Abstract
Throughout the lifespan, humans exhibit varying abilities in perceiving color and luminance with sensitivity peaking at different stages. The interaction between luminance and color perception is likely influenced by the distinct developmental trajectories of the respective visual psychophysical channels. This study aimed to investigate how chromatic noise affects luminance discrimination thresholds in adolescents, young adults, and elderly individuals. Sixty participants with no visual complaints (20/20 or corrected binocular visual acuity, and no indication of color vision impairment in the Ishihara pseudoisochromatic plate test) were divided into three age groups: adolescents (15.7 ± 0.8 years), young adults (20–40 years), and elderly adults (60 + years). Participants underwent a luminance contrast discrimination task with chromatic noise masking using a mosaic stimulus, where four chromatic noise protocols were applied (protan, deutan, tritan, and a no-noise protocol). The results showed that luminance contrast thresholds were significantly elevated with the addition of chromatic noise in all groups compared to those without chromatic masking noise, but adults exhibited smaller differences in thresholds between the conditions with and without noise compared to participants in the adolescent and elderly groups (p < 0.05). The intergroup comparisons revealed that young adults had the lowest thresholds, followed by adolescents and elderly individuals (p ≤ 0.01). Elderly participants exhibited higher luminance thresholds than young adults in all chromatic noise conditions, especially under the tritan protocol. These findings suggest that the maturation of luminance and color interaction is consolidated after adolescence, with sensitivity peaking of the mechanisms of color-luminance interaction in adulthood and declining in the elderly. The study provides insights into the developmental and aging processes of color-luminance interaction mechanisms, highlighting the continued maturation of color processing mechanisms in adolescence and their subsequent decline with age.
Similar content being viewed by others
Introduction
Perceptual interactions between color and luminance have been described under various experimental conditions1,2,3,4,5. One method for studying these interactions is visual masking, which involves presenting spatial color noise over a luminance signal6,7. Miquilini et al.4 developed a mosaic stimulus in which a target defined by a contrast in luminance was masked by chromatic noise, allowing the study of the threshold discrimination of the masked target. It was found that the luminance threshold contrasts were influenced by the saturation of the colors present in the color noise, with lower threshold contrasts observed in conditions of low color saturation or in the absence of color noise, indicating a suppressive effect of chromatic noise on luminance discrimination.
The results of Miquilini et al.4 reveal a mechanism of interactions between color and luminance that has been evaluated under conditions of absent or diminished color vision in samples with congenital color vision loss or deterioration of color vision due to the natural aging process8,9.
Despite extensive literature describing that parallel visual processing pathways for luminance and color extend from the retina to the primary visual cortex without interaction between these information streams10,11,12,13,14, there is a growing body of literature that details cortical mechanisms of interaction between color and luminance3,15,16,17,18,19. There are cells in the primary visual cortex that respond only to color, others that respond only to luminance, and still others that respond to both color and luminance, which are thought to form the anatomophysiological substrate for the mechanisms of color and luminance interaction in the primary visual cortex16. Xing et al. (2015) identified the presence of interaction between brightness and color in the primary visual cortex through the recording of chromatic visual evoked potentials, this interaction arises in an inhibitory network generated by local brightness contrast at the boundary between the target and the background.
Moreover, recent evidence also indicates that the relationship between these mechanisms may not be restricted to the primary visual cortex but may involve higher-order cortical regions and be related to illumination processes and global scene analysis20, just as individual variations in luminance sensitivity can predict differences in chromatic axes21.
Various studies indicate different trends of development, maturation, and aging of the achromatic and chromatic visual pathways (red-green and blue-yellow) throughout life (e.g.,22,23,24,25). Studies using visual evoked potentials have assessed the development and maturation of the opponent pathways for luminance and color22,26,27. The results showed that the main components of the achromatic response are already mature by as early as 3 months, while the components of the chromatic responses continue to change throughout the first year.
Similarly, psychophysical studies25,28,29 have demonstrated the presence of chromatic discrimination in the red-green pathways as early as two months of age. However, findings regarding the development of the blue-yellow opponent system remain controversial, with some studies reporting discrimination at two months and others suggesting it emerges at later stages of development.
Psychophysical studies suggest a similar maturation of chromatic pathways starting around the age of 1024. Both electrophysiological and psychophysical studies have suggested that the performance of chromatic pathways in children only resembles that of adults after puberty, indicating a continuous development of color vision from childhood22,23.
Despite the existing knowledge about the development of chromatic and achromatic visual pathways, little is known about the maturation of mechanisms related to the interaction between these two visual subsystems. Considering that protocols for assessing luminance discrimination masked by chromatic noise represent mechanisms of interaction between color and luminance, they can be used to evaluate how chromatic and achromatic pathways interact across different age groups throughout life. In the present study, we compared luminance contrast discrimination under and without chromatic masking in adolescents, young adults, and older adults.
Methods
Ethical aspects
This research was approved by the Ethics Committee of the Federal University of Pará (#6.546.671). All methods were performed in accordance with relevant guidelines and regulations. Consent from the legal guardians for the participation of minors in the study was obtained through the signing of an informed consent form, and the assent of the adolescents was obtained through their own signatures accompanied by consent of their legal guardians. Participants and their guardians were informed about the research objectives, the protocols to be applied, the potential benefits and risks involved, and the possibility of withdrawing from the study at any time according to their expressed wishes.
Participants
The present study recruited 20 adolescents aged 15–17 years old (mean age: 15.7 ± 0.8 years) and 20 young adult individuals aged 20–40 years old (mean age: 28.5 ± 9.8 years). Additionally, 20 older adults over 60 years (mean age: 60.2 ± 2.4 years) were included. All participants exhibited normal or corrected visual acuity of 20/20, assessed using the Freiburg Visual Acuity Test (FrACT), and had normal color vision based on the Ishihara pseudoisochromatic plate test30. Participants also had no systemic diseases that could influence vision, congenital or acquired color blindness, or frequent exposure to solvents. Individuals were recruited through direct invitation or referrals from selected subjects.
Luminance contrast discrimination test
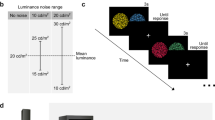
The experiment was conducted using a MacBook Pro LCD 1700 (1690 × 1050 pixel spatial resolution, frame rate of 75 Hz, and 10-bit color resolution). A program was written in MATLAB R2017b (Mathworks, Natick, MA, USA) to conduct the test. A CS-100A colorimeter (Konica Minolta, Osaka, Japan) was used to calibrate the monitor and for all color calculations in the test with all chromaticities calculated for a 2° observer angle and a D65 illuminant.
The test stimulus consisted of mosaics made up of 428 circles with diameters ranging from 0.12 to 0.49° of visual angle, viewed at a distance of 234 cm from the monitor. Sixteen different mosaic arrangements were created, with one of these arrangements randomly selected each time the stimulus was presented.
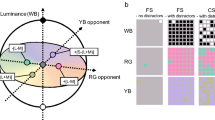
Four configurations of stimuli were presented similarly to previous studies by our group8,9. In three of the four conditions, a luminance contrast in the shape of a Landolt C (external diameter 4.4°, internal diameter 2.2°, gap 1°) was shown in the mosaic, masked by chromatic noise. Three types of chromatic noise were used (protan, deutan, tritan) (Fig. 1. Each type of chromatic noise consisted of 10 chromaticities with a vector magnitude of 0.04 u’v’ units, radially projected from a reference chromaticity (CIE 1976: u’ = 0.1947; v’ = 0.4639. To ensure a more uniform distribution of chromaticities along each confusion line, five chromaticities were spaced by 2 degrees in hue angle on either side of each color confusion line, resulting in five chromatic axes per protocol (see Fig. 2 for chromaticity distribution in the u’v’ color space). These axes were centered on the protan confusion line (protan protocol), the deutan confusion line (deutan protocol), and the tritan confusion line (tritan protocol). An additional condition without chromatic noise (non-noise protocol) was included, using only the reference chromaticity across the mosaic. These confusion lines represent directions in color space along which individuals with color vision deficiencies have difficulty discriminating chromatic differences. They correspond to the main axes of color confusion for protan (associated with L-cones), deutan (M-cones), and tritan (S-cones). These noise types were chosen because they align with the red-green and blue-yellow opponent channels, which are fundamental to human color perception.
Stimuli utilized in each experimental protocol: (A) protan, (B) deutan, (C) tritan, and (D) no-noise protocol. The luminance contrast of the stimuli was represented by a C-shaped target, with chromatic noise applied in protocols (A), (B), and (C) to mask the luminance contrast. In protocol (D), chromatic noise was absent.
The initial luminance of the target was set at 4 cd/m2, while the background had a fixed luminance of 40 cd/m2. A four-alternative forced-choice method (up, down, right, left) was applied to feed a two down/one up adaptive staircase procedure, as seen in Sousa et al.9 and Brito et al.8, corresponding to approximately 71% correct responses. Briefly, an error would lead to a decrease in the target’s luminance, increasing the contrast relative to the background, while two correct responses would result in an increase in the target’s luminance, decreasing the contrast between target and background. A total of 10 reversals were considered for the completion of the test in each protocol, with the last six reversals used to calculate the threshold contrast.
More details on the methodology can be found in previous studies by our group that utilized similar approaches8,9.
Data analysis
To evaluate the effects of age group and protocol (protan, deutan, tritan, and achromatic) on estimated contrast thresholds, we applied Linear Mixed-Effects Models (LMMs). The main model included age group, protocol, and their interaction as fixed effects, with subject ID as a random effect to account for repeated measures. p-values were calculated using Type-III Wald chi-square tests. Post-hoc pairwise comparisons were performed using estimated marginal means, and Tukey’s method was applied to correct for multiple comparisons. Model assumptions were evaluated through visual inspection of residuals using diagnostic plots along with the Shapiro–Wilk normality test and a Bartlett test of homogeneity of variance. Separate models were also fitted for threshold differences, following the same structure of fixed and random effects.
All statistical analyses and visualizations were performed using R software version 4.4.2, along with the following packages: lme4 for model building, lmerTest for p-value computation, car for Type-III Wald chi-square tests, performance for residual diagnostics, emmeans for post-hoc comparisons, and ggplot2 for data visualization. The significance level was set at α = 0.05.
For the statistical analysis, consistent with the approved work plan, the luminance discrimination thresholds in relation to the saturation of the color noise were analyzed using a one-way ANOVA with Tukey’s post hoc test to compare the estimated thresholds in the protan, deutan, tritan, and achromatic protocols of the luminance contrast discrimination test with color masking. A significance level of 0.05 was considered.
Results
The Fig. 3 displays box plots of the estimated luminance contrast thresholds across different stimulus conditions for each age group.
Comparison of luminance contrast thresholds across different stimulus conditions for each age group. Box plots illustrate the distribution of luminance contrast thresholds. Each color corresponds to the protocol under which the thresholds were measured: gray for the no-noise protocol, red for the protan protocol, green for the deutan protocol, and blue for the tritan protocol. *Luminance contrast thresholds are significantly lower than those estimated for all three protocols with chromatic noise (p < 0.05). **Luminance contrast thresholds in the tritan protocol are significantly lower than those measured in the protan and deutan protocols (p < 0.05).
Table 1 shows the results of the comparison of discrimination thresholds considering the factors of stimulation protocols and age group. The results indicated a significant interaction between the age group and stimulation protocol factors.
Post-hoc comparisons showed that for all three age groups significantly lower thresholds were observed in the absence of noise compared to the other stimulus conditions. In the elderly group, not only were significantly lower thresholds noted in the absence of noise compared to the other stimulus conditions, but also a difference was found between thresholds estimated using the tritan protocol and those estimated using the protan and deutan protocols, with thresholds from the tritan protocol being lower than those from the other conditions with chromatic noise.
When considering the fixed factor of the type of stimulation protocol and comparing thresholds across age groups, it was observed that elderly participants exhibited higher threshold contrasts than both young adults and adolescents across all stimulation conditions (p < 0.05). In all stimulation conditions with the presence of chromatic noise, the adolescent group also demonstrated higher threshold contrasts compared to the adult group (p < 0.001); however, this was not observed in the no-noise condition, where adolescents and adults presented threshold contrasts without significant difference (p = 0.738). Figure 4 shows the comparison of luminance thresholds among the different age groups within each stimulation condition.
When assessing the difference between the thresholds estimated in conditions with and without chromatic noise, it was observed that there was a significant effect for the factors age and stimulation protocol, but no significance for the interaction of these factors, as seen in Table 2. Adults exhibited smaller differences in thresholds between the conditions with and without noise compared to participants in the adolescent and elderly groups (p < 0.05). Additionally, a significant difference was noted between the threshold differences when considering the protan and tritan protocols (p = 0.007).
Discussion
This study presented results on the interaction between color and luminance across different age groups (adolescents, adults, and elderly individuals), showing that the mechanisms of color-luminance interaction differ across these age ranges. The findings indicate that the presence of chromatic noise significantly elevated the luminance discrimination thresholds in all age groups, with the largest increase observed in the adolescent and elderly groups. This pattern of results reflects the complexity of the development and aging of the visual systems responsible for processing luminance and color information, which is consistent with previous studies conducted by our research group.
Our group has previously investigated the influence of chromatic masking on luminance threshold discrimination using mosaic stimuli4,8,9. The findings indicate that discrimination thresholds are strongly modulated by the saturation of colors in the noise4, Sousa et al., 2020), and are significantly elevated under mosaic conditions compared to non-mosaic conditions4.This effect may reflect the perceptual strategy employed by observers, as mosaic patterns likely require a scanning process to identify the C-gap orientation. This process could lead to loss of information within the target stimulus, as proposed by Watanabe et al.31. Additionally, the elevated thresholds may also be influenced by the task demands, which involve integrating visual information across spatially distributed elements. This integration likely engages visual grouping mechanisms, and the interaction of these processes with chromatic noise may impose additional perceptual demands, further contributing to the increased thresholds observed.
In previous investigations, we explored how age might affect color-luminance interaction mechanisms, finding that especially under blue-yellow chromatic noise conditions, aging can interfere with chromatic suppression on luminance discrimination. The current study extended the investigation of color-luminance interaction to adolescents. In addition to confirming results previously observed in Brito et al.8 for adult and elderly data, we showed here that adolescents differ significantly from adults in quantitative parameters indicating the interaction between color and luminance.
The similarity in luminance threshold discrimination between adolescents and adults in the absence of chromatic noise suggests that the achromatic mechanism is fully developed, as indicated by earlier studies22,26,27,32. However, it was observed that when exposed to chromatic masking, adolescents exhibited greater chromatic suppression on luminance threshold discrimination than adults, as evidenced by higher threshold contrasts in as evidenced by higher threshold contrasts in the color noise stimulation conditions. This indicates that the mechanisms of color or the interaction between color and luminance in adolescents’ brains have not yet reached full development, as suggested by some studies22,23,24,33.
These findings are also corroborated by studies like those by Fiorentini et al.34, who demonstrated that aging leads to a general decline in contrast sensitivity for both luminance and chromatic signals. In our study, we observed a significant increase in luminance discrimination thresholds in the presence of chromatic noise, especially in elderly individuals, which may be explained by the deterioration of the visual pathways responsible for processing these signals over time. This suggests that the impact of aging on visual perception mechanisms, both for luminance and color, may be interconnected, and the interaction between these mechanisms may be similarly affected with age.
The study by Richardson et al.21, on the other hand, highlighted that individual differences in spectral sensitivity can influence both luminance and chromatic processing. Their findings suggest that these variations may impact the interaction between chromatic axes and luminance, which aligns with our observation that sensitivity to chromatic noise may be mediated by individual differences, especially between adolescents and adults. This interdependence between luminance and color is an important characteristic for understanding how different age groups process chromatic and achromatic stimuli.
Furthermore, Conway20’s research on the role of S-cones in the visual pathways, particularly in the dorsal and ventral cortical areas, offers a crucial perspective for understanding our findings. Conway argues that the activation of S-cones, involved in chromatic contrast perception, is essential for analyzing illumination variations, such as shadows. Aging and the incomplete development of the visual system during adolescence may affect the ability to perceive these illumination variations, which would explain the differences in luminance discrimination thresholds observed between adolescents and adults.
These results indicate that while the achromatic mechanisms are already consolidated in adolescence, the chromatic systems and the interaction between color and luminance continue to develop throughout adolescence and into adulthood, as suggested by Knoblauch et al.23 and Paramei & Oakley33. The gradual deterioration of chromatic sensitivity observed in aging may impact luminance discrimination, particularly under chromatic masking conditions, as observed in our study.
There is an agreement in the literature that throughout adolescence, the brain undergoes significant changes in its volume, gray and white matter, plasticity, and neurotransmitter systems35,36,37. More specifically regarding the visual pathways, studies show that the ventral temporal cortex, which processes information related to faces, size, and color, may undergo important changes during adolescence, such as reduced volume compared to the same region in adults38. Although we do not have direct anatomical or physiological data from the brains of the adolescents tested, the structural and biochemical differences in the adolescent brain may potentially indicate changes in the mechanisms of color and luminance interaction that we have been studying.
A limitation of the present study is the restricted age range of adolescent participants, which was 15–17 years. Future studies should include a broader age range within childhood and adolescence to determine whether this finding represents a continuum of developmental changes. In addition, the differences observed in luminance discrimination thresholds may be attributed to general differences in noise sensitivity rather than specific interactions between color and luminance. Further investigations are needed to explore this possibility, particularly by considering different types of masking.
The mechanisms of color and luminance interaction studied through color masking on the luminance threshold perception show potential for evaluating adolescent neurodevelopment, paving the way for the development of new evaluative methods for assessing the integrity of pathways across different age groups.
Conclusion
The present study, by comparing luminance contrast sensitivity with and without color masking, allowed for a better understanding of how interactions between chromatic and achromatic pathways occur throughout different life stages, extending the investigation to the adolescent age range.
The findings reinforce the hypothesis that the mechanisms of interaction between color and luminance undergo changes throughout life, highlighting the occurrence of a decrease in the suppressive effect of color on the luminance contrast threshold discrimination between adolescence and adulthood. This result suggests that while the achromatic mechanisms (related to luminance perception) are fully developed in adolescence, chromatic mechanisms are still maturing during this phase and may later show a decline due to aging. These findings are important for understanding how visual development and aging impact visual perception and pave the way for future research on visual neurodevelopment and age-related sensory processes.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cooper, B., Sun, H. & Lee, B. B. Psychophysical and physiological responses to gratings with luminance and chromatic components of different spatial frequencies. J. Opt. Soc. Am. A 29(2), A314–A323. https://doi.org/10.1364/JOSAA.29.00A314 (2012).
Cormenzana Méndez, I. et al. Color discrimination is affected by modulation of luminance noise in pseusochromatic stimuli. Front. Psychol. 7, 1006. https://doi.org/10.3389/fpsyg.2016.01006 (2016).
Li, X. et al. Mixing of chromatic and luminance retinal signals in primate area V1. Cereb. Cortex 25(7), 1920–1937. https://doi.org/10.1093/cercor/bhu002 (2015).
Miquilini, L. et al. Influence of spatial and chromatic noise on luminance discrimination. Sci. Rep. 7, 16944. https://doi.org/10.1038/s41598-017-17334-5 (2017).
Souza, G. S. et al. Low number of luminance levels in the luminance noise increases color discrimination thresholds estimated with pseusochromatic stimuli. Front. Psychol. 5, 1291. https://doi.org/10.3389/fpsyg.2014.01291 (2014).
Kim, Y. J. & Mullen, K. Suprathreshold interactions between color and luminance contrast: The effect of cross-oriented luminance contrast on perceived color contrast under dichoptic, monocular, and binocular viewing conditions. J. Vis. 16(12), 1146. https://doi.org/10.1167/16.12.1146 (2016).
Switkes, E., Bradley, A. & De Valois, K. K. Contrast dependence and mechanisms of masking interactions among chromatic and luminance gratings. J. Opt. Soc. Am. A 5(7), 1149–1162. https://doi.org/10.1364/JOSAA.5.001149 (1988).
Brito, R. M. G. et al. Differences in chromatic noise suppression of luminance contrast discrimination in young and elderly people. Vis. Neurosci. 39, E006. https://doi.org/10.1017/S0952523822000050 (2022).
Sousa, B. R. S. et al. Specificity of the chromatic noise influence on the luminance contrast discrimination to the color vision phenotype. Sci. Rep. 10, 17897. https://doi.org/10.1038/s41598-020-74875-3 (2020).
Dacey, D. & Lee, B. The “blue-on” opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature 367, 731–735. https://doi.org/10.1038/367731a0 (1994).
Kaplan, E. & Shapley, R. M. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc. Natl. Acad. Sci. U.S.A. 83(8), 2755–2757. https://doi.org/10.1073/pnas.83.8.2755 (1986).
Lee, B. B., Martin, P. R. & Valberg, A. Amplitude and phase of responses of macaque retinal ganglion cells to flickering stimuli. J. Physiol. 414(1), 95–106. https://doi.org/10.1113/jphysiol.1989.sp017686 (1989).
Lee, B. B., Martin, P. R. & Valberg, A. Nonlinear summation of M- and L-cone inputs to phasic retinal ganglion cells of the macaque. J. Neurosci. 9(4), 1433–1442. https://doi.org/10.1523/JNEUROSCI.09-04-01433.1989 (1989).
Lee, B. B., Martin, P. R. & Valberg, A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. J. Physiol. 414(1), 223–243. https://doi.org/10.1113/jphysiol.1989.sp017685 (1989).
Burns, S. P., Xing, D. & Shapley, R. M. Comparisons of the dynamics of local field potential and multiunit activity signals in macaque visual cortex. J. Neurosci. 30(41), 13739–13749. https://doi.org/10.1523/JNEUROSCI.0743-10.2010 (2010).
Johnson, E. N., Hawken, M. J. & Shapley, R. The spatial transformation of color in the primary visual cortex of the macaque monkey. Nat. Neurosci. 4(4), 409–416. https://doi.org/10.1038/86061 (2001).
Martins, I. C. V. S. et al. Spatial frequency selectivity of the human visual cortex estimated with pseudo-random visual evoked cortical potential (VECP). Vision. Res. 165, 13–21. https://doi.org/10.1016/j.visres.2019.09.004 (2019).
Risuenho, B. B. O., Miquilini, L., Lacerda, E. M. C. B., Silveira, L. C. L. & Souza, G. S. Cortical responses elicited by luminance and compound stimuli modulated by pseudo-random sequences: Comparison between normal trichromats and congenital red-green color blinds. Front. Psychol. 6, 53. https://doi.org/10.3389/fpsyg.2015.00053 (2015).
Shapley, R., Nunez, V. & Gordon, J. Low luminance contrast’s effect on the color appearance of S-cone patterns. Vision. Res. https://doi.org/10.1016/j.visres.2024.108448 (2024).
Conway, B. R. Color signals through dorsal and ventral visual pathways. Vis. Neurosci. 31(2), 197–209. https://doi.org/10.1017/S0952523813000382 (2013).
Richardson, A. J., Lee, K. R., Crognale, M. A. & Webster, M. A. Using equiluminance settings to estimate the cardinal chromatic directions for individuals. J. Opt. Soc. Am. A: 40(3), A169–A177. https://doi.org/10.1364/JOSAA.480055 (2023).
Crognale, M. A. Development, maturation, and aging of chromatic visual pathways: VEP results. J. Vis. 2(6), 438–450. https://doi.org/10.1167/2.6.2 (2002).
Knoblauch, K., Vital-Durand, F. & Barbur, J. L. Variation of chromatic sensitivity across the life span. Vision. Res. 41(1), 23–36. https://doi.org/10.1016/s0042-6989(00)00205-4 (2001).
Ling, B. Y. & Dain, S. J. Development of color vision discrimination during childhood: Differences between blue-yellow, red-green, and achromatic thresholds. J. Opt. Soc. Am. A 35(4), B35–B42. https://doi.org/10.1364/JOSAA.35.000B35 (2018).
Ventura, D. F. Visão de cores no primeiro ano de vida. Psicol. USP 18(2), 83–97. https://doi.org/10.1590/S0103-65642007000200006 (2007).
Hammarrenger, B., Lepore, F., Lippé, S. & Labrosse, M. Magnocellular and parvocellular developmental course in infants during the first year of life. Doc. Ophthalmol. 107(3), 225–233. https://doi.org/10.1023/B:DOOP.0000005331.66114.05 (2003).
Ossenblok, P., Reits, D. & Spekreijse, H. Analysis of striate activity underlying the pattern onset EP of children. Vision. Res. 32(11), 2037–2045. https://doi.org/10.1016/0042-6989(92)90044-J (1992).
Hamer, R. D., Alexander, K. R. & Teller, D. Y. Rayleigh discriminations in young human infants. Vision. Res. 22(5), 575–587 (1982).
Peeples, D. R. & Teller, D. Y. Color-vision and brightness-discrimination in 2- month-old human infants. Science 189(4208), 1102–1103 (1975).
Ishihara, S. Series of plates designed as tests for colour-blindness (Handaya Hongo Harukich, 1997).
Watanabe, A., Pokorny, J. & Smith, V. C. Red-green chromatic discrimination with variegated and homogeneous stimuli. Vision. Res. 38(8), 1033–1040. https://doi.org/10.1016/S0042-6989(97)00455-0 (1998).
Hammarrenger, B. et al. Developmental delay and magnocellular visual pathway function in very-low-birthweight preterm infants. Dev. Med. Child Neurol. 49(1), 28–33. https://doi.org/10.1017/s0012162207000084.x (2007).
Paramei, G. V. & Oakley, B. Variation of color discrimination across the life span. J. Opt. Soc. Am. A: 31(4), A375–A384. https://doi.org/10.1364/JOSAA.31.00A375 (2014).
Fiorentini, A., Burr, D. & Morrone, M. C. Contrast sensitivity in aging: The effect of luminance and chromatic noise. J. Vis. 9(3), 445–455 (1996).
Blakemore, S.-J. Imaging brain development: The adolescent brain. Neuroimage 61(2), 397–406. https://doi.org/10.1016/j.neuroimage.2011.11.080 (2012).
Gogtay, N. et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. 101(21), 8174–8179. https://doi.org/10.1073/pnas.0402680101 (2004).
Pinto, J. G. A., Hornby, K. R., Jones, D. G. & Murphy, K. M. Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan. Front. Cell. Neurosci. 4, 16. https://doi.org/10.3389/fncel.2010.00016 (2010).
Golarai, G., Liberman, A., Yoon, J. M. D. & Grill-Spector, K. Differential development of the ventral visual cortex extends through adolescence. Front. Human Neurosci. 3, 80. https://doi.org/10.3389/neuro.09.080.2009 (2010).
Funding
This research was supported by the following grants: FINEP, IBN-Net #1723, and FAPESP Thematic Project 2022/00191-0. The article processing charge (APC) was financed by the Federal University of Pará (PROPESP/UFPA/PAPQ/PROAP/2025). TCBF received an FAPESPA undergraduate scholarship. J.R.P received a CAPES master’s fellowship. LCPM received a CAPES doctoral fellowship. DFV, MFC and GSS are CNPq Fellows. The CNPq Productivity Grants for DFV, MFC, and GSS are 314630/2020-1, 302552/2017-0, and 309936/2022-5, respectively.
Author information
Authors and Affiliations
Contributions
T.C.B.F, J.R.P, L.C.P.M, G.S.S, L.M contributed to the conception of the work. T.C.B.F, J.R.P, L.C.P.M, B.R.S.S, R.M.G.B, M.I.C.V, G.S.S, L.M was involved in the investigation, methodology, formal analysis and writing – T.C.B.F, J.R.P, L.C.P.M, B.R.S.S, R.M.G.B, M.I.C.V, M.F.C, D.F.V, G.S.S, P.R.K.G, L.M participated in review & editing. T.C.B.F, J.R.P, L.C.P.M, G.S.S., L.M carried out the investigation, designed the experiments, data curation, formal analysis – T.C.B.F, J.R.P, B.R.S.S, G.S.S, L.M original draft, visualization, supervision,. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ferreira, T.C.B., Parente, J.R., Monteiro, L.C.P. et al. Color vision and luminance discrimination throughout the life span. Sci Rep 15, 35616 (2025). https://doi.org/10.1038/s41598-025-19430-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19430-8