Abstract
Anemia in elderly patients presents a significant health concern, necessitating a thorough understanding of its multifaceted etiology and effective predictive strategies. This retrospective cohort study investigates the influential factors contributing to anemia in elderly patients and develops predictive models to aid in early detection and intervention. We analyzed demographic, clinical, and laboratory data from 864 elderly inpatients and developed a predictive model for anemia using multivariate logistic regression. Model performance was evaluated through discrimination and calibration. Notably, advanced age, elevated levels of C-reactive protein (CRP), and activated partial thromboplastin time (APTT), alongside decreased levels of albumin (ALB), calcium (Ca), and 25-Hydroxyvitamin D [25(OH)D], emerged as significant predictors of anemia. Furthermore, multiple drug resistance (MDR) was identified as a notable risk factor. Through meticulous modeling, incorporating demographic, comorbidity, and laboratory parameters, robust predictive frameworks were developed, achieving an area under the receiver operating characteristic curve (AUC) of 0.955, which indicates a high level of accuracy for risk prediction. This study underscores the complex interplay of factors contributing to anemia in elderly patients and provides a practical approach for its early identification and management.
Similar content being viewed by others
Introduction
Anemia, defined by the World Health Organization (WHO) as a condition where hemoglobin levels are below the normal range, resulting in impaired oxygen transport, is a prevalent health issue globally, particularly among the elderly. This condition is associated with a range of adverse outcomes, including increased morbidity and mortality rates, diminished physical function, cognitive decline, and reduced quality of life1,2 elderly population is especially vulnerable to these negative effects, which underscores the importance of understanding the risk factors contributing to anemia in this demographic. Early identification and management of these risk factors are essential to mitigate the adverse impacts of anemia, thereby improving health outcomes and quality of life in older adults.
Numerous studies have examined the risk factors associated with anemia across various populations and clinical contexts. Tehori et al. investigated whether very low hemoglobin levels confer an additional risk compared to low hemoglobin levels, particularly in cases involving gastrointestinal (GI) bleeding3. In another study, Lezhenko et al. identified pathogenetic factors that contribute to anemia of inflammation in young children suffering from acute inflammatory bacterial diseases of the respiratory system, utilizing mathematical modeling to predict its development4. Furthermore, Islam et al. applied machine learning-based approaches to predict anemia among women in Bangladesh, offering critical insights for policymakers to develop targeted interventions aimed at reducing anemia prevalence in this population5.
The association between anemia and various health conditions has been extensively studied, underscoring the complex interplay between anemia and comorbidities. Gelaw et al. explored whether anemia acts as a risk factor for tuberculosis, highlighting the need to understand the broader health implications of anemia and its potential role in exacerbating other diseases6. Similarly, Oh et al. conducted a retrospective cohort study to evaluate the link between anemia and severe outcomes in COVID-19 patients, demonstrating the significant impact of anemia on the progression and severity of this viral infection7.
Moreover, anemia has been recognized as a risk factor for complications across various medical procedures and conditions. Prior research has highlighted the link between anemia and adverse outcomes in ischemic stroke, total hip arthroplasty, and diabetic foot, indicating that anemia may contribute to poorer prognoses in these contexts8,9,10,11. Ma et al. emphasized the critical importance of addressing anemia in the management of these conditions, reinforcing the need for targeted interventions to mitigate its impact12.
Although previous studies have reported on the prevalence and some risk factors of anemia in elderly populations, few have focused specifically on hospitalized elderly patients using comprehensive clinical and laboratory data. Moreover, predictive models tailored to this population remain scarce and are often limited by small sample sizes or a lack of validation. To address these gaps, our study systematically analyzes demographic, clinical, and laboratory variables in a large cohort of hospitalized elderly individuals and develops a predictive model to identify patients at risk of anemia, thereby informing more effective strategies for its prevention and management.
Methods
Study design
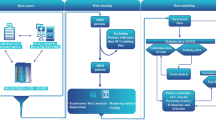
An investigation on the prevalence of anemia and the identification of associated factors among hospitalized elderly patients aged 65 and above was carried out at the Second Hospital of Shandong University, Shandong, China, through a retrospective cohort survey. Eligible cases were derived through systematic application of predefined inclusion and exclusion criteria to the hospital’s electronic medical record system. This rule-driven extraction process reflects standard methodology for retrospective studies using routinely collected clinical data. The study workflow chart is shown in Fig. 1.
Study population
864 elderly patients aged 65 years and above, who were admitted to the Second Hospital of Shandong University between January 1, 2022, and March 31, 2024, were included in the study. The World Health Organization (WHO) guidelines for diagnosing anemia were hemoglobin level < 130 g/L for men and hemoglobin level < 120 g/L for women13. Inclusion criteria for the study comprised hospitalized patients aged 65 years and above who completed the necessary laboratory testing. Patients were excluded if they had missing values for key demographic or clinical variables, including age, sex, hemoglobin level, or primary diagnosis, or if their clinical or laboratory records were incomplete. Individuals with a known history of hematological disorders were also excluded.
Data collection
Sociodemographic characteristics, including age, gender, and comorbidities, were collected via structured interviews and electronic medical records review. Laboratory test results, including hemoglobin levels (HGB), white blood cells (WBC), platelets (PLT), C-reactive protein (CRP), procalcitonin (PCT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), total bilirubin (TBIL), albumin (ALB), creatinine (Cr), urea (Ur), uric acid (UA), prothrombin time (PT), activated partial thromboplastin time (APTT), D-Dimer, serum potassium (K), sodium (Na), chloride (Cl), calcium (Ca), magnesium (Mg), phosphorus (P), N-terminal pro-brain natriuretic peptide (NT-proBNP), 25-Hydroxyvitamin D [25(OH)D], and multiple drug resistance (MDR), were measured by the medical laboratory in the Department of Clinical Laboratory the Second Hospital of Shandong University. All predictor variables were collected either at admission or within the first 72 h of hospitalization, and before the development of anemia in the hospital course. MDR status was determined based on existing microbiological records or initial culture results available shortly after admission. This ensured that all predictors preceded or coincided with the at-risk period and avoided temporal data leakage. Two weeks after admission, hemoglobin levels and other relevant hematological indicators were reassessed to detect the onset of anemia.
Statistical analysis
Descriptive statistics were applied to characterize the study population. Chi-square tests were utilized to examine associations between categorical variables, while continuous variables were analyzed using Mann-Whitney U tests. To determine independent factors associated with anemia among elderly patients, multivariate logistic regression analysis was employed. All the variables were confirmed with variance inflation factors (VIFs) < 5, indicating an acceptable level of multicollinearity. To further assess multicollinearity, we additionally examined condition indices and found no index exceeding 30, suggesting no serious collinearity problem. The regression models were iteratively adjusted for potential confounders, including demographic factors, comorbidities, and laboratory test results. The strength of the association between each variable and anemia was quantified by calculating odds ratios (ORs) with corresponding 95% confidence intervals (CIs).
A predictive model for anemia in hospitalized elderly individuals aged 65 years and older was developed using multivariate logistic regression, incorporating significant predictors identified through the analysis. Continuous variables were assessed for linearity in relation to the logit using lowess smoothing and fractional polynomial plots. No substantial deviation from linearity was observed; thus, continuous predictors were modeled in their original form without transformation. Model performance was evaluated using the area under the ROC curve (AUC), Brier score, calibration slope, and calibration-in-the-large (intercept). The Brier score assesses overall model accuracy, while the calibration slope and intercept evaluate the agreement between predicted and observed probabilities. To reduce the risk of model overfitting and assess internal generalizability, we performed internal validation using a 70/30 split-sample approach, randomly dividing the dataset into a training set (70%) for model development and a testing set (30%) for validation. Model performance metrics were derived from the testing subset. The model was developed and reported according to TRIPOD guidelines.
All statistical analyses were performed using the statistical software packages R (http://www.R-project.org, The R Foundation) and Free Statistics software version 1.8.1. A P-value of less than 0.05 was considered indicative of statistical significance.
Ethics approval and consent to participate
This study protocol was approved in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of the Second Hospital of Shandong University [Approval No. KYLL-2023(KJ)P-1792, October 20, 2023]. Given the retrospective design and the use of anonymized historical clinical data, the requirement for informed consent was waived by the Medical Ethics Committee of the Second Hospital of Shandong University in accordance with relevant guidelines and regulations.
Result
Characteristics of the study population
A total of 864 hospitalized elderly patients aged 65 years and above were enrolled in this study. The sociodemographic characteristics for entire cohort and the differences between the non-anemia group (n = 546) and the anemia group (n = 318) are summarized in Table 1. Among the participants, 448 (51.9%) were male, and 416 (48.1%) were female, with a mean age of 76.3 ± 10.3 years. Various comorbidities were prevalent in the cohort, including hypertension (36.3%), coronary atherosclerotic disease (44.2%), heart failure (21.1%), cerebrovascular disease (38.7%), neurodegenerative diseases (25.7%), diabetes (49.3%), kidney diseases (6.0%), infectious diseases (45.1%) and cancer (8.8%). Laboratory tests revealed significant differences between non-anemic and anemic participants. Hemoglobin levels were markedly lower in the anemia group (97.6 ± 9.3 g/L) compared to the non-anemia group (132.5 ± 14.4 g/L). Similarly, other parameters such as CRP, APTT, D-Dimer, NT-proBNP and MDR exhibited significant variations between the two groups. Furthermore, differences in the intake of vitamin D, iron supplementation, and application of EPO were observed between non-anemic and anemic individuals.
Influential factors of anemia in elderly patients
Multivariate logistic regression analysis revealed that several clinical variables were independently associated with anemia in hospitalized elderly patients, as summarized in Table 2. In the fully adjusted model Model 4, the regression equation included the following predictors: age, CRP, ALB, APTT, D-Dimer, Ca, NT-proBNP, 25(OH)D, and MDR. The estimated regression equation can be expressed as logit(P) = β₀ + β₁×Age + β₂×CRP + β₃×ALB + β₄×APTT + β₅×D-Dimer + β₆×Ca + β₇×25(OH)D + β₈×MDR, where P denotes the probability of anemia.
Among these variables, age was positively associated with anemia (OR = 1.06, 95% CI: 1.04–1.08, P < 0.001), indicating that the odds of anemia increased by approximately 6% with each additional year of age. Higher CRP levels were also linked to increased anemia risk (OR = 1.01, 95% CI: 1.00–1.01, P < 0.001), reflecting the role of systemic inflammation. Conversely, increasing serum albumin was significantly protective (OR = 0.84, 95% CI: 0.81–0.88, P < 0.001), suggesting a relationship between nutritional status and anemia. Longer APTT was associated with greater anemia risk (OR = 1.06, 95% CI: 1.03–1.09, P < 0.001), possibly indicating coagulation pathway disturbances.
Higher D-dimer levels also predicted anemia (OR = 1.16, 95% CI: 1.04–1.28, P = 0.005), highlighting a potential link with hypercoagulability or underlying comorbidities. A strong inverse association was found for lower serum calcium levels (OR = 0.18, 95% CI: 0.07–0.46, P < 0.001), and 25(OH)D levels were likewise inversely associated (OR = 0.88, 95% CI: 0.87–0.90, P < 0.001), suggesting that deficiencies in calcium and vitamin D may contribute to anemia. Notably, patients with multidrug-resistant infections had a substantially increased risk (OR = 2.43, 95% CI: 1.67–3.52, P < 0.001), underscoring the burden of infection-related inflammation and treatment complexity. All continuous predictors were modeled without categorization, and linearity assumptions were assessed prior to inclusion. These associations remained significant in the adjusted Model (Model 1) and partially adjusted Models (Models 2 and 3), indicating the robustness of the findings. The final model equation was: logit(P) = − 9.253 + 0.058×Age + 0.009×CRP − 0.174×ALB + 0.061×APTT + 0.149×D-Dimer − 1.729×Ca − 0.126 × 25(OH)D + 0.889×MDR.
Predictive model performance
Utilizing the results obtained from multivariate logistic regression analyses, we constructed a predictive model aimed at estimating the likelihood of anemia among hospitalized individuals aged 65 years and older. The performance of this predictive model was evaluated using the area under the ROC curve, which yielded an impressive value of 0.955 (95% CI: 0.939 to 0.971, as illustrated in Fig. 2-A). This high AUC value signifies robust discriminatory power, indicating that the model effectively predicts patients with anemia.
Predictive model for anemia in elderly patients. (A) The predictive model demonstrated promising performance in estimating the likelihood of anemia in elderly patients, with AUC values of 0.955 (95% CI 0.939–0.971. (B) The calibration plot indicates good alignment between predicted and observed probabilities, with a calibration slope of 0.97 and intercept of 0.03. The Brier score of 0.086 further confirms model accuracy. (C) The DCA analysis highlights the net benefit of using the predictive model across a range of threshold probabilities. (D) The clinical impact curve illustrates the effective stratification of patients, with a higher number of correct anemia predictions at different risk thresholds.
In addition to the AUC, the Brier score was 0.086, indicating good accuracy. The calibration slope was 0.97 and the calibration intercept was 0.03, suggesting strong calibration and minimal evidence of overfitting. These results support the robustness of the predictive model.
Further validation of the predictive model focused on its calibration and clinical utility. The calibration analysis demonstrated a strong concordance between predicted probabilities and observed outcomes, underscoring the model’s reliability in real-world settings (Fig. 2-B). Additionally, the decision curve analysis confirmed that the model offers a net clinical benefit across various threshold probabilities (Fig. 2-C). This is further supported by the clinical impact curve, which effectively stratified patients and showed a substantial number of accurate anemia predictions across different risk levels (Fig. 2-D). These findings highlight the model’s potential utility in enhancing clinical decision-making processes.
Discussion
Our study provides valuable insights into the prevalence and risk factors of anemia among hospitalized patients aged 65 years and older, addressing a significant public health concern. Significant predictors of anemia identified in our analysis—each treated as a continuous variable—included advanced age, MDR status, CRP, APTT, ALB, Ca, and 25(OH)D. The predictive model, developed through rigorous multivariate analysis incorporating demographic, comorbidities, and laboratory variables, demonstrated a high level of accuracy with the AU of 0.955. This underscores the multifaceted nature of anemia in the elderly and emphasizes the value of comprehensive assessment and targeted intervention strategies for early detection and effective management.
The prevalence of anemia among our cohort was notably high, with 36.81% of patients meeting the WHO criteria for anemia. This prevalence is consistent with other studies documenting the substantial burden of anemia in the elderly, particularly in those with multiple comorbidities and frequent hospitalizations14,15. For instance, recent research by Marton et al.16 corroborates our findings, indicating that anemia remains prevalent among older adults. This underlines the critical need for routine screening and targeted interventions in this demographic to address anemia effectively.
Our study identified several independent predictors of anemia among hospitalized elderly patients. Consistent with previous research, advancing age was a significant determinant of anemia, highlighting the physiological changes and increased susceptibility to nutritional deficiencies and chronic diseases in older adults. Additionally, markers of inflammation, such as higher CRP levels, were strongly associated with anemia, reflecting the role of chronic inflammation in the pathogenesis of anemia of chronic disease. This finding aligns with the work of Salokhiddinovna and Bruserud17,18, who elucidate the impact of chronic inflammation on erythropoiesis and iron metabolism, leading to anemia of chronic disease.
Furthermore, nutritional factors, including low serum albumin and 25(OH)D levels, also emerged as significant predictors of anemia in our study. These findings underscore the importance of addressing nutritional deficiencies and optimizing dietary intake in the management of anemia among elderly patients. Our findings also suggest that increasing 25(OH)D levels may be beneficial in reducing anemia risk, although further causal analysis would be needed to confirm this relationship. Previous studies, such as those by Smith, Tangpricha and Arabi19,20, have indicated potential links between vitamin D status and anemia. This association is further supported by our prior research, which combined observational analysis with Mendelian randomization and demonstrated a causal effect of higher serum 25(OH)D levels in reducing anemia risk21. These findings collectively suggest that optimizing 25(OH)D levels may serve as a strategic approach to alleviate the burden of anemia in elderly populations.
Moreover, the presence of MDR was identified as a novel risk factor for anemia, highlighting the potential impact of polypharmacy and drug interactions on hematologic parameters in the elderly population. This finding is consistent with recent reports by Li et al.22, who described how polypharmacy and inappropriate drug use contribute to adverse health outcomes in the elderly, including anemia.
The development of a predictive model for anemia in hospitalized elderly patients marks a substantial advancement in risk stratification and personalized care, particularly within the context of an aging population increasingly burdened by comorbid conditions. The model’s excellent discriminatory ability, as evidenced by a high AUC of 0.955, underscores its robust performance and potential to accurately identify patients at risk for anemia. By integrating a comprehensive range of demographic characteristics, comorbidities, and laboratory parameters, the model not only demonstrates high predictive accuracy but also enhances its clinical applicability, allowing for early identification of at-risk individuals. Such early detection is crucial, as it enables timely and targeted interventions, potentially mitigating anemia-related complications and improving overall patient outcomes.
Importantly, all continuous predictors in the model were evaluated for linearity prior to inclusion. Lowess smoothing and fractional polynomial plots were used to assess relationships with the logit of anemia, and no significant non-linearity was observed. Therefore, linear functional forms were retained in the model.
It is worth noting that the model was developed to support early risk stratification during hospitalization rather than point-of-admission triage. All included predictors were collected either at admission or within the first 72 h of hospitalization, prior to the onset of anemia in the hospital course. In particular, multidrug-resistant (MDR) status was determined based on pre-existing microbiological culture results or early screening data available shortly after admission. These data represent baseline risk rather than post-outcome variables, and their inclusion does not compromise the temporal integrity of the predictive model.
Moreover, the model’s strong calibration and net clinical benefit, as validated through decision curve and clinical impact analyses, reinforce its reliability and practical utility in real-world settings. These features make it a valuable tool for clinicians, providing a more precise, evidence-based approach to managing anemia in elderly patients. The model aligns with and builds upon previous work by Cheng et al. and Randi23,24, who have also developed predictive frameworks for anemia in the elderly. However, the current model’s integration of a broader set of clinical and laboratory parameters may offer enhanced precision and adaptability across different clinical environments. While the model demonstrated excellent discrimination and calibration, its performance may partially reflect the characteristics of the derivation dataset. To address potential overfitting, internal validation was performed using a 70/30 split-sample approach, in which the model was developed in the training cohort and tested in the hold-out set. The consistent performance metrics across both subsets suggest the model’s internal robustness. However, penalization techniques (e.g., LASSO or ridge regression) were not applied in this analysis. Moreover, external validation in independent cohorts was not conducted, which may limit the generalizability of our findings. Future studies should incorporate such techniques and include data from multiple centers and populations to comprehensively assess the model’s external validity and practical utility.
The predictive model developed in our study holds significant potential for improving both patient outcomes and healthcare resource management. By effectively stratifying elderly patients based on their risk of anemia, this model supports the shift towards personalized, patient-centered care. In healthcare settings where anemia is prevalent, particularly among the elderly, the model can help clinicians prioritize patients who are most in need of intensive monitoring and early interventions, thus optimizing resource allocation. Such proactive management has the potential to reduce anemia-related complications and hospital readmission rates. As personalized medicine continues to gain prominence, the integration of predictive models like this could fundamentally transform anemia management protocols, making them more targeted and efficient. Future research should focus on validating this model in diverse clinical populations, assessing its adaptability, and evaluating its long-term impact on clinical outcomes and healthcare systems.
Beyond its clinical utility, our findings have broader implications for public health policy and healthcare delivery. Given the substantial burden of anemia in elderly populations, there is a clear need for targeted interventions that address modifiable risk factors such as nutritional deficiencies, chronic inflammation, and polypharmacy. Multidisciplinary approaches, including nutritional supplementation, optimized pharmacological treatments, and lifestyle modifications, are essential to reducing the prevalence and severity of anemia in this demographic. As highlighted by Kumar and Bathla25,26, comprehensive care strategies are critical for the effective management of anemia in older adults. By integrating these approaches into both clinical and public health frameworks, healthcare systems can more effectively address anemia in the elderly, leading to improved patient outcomes and enhanced quality of care.
Limitations of this study should be acknowledged. The Clinical observational study design limits causal inference, and residual confounding may affect the interpretation of associations. However, this design also reflects real-world outcomes, providing more practical insights into anemia management in hospitalized elderly patients. Multicollinearity between variables was considered during model development, and highly correlated predictors were carefully assessed to minimize distortion in the estimated effects. Additionally, the single-center design of this study, conducted at a tertiary hospital in Shandong, China, may limit the generalizability of our findings. Local dietary patterns—such as lower meat intake and seasonal variation in fresh produce—may influence the prevalence of nutritional deficiencies like vitamin D or iron, thereby affecting the strength of associations between these factors and anemia. Moreover, regional variation in multidrug-resistant organism prevalence and antibiotic stewardship policies could alter the impact of infection-related predictors. In addition, institutional transfusion thresholds and practices may shape anemia severity and management patterns in ways that differ across healthcare settings. These factors highlight the need for caution when extrapolating the model to other populations. To address these limitations, we plan to conduct external validation studies using datasets from multiple institutions across different regions in China. These efforts will assess the model’s calibration and discrimination in diverse clinical environments and help refine its applicability on a broader scale. Moreover, longitudinal studies are needed to explore the temporal dynamics between identified risk factors and anemia development. Interventional trials should also assess whether targeted strategies based on these predictors can effectively alleviate anemia in hospitalized older adults. Future prospective studies incorporating broader clinical, genetic, lifestyle, and socioeconomic data are warranted to provide a more comprehensive understanding of anemia risk and to refine the predictive model. Lastly, studies involving larger and more heterogeneous populations would benefit from detailed sensitivity analyses, such as exclusion of outliers or subgroup evaluations, to further verify the stability and reliability of the findings.
Conclusion
This study offers significant contributions to understanding the prevalence, risk factors, and predictive modeling of anemia in hospitalized elderly patients aged 65 years and above. Our findings elucidate the complex, multifactorial nature of anemia in this demographic, emphasizing the critical need for comprehensive diagnostic evaluations and tailored intervention strategies. By identifying key predictors of anemia and developing a robust predictive model, this research highlights the potential for enhanced risk stratification and targeted management in clinical practice. The model’s high performance in predicting anemia underscores its practical utility in improving patient outcomes through early detection and personalized care.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Levy, A. et al. Anemia as a risk factor for infectious diseases in infants and toddlers: results from a prospective study. Eur. J. Epidemiol. 20 (3), 277–284. https://doi.org/10.1007/s10654-004-6515-6 (2005).
Vlagopoulos, P. T. et al. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. J. Am. Soc. Nephrol. 16 (11), 3403–3410. https://doi.org/10.1681/ASN.2005030226 (2005).
Tehori, O., Livovsky, D. M., Goldin, E. & Koslowsky, B. The severity of anemia does not correlate with the risk of Gastrointestinal cancer in subsequent evaluation. Scand. J. Gastroenterol. 55 (7), 819–823. https://doi.org/10.1080/00365521.2020.1779804 (2020).
Lezhenko, H. O. & Pogribna, A. O. Prediction of anemia of inflammation development in young children with acute inflammatory bacterial respiratory diseases. CHILD`S HEALTH. 16 (4), 289–295. https://doi.org/10.22141/2224-0551.16.4.2021.236908 (2021).
Islam, M. M. et al. Risk factors identification and prediction of anemia among women in Bangladesh using machine learning techniques. Curr. Women’s Health Reviews. 18 (1), 118–133. https://doi.org/10.2174/1573404817666210215161108 (2022).
Gelaw, Y., Getaneh, Z. & Melku, M. Anemia as a risk factor for tuberculosis: a systematic review and meta-analysis. Environ. Health Prev. Med. 26 (1), 1–15. https://doi.org/10.1186/s12199-020-00931-z (2021).
Oh, S. M. et al. On-admission anemia predicts mortality in COVID-19 patients: A single center, retrospective cohort study. Am. J. Emerg. Med. 48, 140–147. https://doi.org/10.1016/j.ajem.2021.03.083 (2021).
Heo, J., Youk, T. M. & Seo, K. D. Anemia is a risk factor for the development of ischemic stroke and post-stroke mortality. J. Clin. Med. 10 (12), 2556. https://doi.org/10.3390/jcm10122556 (2021).
Zárate Leal, M. F., Martínez Arboleda, J. J., Perez Bermudez, M. J., Londoño, J. F. & Sánchez Vergel, A. A. How does preoperative anemia impact clinical outcomes following primary total hip arthroplasty?? Experience from Colombia. J. Arthroplast. 38 (7), 1303–1308. https://doi.org/10.1016/j.arth.2023.01.025 (2023).
Li, S., Zhao, L., Yu, D. & Ren, H. Attention should be paid to adolescent Girl anemia in china: based on China nutrition and health surveillance (2015–2017). Nutrients 14 (12), 2449. https://doi.org/10.3390/nu14122449 (2022).
Li, J. et al. Association between anemia and the risk and outcomes of diabetic foot in patients with type 2 diabetes mellitus. Experimental Therapeutic Med. 26 (2), 384. https://doi.org/10.3892/etm.2023.12083 (2023).
Ma, J. et al. Dietary patterns and association with anemia in children aged 9–16 years in Guangzhou, China: A cross-sectional study. Nutrients 15 (19), 4133. https://doi.org/10.3390/nu15194133 (2023).
World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1 (Accessed 31 May 2011) (2011).
Boening, A. et al. Anemia before coronary artery bypass surgery as additional risk factor increases the perioperative risk. Ann. Thorac. Surg. 92 (3), 805–810. https://doi.org/10.1016/j.athoracsur.2011.02.076 (2011).
Scrascia, G. et al. Incremental value of anemia in cardiac surgical risk prediction with the European system for cardiac operative risk evaluation (EuroSCORE) II model. Ann. Thorac. Surg. 98 (3), 869–875. https://doi.org/10.1016/j.athoracsur.2014.04.124 (2014).
Marton, I., Agócs, S. & Babik, B. Epidemiology of anemia. Orv. Hetil. 161 (37), 1569–1573. https://doi.org/10.1556/650.2020.31916 (2020).
Pavlova, V. Y. & Kazakovtseva, E. V. Anemia of chronic diseases. J. Volgograd. State Med. Univ. 21 (2), 21–28. https://doi.org/10.19163/1994-9480-2024-21-2-21-28 (2024).
Bruserud, Ø., Vo, A. K. & Rekvam, H. Hematopoiesis, inflammation and Aging—The biological background and clinical impact of anemia and increased C-Reactive protein levels on elderly individuals. J. Clin. Med. 11 (3), 706. https://doi.org/10.3390/jcm11030706 (2022).
Smith, E. M. & Tangpricha, V. Vitamin D and anemia: insights into an emerging association. Curr. Opin. Endocrinol. Diabetes Obes. 22 (6), 432–438. https://doi.org/10.1097/MED.0000000000000199 (2015).
Arabi, S. M., Ranjbar, G., Bahrami, L. S., Vafa, M. & Norouzy, A. The effect of vitamin D supplementation on hemoglobin concentration: a systematic review and meta-analysis. Nutr. J. 19, 1–11. https://doi.org/10.1186/s12937-020-0526-3 (2020).
Bi, S., Zhang, J., Wei, N., Zhou, Q. & Wang, C. Association between serum 25-hydroxyvitamin D level and risk of anemia: An observational and Mendelian randomization study. Int. J. Gen. Med. 2024 (17), 3893–3905. https://doi.org/10.2147/IJGM.S479039 (2023).
Li, Y. et al. Association between polypharmacy and mortality in the older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 100, 104630. https://doi.org/10.1016/j.archger.2022.104630 (2022).
Cheng, L. et al. An ensemble machine learning model for predicting one-year mortality in elderly coronary heart disease patients with anemia. J. Big Data 11 (1), 99 (2024).
Randi, M. L. et al. Prevalence and causes of anemia in hospitalized patients: impact on diseases outcome. J. Clin. Med. 9 (4), 950 (2020).
Kumar, S. B., Arnipalli, S. R., Mehta, P., Carrau, S. & Ziouzenkova, O. Iron deficiency anemia: efficacy and limitations of nutritional and comprehensive mitigation strategies. Nutrients 14 (14), 2976. https://doi.org/10.3390/nu14142976 (2022).
Bathla, S. & Arora, S. Prevalence and approaches to manage iron deficiency anemia (IDA). Crit. Rev. Food Sci. Nutr. 62 (32), 8815–8828. https://doi.org/10.1080/10408398.2021.1935442 (2022).
Acknowledgements
We would like to thank all participants for their time and excellent cooperation, especially sincerely thank the Physician Scientist Team for their enthusiastic and meticulous teaching and guidance.
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was not required, as the data were sourced from historical records and anonymized.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Medical Ethics Committee of the Second Hospital of Shandong University [No. KYLL-2023(KJ)P-1792, 2023-10-20].
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bi, S., Meng, G., Wei, N. et al. Influential factors and predictive model of anemia in hospitalized elderly patients. Sci Rep 15, 35324 (2025). https://doi.org/10.1038/s41598-025-19397-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19397-6