress related pathways, like oxidative stress or ER stress.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author, Dr. Herman H.W. Silljé (h.h.w.sillje@umcg.nl), upon reasonable request. The RNA-sequencing data of the current study is available in the NCBI Sequence Read Archive (SRA) under the accession number PRJNA1285300. https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1285300.
References
Glaves, J. P., Primeau, J. O., Espinoza-Fonseca, L. M., Lemieux, M. J. & Young, H. S. The phospholamban pentamer alters function of the sarcoplasmic reticulum calcium pump SERCA. Biophys. J. 116, 633–647. https://doi.org/10.1016/j.bpj.2019.01.013 (2019).
Vafiadaki, E., Glijnis, P. C., Doevendans, P. A., Kranias, E. G. & Sanoudou, D. Phospholamban R14del disease: the past, the present and the future. Front. Cardiovasc. Med. 10, 1162205. https://doi.org/10.3389/fcvm.2023.1162205 (2023).
Haghighi, K. et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc. Natl. Acad. Sci. U S A. 103, 1388–1393. https://doi.org/10.1073/pnas.0510519103 (2006).
van der Zwaag, P. A. et al. Recurrent and founder mutations in the Netherlands-Phospholamban p.Arg14del mutation causes arrhythmogenic cardiomyopathy. Neth. Heart J. 21, 286–293. https://doi.org/10.1007/s12471-013-0401-3 (2013).
Stege, N. M., de Boer, R. A., Makarewich, C. A., van der Meer, P. & Silljé, H. H. W. Reassessing the mechanisms of PLN-R14del cardiomyopathy. JACC: Basic. Translational Sci. https://doi.org/10.1016/j.jacbts.2024.02.017 (2024).
Stege, N. M. et al. DWORF extends life span in a PLN-R14del cardiomyopathy mouse model by reducing abnormal sarcoplasmic reticulum clusters. Circ. Res. 133, 1006–1021. https://doi.org/10.1161/CIRCRESAHA.123.323304 (2023).
Te Rijdt, W. P. et al. Phospholamban p.Arg14del cardiomyopathy is characterized by phospholamban aggregates, aggresomes, and autophagic degradation. Histopathology 69, 542–550. https://doi.org/10.1111/his.12963 (2016).
van der Zwaag, P. A. et al. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur. J. Heart Fail. 14, 1199–1207. https://doi.org/10.1093/eurjhf/hfs119 (2012).
Posch, M. G. et al. Genetic deletion of arginine 14 in phospholamban causes dilated cardiomyopathy with attenuated electrocardiographic R amplitudes. Heart Rhythm. 6, 480–486. https://doi.org/10.1016/j.hrthm.2009.01.016 (2009).
van Lint, F. H. M. et al. Exercise does not influence development of phenotype in PLN p.(Arg14del) cardiomyopathy. Neth. Heart J. 31, 291–299. https://doi.org/10.1007/s12471-023-01800-4 (2023).
van der Voorn, S. M. et al. Lack of evidence for the role of the p.(Ser96Ala) polymorphism in histidine-rich calcium binding protein as a secondary hit in cardiomyopathies. Int. J. Mol. Sci. 24. https://doi.org/10.3390/ijms242115931 (2023).
Lopera-Maya, E. A. et al. Phenotypic and genetic factors associated with absence of cardiomyopathy symptoms in PLN:c.40_42delAGA carriers. J. Cardiovasc. Transl. Res. 16, 1251–1266. https://doi.org/10.1007/s12265-022-10347-5 (2023).
Eijgenraam, T. R. et al. The phospholamban p.(Arg14del) pathogenic variant leads to cardiomyopathy with heart failure and is unreponsive to standard heart failure therapy. Sci. Rep. 10, 9819. https://doi.org/10.1038/s41598-020-66656-9 (2020).
Funk, F. et al. Phospholamban pentamerization increases sensitivity and dynamic range of cardiac relaxation. Cardiovasc. Res. 119, 1568–1582. https://doi.org/10.1093/cvr/cvad037 (2023).
Cleary, S. R. et al. Dilated cardiomyopathy variant R14del increases phospholamban pentamer stability, blunting dynamic regulation of calcium. J. Biol. Chem. 301, 108118. https://doi.org/10.1016/j.jbc.2024.108118 (2025).
Gurha, P. et al. Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction. Circulation 125, 2751–2761. https://doi.org/10.1161/CIRCULATIONAHA.111.044354 (2012).
Hu, C. et al. Peroxiredoxin-5 knockdown accelerates pressure overload-Induced cardiac hypertrophy in mice. Oxid. Med. Cell. Longev. 2022, 5067544. https://doi.org/10.1155/2022/5067544 (2022).
Shirakabe, A. et al. Drp1-dependent mitochondrial autophagy plays a protective role against pressure Overload-Induced mitochondrial dysfunction and heart failure. Circulation 133, 1249–1263. https://doi.org/10.1161/CIRCULATIONAHA.115.020502 (2016).
Melleby, A. O. et al. A novel method for high precision aortic constriction that allows for generation of specific cardiac phenotypes in mice. Cardiovasc. Res. 114, 1680–1690. https://doi.org/10.1093/cvr/cvy141 (2018).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18, e3000410. https://doi.org/10.1371/journal.pbio.3000410 (2020).
van Rijsingen, I. A. et al. Outcome in phospholamban R14del carriers: results of a large multicentre cohort study. Circ. Cardiovasc. Genet. 7, 455–465. https://doi.org/10.1161/CIRCGENETICS.113.000374 (2014).
de Brouwer, R. et al. Sex-specific aspects of phospholamban cardiomyopathy: the importance and prognostic value of low-voltage electrocardiograms. Heart Rhythm. 19, 427–434. https://doi.org/10.1016/j.hrthm.2021.11.009 (2022).
Eijgenraam, T. R. et al. Antisense therapy attenuates phospholamban p.(Arg14del) cardiomyopathy in mice and reverses protein aggregation. Int. J. Mol. Sci. 23. https://doi.org/10.3390/ijms23052427 (2022).
Knipp, B. S. et al. Ultrasound measurement of aortic diameters in rodent models of aneurysm disease. J. Surg. Res. 112, 97–101. https://doi.org/10.1016/s0022-4804(03)00114-8 (2003).
Zacchigna, S. et al. Towards standardization of echocardiography for the evaluation of left ventricular function in adult rodents: a position paper of the ESC working group on myocardial function. Cardiovasc. Res. 117, 43–59. https://doi.org/10.1093/cvr/cvaa110 (2021).
Kranias, E. G. & Hajjar, R. J. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ. Res. 110, 1646–1660. https://doi.org/10.1161/CIRCRESAHA.111.259754 (2012).
Vafiadaki, E., Kranias, E. G., Eliopoulos, A. G. & Sanoudou, D. The phospholamban R14del generates pathogenic aggregates by impairing autophagosome-lysosome fusion. Cell. Mol. Life Sci. 81, 450. https://doi.org/10.1007/s00018-024-05471-1 (2024).
Taegtmeyer, H., Sen, S. & Vela, D. Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann. N Y Acad. Sci. 1188, 191–198. https://doi.org/10.1111/j.1749-6632.2009.05100.x (2010).
Tracy, E., Rowe, G. & LeBlanc, A. J. Cardiac tissue remodeling in healthy aging: the road to pathology. Am. J. Physiol. Cell. Physiol. 319, C166–C182. https://doi.org/10.1152/ajpcell.00021.2020 (2020).
Okada, K. et al. Prolonged Endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of Endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110, 705–712. https://doi.org/10.1161/01.CIR.0000137836.95625.D4 (2004).
Park, C. S., Cha, H., Kwon, E. J., Sreenivasaiah, P. K. & Kim, D. H. The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 421, 578–584. https://doi.org/10.1016/j.bbrc.2012.04.048 (2012).
Feyen, D. A. M. et al. Unfolded protein response as a compensatory mechanism and potential therapeutic target in PLN R14del cardiomyopathy. Circulation 144, 382–392. https://doi.org/10.1161/CIRCULATIONAHA.120.049844 (2021).
Myrthe, Y. C. et al. Identifying predictors for heart failure outcomes in phospholamban p.(Arg14del)-Positive individuals. JACC Heart Fail. 13 https://doi.org/10.1016/j.jchf.2025.102558 (2025).
Funding
This work was supported by the Dutch Heart Foundation [2018-30 (CVON-PREDICT2) to H.H.W.S. and V.O.N.T., 01-003-2022-0358 (CarMa) to H.H.W.S. and V.O.N.T]; China Scholarship Council Grant [202008230186 to L.S.].
Author information
Authors and Affiliations
Contributions
L.S, V.O.N.T and H.H.W.S. conceived and designed research; L.S., V.O.N.T. and E.M.S. performed experiments; L.S. and K.A.G. analyzed data; L.S., V.O.N.T, and H.H.W.S. interpreted results of experiments; L.S. and K.A.G. prepared figures; L.S. drafted manuscript; L.S., E.M.S., K.A.G., V.O.N.T. and H.H.W.S. edited and revised manuscript; all authors approved final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, L., Schouten, E.M., Gomez, K.A. et al. Cardiac remodeling pathways do not accelerate disease onset and severity in a mouse model of PLN-R14del cardiomyopathy. Sci Rep 15, 35393 (2025). https://doi.org/10.1038/s41598-025-19321-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19321-y